Abstract
We have examined the capacity of the major cytoplasmic membrane protein (MCMP) of Legionella pneumophila, a genus common antigen and member of the hsp 60 family of heat shock proteins, to induce protective immunity in a guinea pig model of Legionnaires' disease. We purified MCMP to homogeneity from L. pneumophila by buffer extraction, ion-exchange chromatography, and molecular sieve chromatography. Guinea pigs immunized with MCMP developed a strong cell-mediated immune response to the immunogen manifest by marked cutaneous delayed-type hypersensitivity. Guinea pigs immunized with MCMP and then challenged with a lethal aerosol dose of L. pneumophila exhibited a high level of protective immunity. Altogether, in four independent experiments, 55 of 64 (86%) animals immunized three times with 0.6-40 micrograms MCMP including 11 of 11 (100%) animals immunized three times with 40 micrograms MCMP survived aerosol challenge with L. pneumophila compared with 1 of 29 (3%) sham-immunized control animals (P < 0.0001, Cochran-Mantel-Haenszel X2 statistic for pooled data). To our knowledge, MCMP is the first member of the hsp 60 family of proteins shown to induce protective immunity to a microbial pathogen. MCMP has potential as a vaccine against Legionnaires' disease. Since MCMP is a genus common antigen, vaccination with a combination of MCMPs derived from different Legionella species has the potential of inducing protective immunity against all the major Legionella species causing human disease.
Full text
PDF

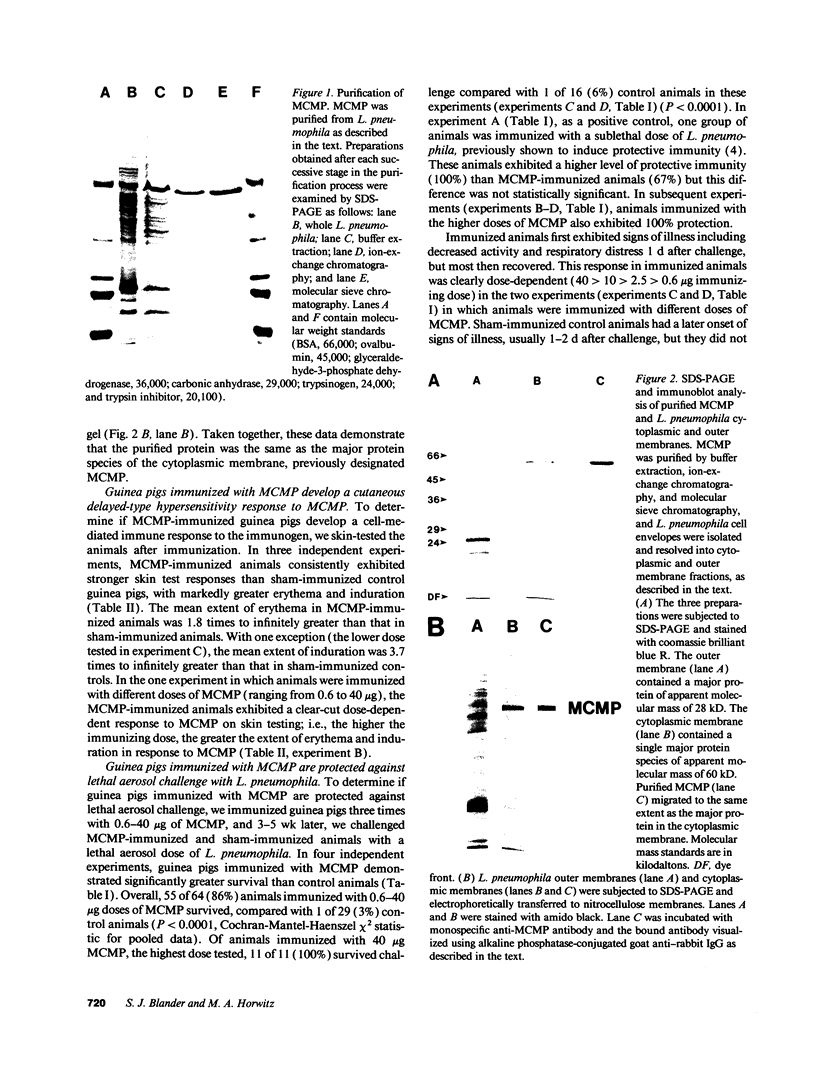
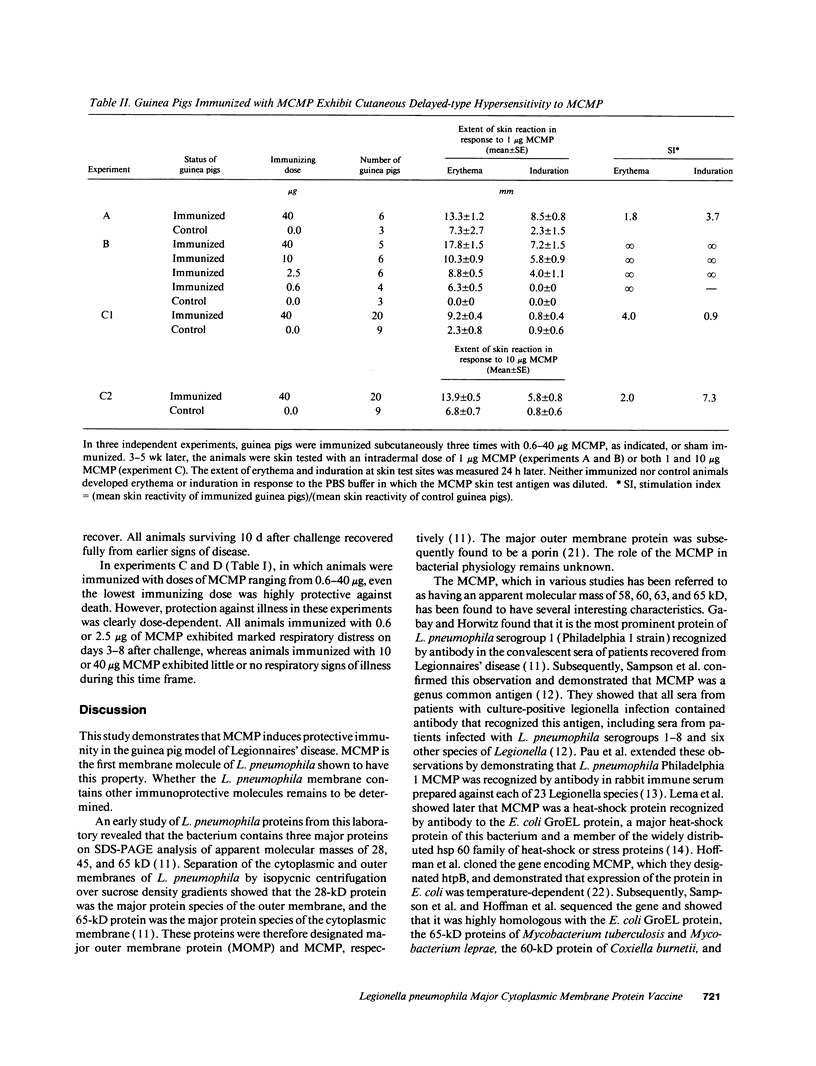


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Billingham M. E., Carney S., Butler R., Colston M. J. A mycobacterial 65-kD heat shock protein induces antigen-specific suppression of adjuvant arthritis, but is not itself arthritogenic. J Exp Med. 1990 Jan 1;171(1):339–344. doi: 10.1084/jem.171.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Breiman R. F., Horwitz M. A. A live avirulent mutant Legionella pneumophila vaccine induces protective immunity against lethal aerosol challenge. J Clin Invest. 1989 Mar;83(3):810–815. doi: 10.1172/JCI113962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with Legionella pneumophila membranes induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. Protective immunity independent of the major secretory protein of Legionella pneumophila. J Clin Invest. 1991 Mar;87(3):1054–1059. doi: 10.1172/JCI115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with the major secretory protein of Legionella induces humoral and cell-mediated immune responses and protective immunity across different serogroups of Legionella pneumophila and different species of Legionella. J Immunol. 1991 Jul 1;147(1):285–291. [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. J Exp Med. 1989 Mar 1;169(3):691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Szeto L., Shuman H. A., Horwitz M. A. An immunoprotective molecule, the major secretory protein of Legionella pneumophila, is not a virulence factor in a guinea pig model of Legionnaires' disease. J Clin Invest. 1990 Sep;86(3):817–824. doi: 10.1172/JCI114779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman R. F., Horwitz M. A. Guinea pigs sublethally infected with aerosolized Legionella pneumophila develop humoral and cell-mediated immune responses and are protected against lethal aerosol challenge. A model for studying host defense against lung infections caused by intracellular pathogens. J Exp Med. 1987 Mar 1;165(3):799–811. doi: 10.1084/jem.165.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Blake M., Niles W. D., Horwitz M. A. Purification of Legionella pneumophila major outer membrane protein and demonstration that it is a porin. J Bacteriol. 1985 Apr;162(1):85–91. doi: 10.1128/jb.162.1.85-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay J. E., Horwitz M. A. Isolation and characterization of the cytoplasmic and outer membranes of the Legionnaires' disease bacterium (Legionella pneumophila). J Exp Med. 1985 Feb 1;161(2):409–422. doi: 10.1084/jem.161.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Jenner P. J., Colston M. J., Bacon P. A. Recognition of a mycobacteria-specific epitope in the 65-kD heat-shock protein by synovial fluid-derived T cell clones. J Exp Med. 1990 Mar 1;171(3):831–841. doi: 10.1084/jem.171.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., Butler C. A., Quinn F. D. Cloning and temperature-dependent expression in Escherichia coli of a Legionella pneumophila gene coding for a genus-common 60-kilodalton antigen. Infect Immun. 1989 Jun;57(6):1731–1739. doi: 10.1128/iai.57.6.1731-1739.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., Houston L., Butler C. A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990 Oct;58(10):3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Cell-mediated immunity in Legionnaires' disease. J Clin Invest. 1983 Jun;71(6):1686–1697. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Characterization of avirulent mutant Legionella pneumophila that survive but do not multiply within human monocytes. J Exp Med. 1987 Nov 1;166(5):1310–1328. doi: 10.1084/jem.166.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Lema M. W., Brown A., Butler C. A., Hoffman P. S. Heat-shock response in Legionella pneumophila. Can J Microbiol. 1988 Oct;34(10):1148–1153. doi: 10.1139/m88-202. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Modrow S., Karr R. W., Young R. A., Kaufmann S. H. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989 Nov 1;143(9):2844–2849. [PubMed] [Google Scholar]
- Nash T. W., Libby D. M., Horwitz M. A. Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest. 1984 Sep;74(3):771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau C. P., Plikaytis B. B., Carlone G. M., Warner I. M. Purification, partial characterization, and seroreactivity of a genuswide 60-kilodalton Legionella protein antigen. J Clin Microbiol. 1988 Jan;26(1):67–71. doi: 10.1128/jcm.26.1.67-71.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Sampson J. S., O'Connor S. P., Holloway B. P., Plikaytis B. B., Carlone G. M., Mayer L. W. Nucleotide sequence of htpB, the Legionella pneumophila gene encoding the 58-kilodalton (kDa) common antigen, formerly designated the 60-kDa common antigen. Infect Immun. 1990 Sep;58(9):3154–3157. doi: 10.1128/iai.58.9.3154-3157.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson J. S., Plikaytis B. B., Wilkinson H. W. Immunologic response of patients with legionellosis against major protein-containing antigens of Legionella pneumophila serogroup 1 as shown by immunoblot analysis. J Clin Microbiol. 1986 Jan;23(1):92–99. doi: 10.1128/jcm.23.1.92-99.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991;167:145–160. doi: 10.1007/978-3-642-75875-1_9. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar E. A., Schachter J., Bavoil P., Stephens R. S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990 Oct;162(4):922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- Young D. B. Structure of mycobacterial antigens. Br Med Bull. 1988 Jul;44(3):562–583. doi: 10.1093/oxfordjournals.bmb.a072268. [DOI] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]
- van den Broek M. F., Hogervorst E. J., Van Bruggen M. C., Van Eden W., van der Zee R., van den Berg W. B. Protection against streptococcal cell wall-induced arthritis by pretreatment with the 65-kD mycobacterial heat shock protein. J Exp Med. 1989 Aug 1;170(2):449–466. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]