Abstract
Mangroves are intertidal ecosystems that are particularly vulnerable to climate change. At the low tidal limits of their range, they face swamping by rising sea levels; at the high tidal limits, they face increasing stress from desiccation and high salinity. Facilitation theory may help guide mangrove management and restoration in the face of these threats by suggesting how and when positive intra- and interspecific effects may occur: such effects are predicted in stressed environments such as the intertidal, but have yet to be shown among mangroves. Here, we report the results of a series of experiments at low and high tidal sites examining the effects of mangrove density and species mix on seedling survival and recruitment, and on the ability of mangroves to trap sediment and cause surface elevation change. Increasing density significantly increased the survival of seedlings of two different species at both high and low tidal sites, and enhanced sediment accretion and elevation at the low tidal site. Including Avicennia marina in species mixes enhanced total biomass at a degraded high tidal site. Increasing biomass led to changed microenvironments that allowed the recruitment and survival of different mangrove species, particularly Ceriops tagal.
Keywords: mangrove, facilitation, intertidal, salinity, density, sediment
1. Introduction
Mangroves are forests that grow at sheltered intertidal sites from the tropics to warm temperate latitudes. They are among the most productive of all ecosystems, and provide a range of goods and services including coastal protection, fishery nursery habitat and carbon sequestration (Ewel et al. 1998; McLeod & Salm 2006; Huxham et al. 2007; Bouillon et al. 2008). Although still widespread, they are under enormous pressure from human impacts, with around 35 per cent of the original area degraded or destroyed since 1980 and current global rates of loss running between 1 and 2 per cent per annum (Valiela et al. 2001; FAO 2007). While direct exploitation for timber, fuel wood and aquaculture are the main current threats, climate change (interacting in multiple ways with other pressures) is likely to become the largest cause of mangrove destruction in the future (Gilman et al. 2008).
Mangrove ecosystems are particularly vulnerable to the predicted impacts of climate change for three reasons. First, and most generally, they inhabit an environment subject to extreme and fluctuating pressures, including the physical impacts of waves, variations in salinity, anoxic sediments and low nutrient availability (Krauss et al. 2008). Hence, mangrove trees are often able to tolerate stress but grow at sub-optimal rates in response, making the mangrove ecosystem vulnerable to phase shifts to new (and less desirable) stable states including salt-flats (Kirui et al. 2008) and eroding, low oxygen mud flats (Cahoon et al. 2003). While mangroves often show resistance and resilience in the face of disturbances, the stresses brought by climate change combined with those they already face may cause sudden and irreversible loss at many sites. Second, mangroves occupy a narrow ecological niche (lying most frequently between mid- to high-intertidal zones) and many of the areas they currently inhabit will be inundated by sea level rise in this century. Mangroves may survive rising sea levels by migrating inland or by facilitating soil surface elevation increases (McLeod & Salm 2006; McKee et al. 2007a); where neither is possible, they will drown out and die. Third, hypersaline conditions are common at the high-tidal limits of mangrove forests and salinity is often the main factor limiting growth and recovery following disturbance (Jayatissa et al. 2008; Kirui et al. 2008). All climate change scenarios predict increased temperatures, implying faster evaporation of seawater. Regional predictions of precipitation are currently very uncertain. Many show either reduced or more sporadic rainfall, implying lower fresh water inputs for some or all of the year (IPCC 2007). A general pattern of drying in the subtropics and increases (or little change) in precipitation in the tropics is apparent in many models—see for example those for East Africa (Doherty et al. 2009). Where temperature increases combine with reduced total freshwater input or longer seasonal droughts, increases in the salinity of sediments in those high tidal areas into which mangroves must migrate can be expected. Hence, mangroves may face a ‘climate change squeeze’, with low tidal sites threatened by submersion and high tidal areas becoming increasingly saline.
What can be done to mitigate these threats? Managers can look to a growing literature on mangrove restoration to inform plans to enhance the resilience of mangrove areas. Resilience implies recovery from natural or anthropogenic disturbance, which often needs some active intervention. Ensuring a natural hydrological regime, with regular tidal flushing, is important and may be sufficient to ensure recovery in areas with good seed or propagule supply (Field 1998; Lewis 2005). Managers must also protect high-quality forests by reducing human impacts, for example by developing alternative livelihoods for those dependent on mangrove exploitation (McLeod & Salm 2006). However, the direct planting of new trees is necessary in areas that cannot recover otherwise (Kairo et al. 2001; Bosire et al. 2008; Kirui et al. 2008). It is likely to become more important if managers are to keep pace with the scale of changes predicted under most climate change scenarios; for example, it will be necessary to establish mangroves in previously unforested areas as current habitat disappears. Such direct intervention raises many questions, including what species should be selected for planting, how should seedlings be raised, what spatial positioning (regular or clumped; high or low density) should be adopted and how can new plantations be designed for maximum resilience to future climate change?
Mangrove planting has usually resulted in monospecific plantations of seedlings in evenly spaced rows, reflecting an assumption that competition between seedlings must be minimized to ensure survival (Gedan & Silliman 2009). Facilitation theory questions this assumption, suggesting that positive interactions among individuals and species may enhance survival and growth, particularly in harsh environments such as the intertidal zone (Bertness & Hacker 1994; Brooker et al. 2008). Experiments in the Caribbean (McKee et al. 2007b) and Florida (Milbrandt & Tinsley 2006) demonstrate that small saltmarsh plants may act as benefactor species enhancing recruitment and survival of mangroves at high intertidal sites. There are no published experimental demonstrations of positive intra- or interspecific effects of mangrove species themselves on mangrove survival, recruitment or growth. Assessing these effects (intra-specifically at low and high tidal sites, and interspecifically at a high tidal site) is the primary goal of the current paper.
One probable effect of using high planting densities at low- and mid-tidal sites is enhanced sediment trapping. This may itself be a facilitatory effect, with more sediment bringing with it more nutrients for growth. However, it could also lead to the smothering of pneumatophores and stunting or death of the trees. Field observations (e.g. Krauss et al. 2003) and experiments using artificial structures (Young & Harvey 1996) support the idea that higher densities enhance sedimentation, but it is also possible that they lead to scouring and erosion (Furukawa & Wolanski 1996). Sediment accretion is one of the processes by which mangroves may raise the soil surface, thus responding to sea level rise (Cahoon & Lynch 1997), and may also contribute to the ability of mangroves to sequester carbon (Bouillon et al. 2008). Management for climate change therefore requires information on whether planting at high densities will indeed cause sediment accretion and if so whether this will involve decreased survival. The ‘severity gradient hypothesis’ predicts that the balance of facilitative to competitive effects will tip towards the former with increasing environmental harshness (Brooker et al. 2008); in the context of mangroves, this may imply that facilitation is less likely at protected, low tidal sites with freshwater input than at arid, high tidal sites. Managers may therefore need to adopt different approaches depending on tidal position. We report results of experiments testing the effects of mangrove density on seedling survival at both types of site, and explore the effects of density on sediment dynamics at a low tidal site.
Positive interactions may also occur between different species of mangroves. Numerous, mostly terrestrial, experiments have now shown how higher species richness can lead to enhanced ecosystem functioning (such as higher productivity, e.g. Fargione et al. 2007), but this has yet to be shown in mangrove systems. We present summary results from experiments involving mixed species plantations at a high tidal site that demonstrates interspecific facilitation of recruitment and ecosystem recovery. Recruitment of new mangrove plants under established trees could be due to the amelioration of physical conditions. It might also reflect the reduction of biotic stresses, such as herbivory (Alberti et al. 2008) or could simply imply a passive trapping of seeds or propagules rather than direct facilitation. Additional experimental work therefore examined the effects of mixing species on rates of herbivory and the survival of propagules directly planted into plots of different species compositions in order to better understand the mechanisms involved.
2. Material and methods
(a). Study sites
(i). Low tidal site, Sri Lanka
The study site was Palakuda, situated in Puttalam lagoon, Sri Lanka (8°14′ N, 79°73′ E). Maximum tidal range in the lagoon is 60 cm, and the site lies approximately 0.2 m above chart datum; however, the lagoonal topography modifies normal tidal patterns, and between October and May the site is usually permanently inundated, while between June and September the site experiences exposure diurnally or for longer periods. Salinity averages 24 PSU, although it may range from 15 to 35 depending on the rainfall. Average rainfall is 1200 mm yr−1 and maximum daily temperatures vary between 30.4°C and 33.6°C (Arulananthan et al. 1995). The substrate is a muddy sand (2.8% carbon content, 6.5% silt) and the surface is permanently wet.
(ii). High tidal sites, Kenya
The study sites were in Gazi bay, located on the southern coast of Kenya (4°28′ S and 39°29′ E). The bay is a shallow coastal ecosystem with extensive mangrove formations intersected by two creeks. The total annual rainfall ranges between 1000 and 1600 mm. The average annual temperature is 26°C with daily variation between 24°C and 39°C (Kenya Meteorological Department, Mombasa). Mangroves in Gazi have been exploited for many years, mainly as sources of fuel wood and building poles for the local community as well as to provide wood for brick and calcium industries in the 1970s (Kairo 1995). Clear felling left some areas of the bay completely denuded (Bosire et al. 2003) and these sites still show few signs of natural regeneration. Two of these cleared sites were used for the present study. They are high tidal (approx. 3.2 m above chart datum), high salinity (59 average pore water salinity) sandy areas, inundated only during spring tides; the surface of the sediment often appears completely dry. The three species used in the current experiments grow sympatrically in the remaining natural forest contiguous to the experimental sites and were present on the sites before clear felling.
(b). Experiment 1: intraspecific effects on sediment dynamics and survival at a low tidal site
Propagules of Rhizophora mucronata (a common mangrove species in Sri Lanka that grows naturally at the experimental site) were collected from wild trees growing nearby and used for planting. Fifteen 7.2 × 7.2 m2 plots, arranged in three blocks of five plots each, with a minimum gap of 1.2 m between plots, were demarcated in May 2006 in an area of bare mudflat. Plots were randomly allocated to one of five treatments: four different planting densities (6.96, 3.26, 1.93 and 0.95 plants m−2), and an unplanted control. Hence, each treatment was replicated three times within a randomized block design, and a total of 2037 trees were planted. Sediment accretion was determined by laying a mixture of 50 per cent powdered feldspar and 50 per cent sand over a 30 × 30 cm2 surface area in the centre of each plot and periodically taking soil plugs (four per plot) cut out of the sediment containing the marker horizon. Soil elevation changes were measured simultaneously with accretion measurements; elevation change differs from sediment accretion because it incorporates sub-surface processes, such as root growth and expansion, as well as above-surface processes such as accretion and erosion. At the two opposite corners of the feldspar marker horizon in each plot, two 1 m long × 0.64 cm diameter stainless steel pins were driven 80 cm into the soil. The distances from the top of each pin to the soil surface were periodically measured to the nearest 0.1 cm to give cumulative and annual changes in elevation. Data were collected between August 2006 and September 2008 in planted plots and controls. Percentage survival of trees in all plots was recorded in April 2008. Mean annual accretion and elevation rates and mean percentage survival values for all treatments were compared using two-way analysis of variances (ANOVAs, with block included as a random factor in these analyses) followed by Tukey post hoc tests; these and subsequent statistical tests were conducted using Minitab 15, SPSS 14 or SAS 9.2 software.
(c). Experiment 2: intraspecific effects on survival at a high tidal site
Wild seedlings of Avicennia marina, averaging 18 cm in height, were collected from mangrove stands adjacent to the field site in July 2007 and randomly assigned to one of the four density treatments: 0.44, 2.25, 4.0 and 8.0 plants m−2. Treatments were randomly allocated to 16, 6 × 6 m2 plots arranged into four blocks, with one replicate for each treatment per block (constraints on space precluded replicating within blocks), giving 2120 trees in total. Blocks were arranged on a tidal gradient ranging from 3.15 to 3.40 m above chart datum. Survival of trees was recorded after six months in February 2008, and the effects of blocks and density examined using a General Linear Model.
(d). Experiment 3: interspecific effects on growth and recruitment at a high tidal site
The effects of mangrove species identity and richness, as well as of environmental variables, on aboveground biomass and ecosystem recovery were investigated in a replanted area of 0.28 ha. Three species of mangrove, A. marina, Bruguiera gymnorrhiza and Ceriops tagal were used in the experiment. The eight treatments consisted of each species on its own (henceforth A, B and C), all two species mixes (AB, AC and BC), the three species mix (ABC) and unplanted control plots (Cont). Plots were 6 × 6 m2, with a planting distance of 0.6 m between trees; hence each planted plot consisted of 121 trees (at a density of 0.3 plants m−2), giving 3388 trees in total. Interplot distances were kept to a minimum of 6 m; this is greater than the home range size of Uca spp. crabs, the dominant epifauna at the site, hence helping to ensure independence of plots (Skov & Hartnoll 2002). Treatments were randomly allocated to plots in a replicated random block design, with two blocks (separated by approx. 100 m) each containing two replicates; hence, there was a replication of four for each treatment, and a total of 32 plots. Seeds and propagules of the three species were collected from the surrounding forest in early 2004, and grown in nursery plots before planting in the treatment plots in July/August 2004. All planted saplings were approximately 30 cm in height, of the same age and were randomly allocated to relevant plots.
A range of environmental variables, including sediment grain size, temperature, moisture content and height above sea level, were measured in all plots before planting, and at regular intervals afterwards; further details on methods are given in Kirui et al. (2008). Aboveground biomass in plots was measured in August 2005, 2006, 2007 and 2008. Eighteen plants were randomly selected in each plot. In plots with two or three species, stratified random sampling was used to give nine or six plants per species, respectively. Heights were measured from the ground level to the point at the top where the last set of leaves joined the stem (apical bud). Stem diameter was measured at 30 cm above the ground level for A. marina, and in the middle between the first and second internode above the propagule for the other two species. Diameter and height were used as predictor variables in regression equations developed from wild trees of representative size to estimate the aboveground biomass of each of the three species (see Kirui (2008) for more details). Leaf damage was recorded as evidence of herbivory. The proportion of damaged leaves on the 18 randomly selected trees in each plot was recorded annually along with the degree of damage. The number and species of any ‘wildlings’ (wild, unplanted saplings) recruiting to each plot were recorded in August 2007. ANOVA and regressions were used to examine differences between treatments in biomass and relationships between biomass and environmental variables.
(e). Experiment 4: effects of saplings on survival of propagules at the high tidal site
Recruitment of naturally dispersed propagules (‘wildings’) was observed in the experimental plots described under ‘experiment 3’, despite the absence of such recruitment in the experimental area for over 30 years. In order to better understand the processes involved in this, propagules of C. tagal were collected from nearby wild trees in August 2008 and 12 were randomly allocated to each experimental planted and control plot (giving 384 in total). All were labelled (using monofilament) and planted using a stratified random approach with four plants at randomly chosen positions within each quarter of each plot. Survival was then monitored at approximately monthly intervals over the next six months. Differences between treatments in the numbers of wildings were examined using ANOVA and in survival curves of planted propagules using Cox regression, a semi-parametric multiple regression technique designed to deal with non-normal survival data (Collet 1994).
3. Results
(a). Experiment 1: intraspecific effects on sediment dynamics and survival at the low tidal site
Mean annual rates of sediment accretion (measured using the marker horizon technique) and surface elevation (measured using the pins) over the first 25 months of the experiment ranged from 7.3 to 15.6 mm yr−1 and −0.4 to 2.1 mm yr−1, respectively (figure 1).
Figure 1.
Mean (±s.e.) annual sediment accretion (open diamonds) and elevation (filled circles) rates measured for five treatments of mangrove seedling density over 28 months at Palakuda, Sri Lanka.
Annual accretion and elevation rates both varied significantly between densities (ANOVA: F4,8 = 15.1, p < 0.001; F4,8 = 4.4, p = 0.03 accretion and elevation, respectively) but not between blocks. Both showed the same trend of increasing rates with increasing density, although rates of accretion were between 7 and approximately 19 times faster than rates of soil elevation (figure 1). All treatments showed positive elevation change with the exception of the unplanted controls, which experienced an average erosion/subsidence of 0.4 mm during the period of the experiment. Mean percentage survival after 702 days was highest in the highest density treatment and lowest in the lowest density treatment (table 1); mean survival differed significantly between the lowest density and the other treatments (table 1; ANOVA: F4,8 = 22.8, p < 0.001).
Table 1.
Mean (±s.e.) % survival after 24 months of R. mucronata planted at four different densities at Palakuda, Sri Lanka; means with different superscripts showed significant differences.
| density (plants m−2) | % survival |
|---|---|
| 6.96 | 93.7 ± 1.2a |
| 3.26 | 88.8 ± 1.7a |
| 1.93 | 89.0 ± 0.6a |
| 0.95 | 79.6 ± 1.2b |
(b). Experiment 2: intraspecific effects on survival at the high tidal site
After six months, the mortality rates of the A. marina seedlings ranged from 1 to 100 per cent per plot, with large differences between density treatments and blocks. Mean per cent mortality rates were 58, 44, 29 and 24 in the 0.44, 2.25, 4.0 and 8.0 seedlings m−2 treatments, respectively (figure 2). There was a clear interaction between block and density treatments (figure 2). Lack of within-block replication meant it was not possible to look for significant interactions in a standard two-way ANOVA. Data were therefore analysed using an ANCOVA model, with density as a covariable and block as the factor; this gave highly significant density (F1,8 = 12.7, p = 0.007) and block (F3,8 = 11.3, p = 0.003) effects, and a significant interaction (F3,8 = 5.4, p = 0.025). Block 4 was the lowest on the shore and was close (approx. 4 m) to the edge of the remaining natural forest; sesarmid crabs living in the forest inflicted high mortalities on seedlings in this block (we observed crabs attacking planted seedlings; seedlings that had suffered crab attack were easily distinguishable from those that died from other causes).
Figure 2.
Percentage mortality of A. marina seedlings after growing for six months at four different densities and in four different blocks; block 4 was at the lowest tidal level (close to the forest fringe) and block 1 was the highest. Filled diamond, block 1; open square, block 2; filled triangle, block 3; crosses, block 4.
(c). Experiment 3: interspecific effects on growth and recruitment at the high tidal site
Large differences in mean biomass had developed between treatments after 4 years of growth (figure 3). A. marina showed the best survival of the three species used, because of its tolerance to the high salinity conditions characteristic of the initial plots (Kirui et al. 2008). Treatments with A. marina in the species mix thus showed the highest biomasses; there was a significant overall difference between treatments (ANOVA: F6,14 = 19.9, p = 0.001) and post hoc analyses showed that treatments B and BC had significantly less biomass than the others. Despite the much greater vigour of A. marina compared with the other two species, there was no evidence for negative effects of competition on the survival of C. tagal and B. gymnorrhiza (Kirui et al. 2008) or growth (Kirui 2008). Instead, mixed treatments containing A. marina showed evidence of over-yielding (Kirui 2008). Evidence of herbivory differed significantly between species (repeated-measures ANOVA: F2,27 = 6.1, p = 0.007) with A. marina showing the highest proportion of damaged leaves, followed by B. gymnorrhiza and then C. tagal (19.3 ± 1.5, 13.2 ± 1.4, 11.7 ± 1.4; mean ± s.e. percentage damaged leaves for the three species, respectively, data pooled across all treatments and years). There were no differences in recorded herbivory within species growing in different species mixes.
Figure 3.
Mean (±s.e.) aboveground dry weight in species mix treatments after 4 years of growth. A, A. marina; B, B. gymnorrhiza; C, C. tagal; all other treatments are possible combinations of these three species.
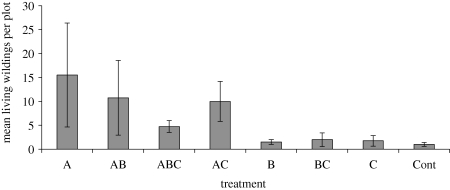
By 2007 (after 3 years of growth), there were large differences in the numbers of wildings found in the treatments; while treatments containing A. marina showed 28 or more living recruits, those without had 10 or less, with the bare controls showing the lowest number (figure 4). This facilitatory effect on recruitment was largely interspecific: four species had recruited into the plots, with C. tagal predominating (92.1%). Other species recruited were R. mucronata (4.2%), Xylocarpus granatum (3.3%) and A. marina (0.4%).
Figure 4.
Mean (±s.e.) number of mangrove wildings in species mix treatments after 3 years of growth. A, A. marina; B, B. gymnorrhiza; C, C. tagal; all other treatments are combinations of these species and an unplanted control (Cont).
Measurements of a range of physical variables (see Kirui et al. (2008) for more details) showed that the environment within the plots changed in a number of ways in response to planting. For example, by 2007, plots with greater biomass tended to have wetter sediment, which was probably related to lower sediment temperatures in these plots (figure 5).
Figure 5.
Relationships between aboveground biomass (kg dry weight) and (a) sediment water content (% by weight in top 1 cm) in 2007 and (b) sediment temperature (1 cm depth) in 2008. Regression equations were (a) per cent moisture = 15.9 + 0.000274 (biomass), R2 = 0.27, p = 0.001. (b) temp = 33.8–0.000091 (biomass), R2 = 0.64, p < 0.001.
(d). Experiment 4: effects of saplings on survival of propagules at a high tidal site
Cox (proportional hazards) regression was performed to compare the survival curves of propagules in the eight treatments: only those that were recorded as dead were included; missing propagules were censored (that is removed from the dataset). The global test for differences between curves was highly significant (χ2 = 20.5, d.f. = 7, p = 0.005). The three species treatment (ABC) showed the lowest mortality. Treatments Cont, BC and C all had significantly (p < 0.05) higher mortality risks, with hazard ratios of 2.05, 2.10 and 2.23, respectively.
4. Discussion
Gedan & Silliman (2009) recently predicted that mangrove restorations are likely to be more successful if they assume facilitative rather than competitive interactions during initial ecosystem development. For example, they suggest that planting seedlings in high-density clusters is a better strategy than spacing them to avoid competition. Two field studies provide indirect evidence in support of this by showing facilitation between mangroves and other species at high tidal sites (Milbrandt & Tinsley 2006; McKee et al. 2007b). Theoretical support comes from the stress gradient hypothesis, at least in general terms (Brooker et al. 2008): defining ‘stress’, however, is difficult. Early work on facilitation in the intertidal suggested that competitive effects might predominate at relatively benign low tidal sites, while facilitation was likely to operate at the upper intertidal (Bertness 1991). Our results from single species plots show positive effects of density on survival at both high (hypersaline, dry, low sediment supply) and low (brackish, wet, high sediment supply) tidal sites, with two different species and on two different continents, suggesting that facilitative effects may be common across a wide range of mangrove habitats and that expecting stress to increase predictably along the tidal gradient is simplistic. At present, we do not know the mechanisms underlying this enhanced survival. The amelioration of local microclimate through self-shading and rhizosphere oxygenation (McKee et al. 2007b) or optimization of soil surface elevation (Morris 2007) are possibilities, particularly at the high tidal site.
The sediment accretion rate of 15.6 mm yr−1 at our high-density treatment in Sri Lanka is among the highest on record for a healthy mangrove site (that does not involve sudden discharges of sediment from eroding watersheds, e.g. Cahoon et al. 2003). High rates of sediment accretion can kill mangroves (Terrados et al. 1997) but might also be necessary to help them keep pace with sea level rise (Cahoon et al. 2006). Managers could face a trade-off between encouraging sedimentation by planting at high densities and suffering higher mortality as a result. However, our results are reassuring in this respect: increasing seedling density enhanced both sedimentation and survival. In fact, the facilitatory mechanism involved may have been the enhanced trapping of relatively nutrient-rich sediment (Morris et al. 2002). Surface elevation in mangroves may depend as much on the growth of below-ground roots, expanding and pushing the surface upwards, as on the accretion of sediment (Rogers et al. 2005; McKee et al. 2007a,b). Hence, measuring only accretion may give a misleading picture of how the surface level is changing and thus subsurface processes (ideally measured using the bedrock as the baseline) must be considered. Here, we used pins of only 80 cm depth; more than sufficient to exceed the developing rhizosphere of the plants but potentially open to movement from other causes (Whelan et al. 2005). Soil surface measurements showed dramatically slower rates of elevation change when compared with accretion, and revealed net subsidence in the unplanted control treatment. The IPCC predicts sea level rise of 18–59 cm in the current century (IPCC 2007), although many authorities believe these are conservative projections (Edwards 2007). The highest rate of elevation change measured here—2.1 mm yr−1 in the highest density treatment—is sufficient to keep pace only with the lower end of projections. However, our trees were only 29 months old at the last measurements and rates of elevation change are likely to increase as the roots develop. Furthermore, increasing levels of atmospheric CO2 may further facilitate root zone expansion in C3 mangrove species, especially at high plantation densities and higher salinities, similar to that shown for marsh plants, where higher CO2 levels tended to ameliorate salinity stress and promote greater root increment (Cherry et al. 2009; Langley et al. 2009).
Biomass accumulation at the high tidal-mixed species plots was driven largely by A. marina. There was no evidence that this species was reducing the growth or survival of the other species (Kirui et al. 2008). Increasing biomass was associated with changing environmental conditions, which probably explains the recruitment and growth of wild propagules in the planted plots: before the current work, the experimental site had been barren after cutting for approximately 35 years. Hence, A. marina is acting as a nurse species allowing the recruitment of other species (predominantly C. tagal). While amelioration of abiotic stress is not the only mechanism that can produce facilitation, it is the most likely one here. Facilitation can occur through protection from herbivory. For example, Alberti et al. (2008) showed that Spartina densiflora had lower percentage of leaf damage when associated with a less palatable pioneer. However, there was no evidence for such an effect in the current work. Our ‘pioneer’ A. marina had significantly higher rates of damage than the other two species and there was no reduction in their leaf damage when grown in mixtures. Increased recruitment may also arise through the passive trapping of floating seeds and propagules (McKee et al. 2007b). However, the significantly higher mortality rates of experimental C. tagal propagules planted in control, C and BC treatments compared with treatments containing A. marina suggest that environmental amelioration is important. Propagule mortality rates in monospecific B. gymnorrhiza treatments did not differ from those containing A. marina despite the low biomass in B treatments; this surprising result further suggests that species-specific mechanisms may also be operating.
While predictions of the effects of climate change for specific mangrove sites are uncertain, some general trends over the next century command widespread assent: sea level will rise by an average of more than 1 mm yr−1, average temperatures will increase and freshwater inputs will generally become more variable. Mangroves in many areas may disappear in the face of these physical changes. For example, most mangrove sites are losing surface elevation relative to sea level rise, and low tidal areas are therefore highly vulnerable to submersion (Cahoon et al. 2006). However, biogenic responses can enhance mangrove resistance and resilience, and are already responsible for the maintenance of some mangroves above sea level (e.g. McKee et al. 2007a,b). Knowledge of these biogenic mechanisms will help managers mitigate climate change effects. Managers might, for instance, reduce nitrogen inputs, since elevated nitrogen may lead to decreased root productivity (Langley et al. 2009) and enhanced root decomposition (Huxham et al. in press). They might control the size and location of harvested areas to prevent ‘peat collapse’ (Cahoon et al. 2003), and encourage coverage of high tidal areas by ‘nurse species’ (McKee et al. 2007a,b). The current work shows how knowledge of positive interactions between mangrove trees may add to these management options, for example by encouraging high-density planting when restoring degraded areas.
In conclusion, we have demonstrated how intra- and interspecific facilitation can operate at low and high tidal sites among mangroves. Mangrove conservation and restoration must consider how to enable forests to keep pace with sea level rise and to migrate to new, often degraded sites. Developing coastal management strategies to encourage facilitatory effects such as these can help such efforts to succeed.
Acknowledgements
We also thank Laitani Suleimani, the many Earthwatch volunteers and the KMFRI staff who helped in the field and the comments of two anonymous referees. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the US Government. We are grateful to the Earthwatch Institute, the Leverhulme Trust and Zurich International who helped fund this work.
Footnotes
One contribution of 14 to a Theme Issue ‘The effects of climate change on biotic interactions and ecosystem services’.
References
- Alberti J., Escapa M., Iribarne O., Silliman B., Bertness M.2008Crab herbivory regulates plant facilitative and competitive processes in Argentinean marshes. Ecology 89, 155–164 (doi:10.1890/07-0045.1) [DOI] [PubMed] [Google Scholar]
- Arulananthan K., Rydberg L., Cederlöf U., Wiyeratne E.1995Water exchange in a hypersaline tropical estuary, the Puttalam lagoon, Sri Lanka. Ambio 24, 438–443 [Google Scholar]
- Bertness M. D.1991Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology 72, 125–137 (doi:10.2307/1938908) [Google Scholar]
- Bertness M. D., Hacker S. D.1994Physical stress and positive associations among plants. Am. Nat. 144, 363–372 (doi:10.1086/285681) [Google Scholar]
- Bosire J. O., Dahdouh-Guebas F., Kairo J. G., Koedam N.2003Colonization of non-planted mangrove species into restored mangrove stands in Gazi Bay, Kenya. Aquat. Bot. 76, 267–279 (doi:10.1016/S0304-3770(03)00054-8) [Google Scholar]
- Bosire J., Dahdouh-Guebas F., Walton M., Crona B. I., Lewis R. R., III, Field C., Kairo J. G., Koedam N.2008Functionality of restored mangroves: a review. Aquat. Bot. 89, 251–259 (doi:10.1016/j.aquabot.2008.03.010) [Google Scholar]
- Bouillon S., et al. 2008Mangrove production and fate: a revision of budget estimates. Global Biogeoch. Cycles 22, GB2013 (doi:10.1029/20007GB003052) [Google Scholar]
- Brooker R. W., et al. 2008Facilitation in plant communities: the past, the present, and the future. J. Ecol. 96, 18–34 [Google Scholar]
- Cahoon D. R., Lynch J. C.1997Vertical accretion and shallow subsidence in a mangrove forest of southwestern Florida, USA. Mang. Salt Marsh. 1, 173–186 (doi:10.1023/A:1009904816246) [Google Scholar]
- Cahoon D. R., Hensel P., Rybczyk J., McKee K., Edward Proffitt C., Perez C.2003Mass tree mortality leads to mangrove peat collapse at Bay Islands, Honduras after Hurricane Mitch. J. Ecol. 91, 1093–1105 (doi:10.1046/j.1365-2745.2003.00841.x) [Google Scholar]
- Cahoon D. R., Hensel P., Spencer T., Reed D., McKee K. L., Saintilan N.2006Coastal wetland vulnerability to relative sea-level rise: wetland elevation trends and process controls. In Wetlands and natural resource management. Ecological Studies, vol. 190 (eds Verhoeven J. T. A., Beltman B., Bobbink R., Whigham D.). Berlin Heidelberg, Germany: Springer [Google Scholar]
- Cherry J. A., McKee K. L., Grace J. B.2009Elevated CO2 enhances biological contributions to elevation change in coastal wetlands by offsetting stressors associated with sea-level rise. J. Ecol. 97, 67–77 (doi:10.1111/j.1365-2745.2008.01449.x) [Google Scholar]
- Collet D.1994Modelling survival data in medical research London, UK: Chapman & Hall [Google Scholar]
- Doherty R. M., Sitch S., Smith B., Lewis S. L., Thornton P. K.2009Implications of future climate and atmospheric CO2 content for regional biogeochemistry, biogeography and ecosystem services across East Africa. Global Change Biol. 16, 617–640 (doi:10.1111/j.1365-2486.2009.01997.x) [Google Scholar]
- Edwards R.2007Sea levels: resolution and uncertainty. Prog. Phys. Geogr. 31, 621–632 (doi:10.1177/0309133307087086) [Google Scholar]
- Ewel K. C., Twilley R. R., Ong J. E.1998Different kinds of mangrove forests provide different goods and services. Global Ecol. Biogeogr. Lett. 7, 83–94 (doi:10.2307/2997700) [Google Scholar]
- FAO 2007The world's mangroves: 1980–2005 Rome, Italy: Food and Agriculture Organization of the United Nations [Google Scholar]
- Fargione J., Tilman D., Dybzinski R., Lambers J. H. R., Clark C., Harpole W. S., Knops J. M. H., Reich P. B., Loreau M.2007From selection to complementarity: shifts in the causes of biodiversity–productivity relationships in a long-term biodiversity experiment. Proc. R. Soc. B 274, 871–876 (doi:10.1098/rspb.2006.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field C. D.1998Rehabilitation of mangrove ecosystems: an overview. Mar. Poll. Bull. 37, 383–392 [Google Scholar]
- Furukawa K., Wolanski E.1996Sedimentation in mangrove forests. Mang. Salt Marshes 1, 3–10 (doi:10.1023/A:1025973426404) [Google Scholar]
- Gedan K., Silliman B.2009Using facilitation theory to enhance mangrove restoration. Ambio 38, 109 (doi:10.1579/0044-7447-38.2.109) [DOI] [PubMed] [Google Scholar]
- Gilman E., Ellison J. C., Duke N. C., Field C.2008Threats to mangroves from climate change and adaptation options: a review. Aquat. Bot. 89, 237–250 (doi:10.1016/j.aquabot.2007.12.009) [Google Scholar]
- Huxham M., Kimani E., Newton J., Augley J.2007Stable isotope records from otoliths as tracers of fish migration in a mangrove system. J. Fish Biol. 70, 1554–1567 (doi:10.1111/j.1095-8649.2007.01443.x) [Google Scholar]
- Huxham M., Langat J., Tamooh F., Kenedy H., Mencuccini M., Skov M. W., Kairo J.In press Decomposition of mangrove roots: effects of location, nutrients, species identity and mix in a Kenyan forest. Est. Coast Shelf Sci. [Google Scholar]
- IPCC 2007Climate change 2007: synthesis report. A report of the Intergovernmental Panel on Climate Change Cambridge, UK: Cambridge University Press [Google Scholar]
- Jayatissa L. P., Wickramasinghe W. A. A. D. L., Dahdouh-Guebas F., Huxham M.2008Interspecific variations in responses of mangrove seedlings to two contrasting salinities. Int. Rev. Hydrobiol. 93, 700–710 (doi:10.1002/iroh.200711017) [Google Scholar]
- Kairo J. G.1995. In Community participatory forestry for rehabilitation of deforested mangrove areas of Gazi Bay (Kenya). A first approach. Final technical report Nairobi, Kenya: World Wide Fund for Nature-US and University of Nairobi [Google Scholar]
- Kairo J. G., Dahdouh-Guebas F., Bosire J., Koedam N.2001Restoration and management of mangrove systems—a lesson for and from the East African region. S. A. J. Bot. 67, 383–389 [Google Scholar]
- Kirui B.2008Influence of species diversity on the return of ecosystem functions in replanted mangroves in Kenya. PhD thesis, Edinburgh Napier University, Edinburgh, UK [Google Scholar]
- Kirui B., Huxham M., Kairo J., Skov M.2008Influence of species richness and environmental context on early survival of replanted mangroves at Gazi Bay, Kenya. Hydrobiologia 603, 171–181 (doi:10.1007/s10750-007-9270-3) [Google Scholar]
- Krauss K. W., Allen J. A., Cahoon D. R.2003Differential rates of vertical accretion and elevation change among aerial root types in Micronesian mangrove forests. Est. Coast. Shelf Sci. 56, 251–259 [Google Scholar]
- Krauss K. W., Lovelock C. E., McKee K. L., López-Hoffman L., Ewe S. M. L., Sousa W. P.2008Environmental drivers in mangrove establishment and early development: a review. Aquat. Bot. 89, 105–127 (doi:10.1016/j.aquabot.2007.12.014) [Google Scholar]
- Langley J. A., McKee K. L., Cahoon D. R., Cherry J. A., Megonigal J. P.2009Elevated CO2 stimulates marsh elevation gain, counterbalancing sea-level rise. Proc. Natl Acad. Sci. USA 106, 6182–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R., III2005Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 24, 403–418 [Google Scholar]
- McKee K. L., Cahoon D. R., Feller I. C.2007aCaribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Global Ecol. Biogeogr. 16, 545–556 (doi:10.1111/j.1466-8238.2007.00317.x) [Google Scholar]
- McKee K. L., Rooth J. E., Feller I. C.2007bMangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecol. Appl. 17, 1678–1693 (doi:10.1890/06-1614.1) [DOI] [PubMed] [Google Scholar]
- McLeod E., Salm R. V.2006Managing mangroves for resilience to climate change Gland, Switzerland: IUCN [Google Scholar]
- Milbrandt E. C., Tinsley M. N.2006The role of saltwort (Batis maritima L.) in regeneration of degraded mangrove forests. Hydrobiologia 568, 369–377 (doi:10.1007/s10750-006-0203-3) [Google Scholar]
- Morris J. T.2007Ecological engineering in intertidal saltmarshes. Hydrobiologia 577, 161–168 (doi:10.1007/s10750-006-0425-4) [Google Scholar]
- Morris J. T., Sundareshwar P. V., Nietch C. T., Kjerfve B., Cahoon D. R.2002Responses of coastal wetlands to rising sea level. Ecology 83, 2869–2877 (doi:10.1890/0012-9658(2002)083[2869:ROCWTR]2.0.CO;2) [Google Scholar]
- Rogers K., Saintilan N., Cahoon D.2005Surface elevation dynamics in a regenerating mangrove forest at Homebush Bay, Australia. Wetlands Ecol. Man. 13, 587–598 (doi:10.1007/s11273-004-0003-3) [Google Scholar]
- Skov M. W., Hartnoll R. G.2002Paradoxical selective feeding on a low-nutrient diet: why do mangrove crabs eat leaves? Oecologia 131, 1–7 (doi:10.1007/s00442-001-0847-7) [DOI] [PubMed] [Google Scholar]
- Terrados J., Thampanya U., Srichai N., Kheowvongsri P., Geertz-Hansen O., Boromthanarath S., Panapitukkul N., Duarte C. M.1997The effect of increased sediment accretion on the survival and growth of Rhizophora apiculata seedlings. Est. Coast. Shelf Sci. 45, 697–701 [Google Scholar]
- Valiela I., Bowen J. L., York J. K.2001Mangrove forests: one of the world's threatened major tropical environments. BioScience 51, 807–815 (doi:10.1641/0006-3568(2001)051) [Google Scholar]
- Whelan K. R. T., Smith T. J., III, Cahoon D. R., Lynch J. C., Anderson G. H.2005Groundwater control of mangrove surface elevation: shrink and swell varies with soil depth. Estuaries 28, 833–843 (doi:10.1007/BF02696013) [Google Scholar]
- Young B. M., Harvey L. E.1996A spatial analysis of the relationship between mangrove (Avicennia marina var. australasica) physiognomy and sediment accretion in the Hauraki Plains, New Zealand. Est. Coast. Shelf Sci. 42, 231–246 (doi:10.1006/ecss.1996.0017) [Google Scholar]