Abstract
Many animals experience marked seasonal fluctuations in environmental conditions. In response, animals display adaptive alterations in physiology and behaviour, including seasonal changes in immune function. During winter, animals must reallocate finite energy stores from relatively costly, less exigent systems (e.g. reproduction and immunity) to systems critical for immediate survival (e.g. thermoregulation). Seasonal changes in immunity are probably mediated by neuroendocrine factors signalling current energetic state. One potential hormonal candidate is insulin, a metabolic hormone released in response to elevated blood glucose levels. The aim of the present study was to explore the potential role of insulin in signalling energy status to the immune system in a seasonally breeding animal, the Siberian hamster (Phodopus sungorus). Specifically, exogenous insulin was administered to male hamsters housed in either long ‘summer-like’ or short ‘winter-like’ days. Animals were then challenged with an innocuous antigen and immune responses were measured. Insulin treatment significantly enhanced humoural immune responses in short, but not long days. In addition, insulin treatment increased food intake and decreased blood glucose levels across photoperiodic treatments. Collectively, these data support the hypothesis that insulin acts as an endocrine signal integrating seasonal energetic changes and immune responses in seasonally breeding rodents.
Keywords: immunity, energy balance, insulin, antibody response
1. Introduction
Most non-tropical animals experience marked seasonal fluctuations in environmental conditions, which can act as seasonal stressors. During winter, animals typically experience reduced food availability and low ambient temperatures that can limit total energy budgets by decreasing available energy or increasing energy output, respectively. To cope with these potential seasonal stressors, many small mammals have evolved mechanisms to use photoperiodic (day length) cues to appropriately alter energy investment into physiological systems to match current environmental conditions. For example, long-day, reproductively active rodents downregulate their reproductive neuroendocrine axes and cease breeding activity during the short days of winter; reproductive activity is restored following the return of long day lengths associated with spring and summer (Bronson & Heideman 1994). In addition, marked seasonal changes in foraging, food intake and body mass occur in a wide range of vertebrate species (Bartness & Demas 2004).
A growing body of evidence suggests that immunity, like reproduction, is energetically costly and plays a key role in physiological trade-offs (Lochmiller & Deerenberg 2000; Demas 2004; Martin et al. 2008). If immune function is costly, we would predict seasonal changes in immune function in response to changing photoperiods. Empirical support for this hypothesis exists; long-day breeding mammals in seasonal environments modulate immune function during the short days of winter by altering circulating antibody responses, lymphocyte proliferation, delayed-type hypersensitivity, wound healing and numbers of circulating leucocytes and lymphocytes (Yellon et al. 1999; Drazen et al. 2000; Bilbo et al. 2002; Kinsey et al. 2003; Weil et al. 2007). Despite these observations, considerably less is known regarding the physiological mechanisms mediating photoperiodic changes in immune function. Although changes in gonadal steroid hormones (e.g. testosterone and oestradiol) and pineal melatonin can explain some of the photoperiodic effects on immunity (Demas & Nelson 1998a,b; Bilbo & Nelson 2001; Drazen et al. 2001), additional endocrine factors are probably involved, including hormones that regulate energy balance (Demas 2004). For example, leptin, a hormone secreted predominantly by adipose tissue, modulates immune responses in several rodent species (Lord et al. 1998; Demas & Sakaria 2005). Furthermore, seasonal changes in leptin concentrations appear to mediate, at least in part, seasonal changes in immunity (Drazen et al. 2001). Although leptin increases immune responses in short-day hamsters, antibody responses are not fully restored to normal long-day levels, suggesting that additional endocrine factors are probably involved in mediating this response.
The pancreatic peptide insulin is secreted in response to increased energy intake and regulates the storage of excess energy. It is well known that insulin signals energy availability to peripheral tissues and the central nervous system (Schwartz & Kahn 1999; Benoit et al. 2004). The role of insulin in signalling energy status to the immune system and modulating subsequent responses, however, remains unknown. Because insulin is secreted in response to changes in blood glucose levels, it serves as an important indicator of current energy availability within an animal (Benoit et al. 2004). Furthermore, insulin levels are correlated with adiposity in most mammals studied thus far, including Siberian hamsters (Phodopus sungorus; Bartness et al. 1995). Therefore, insulin may serve as both an indicator of short-term available energy (i.e. glucose) as well as long-term energy stores (i.e. adiposity). Although insulin appears to play an important role in signalling peripheral energy stores, little is known regarding the potential role of this hormone in regulating immune responses. Insulin receptors are expressed by immune cells (e.g. macrophages, B and T lymphocytes), and stimulation of these receptors with exogenous hormone affects glucose transport and can alter immune responses (reviewed in Wolowczuk et al. 2008). It has also been suggested that the insulin receptor plays a critical role in the proliferation and survival of immune cells (Knutson 1991). To our knowledge, however, virtually nothing is known regarding the role of insulin in regulating seasonal changes in immune responses.
The aim of the current investigation was to test the hypothesis that insulin plays a role in regulating seasonal changes in immunity. Specifically, if insulin acts as a link between energy balance and immunity, then experimental increases in the insulin signal, indicating an excess of available energy, should enhance immune function and attenuate short-day decreases in immunity. To test this hypothesis, we housed Siberian hamsters in either long or short days, manipulated insulin levels via treatment with exogenous hormone and examined the effects of these manipulations on both innate and acquired immune responses.
2. Material and methods
(a). Animals and housing conditions
Adult (greater than 60 days old) male Siberian hamsters (n = 50) were obtained from our breeding colony maintained on long days (L : D 16 : 8) at Indiana University. Hamsters were individually housed in polypropylene cages (23.3 × 15.3 × 15.9 cm) for one week prior to the start of the experiment. Food (Purina rat chow, St Louis, MO, USA) and tap water were provided ad libitum throughout the experiment. Room temperature was maintained at 21 ± 2°C and relative humidity at 50 ± 10%. Animals were then randomly assigned to either long (L : D 16 : 8) (n = 18) or short days (L : D 8 : 16) (n = 32) and initial body mass was measured. All procedures were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC).
(b). Responsiveness to photoperiod
Frequently, a subset of hamsters fails to show photo-responsiveness (i.e. do not display typical gonadal regression, reductions in fat stores or changes in pelage density and coloration) despite prolonged maintenance in short days; these animals are known as photoperiodic non-responders (Puchalski & Lynch 1986). After eight weeks of exposure to short-day photoperiods, 15 animals were determined to be non-responders (defined by a less than or equal to 10% reduction in body mass and maintenance of summer pelage coloration) and were removed from all subsequent analyses (Greives et al. 2008). At the conclusion of the experiment, necropsies were performed on the remaining animals and paired testes were weighed to confirm responsiveness (defined as a paired gonadal mass < 0.15 g; Greives et al. 2007). One additional hamster was determined to be a non-responder based on paired testes mass (paired testes mass of 0.63 g) and was removed from subsequent analyses.
(c). Food intake and insulin administration
Weekly body masses and daily food intake were measured for all hamsters prior to insulin treatment. Following one week of baseline food and body mass measurements, insulin injections were initiated. Long- and short-day hamsters were divided into one of two injection groups: control animals received 0.1 ml of 0.9 per cent saline vehicle (long day, n = 9; short day, n = 8), whereas experimental animals received 0.1 ml of 40 U kg−1 protamine zinc insulin (PZI) (IDEXX Pharmaceuticals, Greensboro, NC, USA) (long day, n = 9; short day, n = 8) dissolved in saline. PZI was used because it is a long-lasting form of insulin that has been shown to affect energetic state in rodents, including hamsters. The 40 U kg−1 dose was chosen based on a pilot study from our laboratory that revealed lower doses (20 U kg−1) had no effect on blood glucose levels or subsequent immune measures (D. A. Zysling & G. E. Demas 2008, unpublished data), as well as previously published research in this species (Bartness et al. 1991). The dose of insulin was gradually increased until over a period of 14 days to 40 U kg−1 to avoid severe insulin-induced hypoglycaemia that could result in death (Bartness et al. 1991; Bartness & Clein 1994; Demas & Bartness 1999). To this end, daily subcutaneous (s.c.) injections of PZI were administered for 24 days according to the following injection schedule: day 1: 0.625 U kg−1; days 2–3: 1.25 U kg−1; day 4–6: 5 U kg−1; days 7–10: 10 U kg−1; days 11–14: 20 U kg−1 and days 15–24: 40 U kg−1.
(d). Immunizations
Fifteen days after the first injection (i.e. once experimental animals had reached the desired 40 U kg−1 dose of insulin), all hamsters received a single s.c. injection of 100 µg of the antigen keyhole limpet haemocyanin (KLH) suspended in 0.1 ml sterile saline (day 0; Zysling et al. 2006). The animals were previously naive and were returned to the colony room following injections. KLH is an innocuous respiratory protein derived from the giant keyhole limpet (Megathura crenulata). KLH was used because it generates a robust antigenic response in rodents, but does not make the animals sick (e.g. prolonged inflammation or fever; Dixon et al. 1966). Blood was drawn from the retro-orbital sinus at two different sampling periods (days 5 and 10 post-immunization). These sampling periods were chosen to capture basal (day 5) and peak (day 10) immunoglobulin G (IgG) production (the predominant Ig class present in blood) during the course of the immune response to KLH (Demas et al. 1997a; Drazen et al. 2000). Day 5 also corresponds to the peak of immunoglobulin M (IgM) production, which is the initial antibody class produced in response to an infection (Demas et al. 1997a,b; Drazen et al. 2000). On each sampling day, animals were brought into the surgery room, lightly anaesthetized with isoflurane vapours (Aerrane, Henry Schein, Melville, NY, USA), and blood samples were drawn from the retro-orbital sinus between 1000 h and 1200 h EST. Samples were allowed to clot for 1 h, the clots were removed and the samples were centrifuged (at 4°C) for 30 min at 2500 rpm. Serum aliquots were aspirated and stored in sealable polypropylene microcentrifuge tubes at −80°C until assayed for anti-KLH immunoglobulins and leptin.
(e). Blood glucose measurement
Blood glucose levels were measured 10 days after KLH injection (i.e. after 25 days of daily insulin treatment). Blood samples were collected 1 h after insulin or vehicle administration and blood glucose levels were determined using a digital glucose meter (Roche Diagnostics, Indianapolis, IN, USA). Specifically, 5 µl of whole blood was transferred onto the meter's check strips and the readout was recorded. The meter was previously calibrated using an internal standard provided by the manufacturer.
(f). Antibody response to KLH
Serum samples were analysed by enzyme-linked immunosorbent assay (ELISA) to determine IgM and IgG concentrations (Klein-Schneegans et al. 1989). Microtiter plates were coated with KLH in sodium bicarbonate buffer (pH 9.6), incubated overnight at 4°C and washed with phosphate buffered saline (PBS) with 0.05 per cent Tween 20 (PBS-T; pH 7.4). Plates were then blocked with 5 per cent non-fat dry milk in PBS and washed again with PBS-T. Serum samples were diluted 1 : 20 with PBS and added to the plate wells in duplicate. Negative control samples (i.e. serum from KLH-naive hamsters) and positive control samples (i.e. serum pooled from hamsters previously shown to have significantly high anti-KLH antibody response) were also diluted 1 : 20 with PBS and added to the plate wells in duplicate. Plates were incubated at 37°C for 3 h and then washed with PBS-T. Secondary antibody was then added to the wells and the plates were incubated for 1.5 h for day 5 (IgM) samples and 1 h for day 10 (IgG) samples. Alkaline phosphatase-conjugated anti-mouse IgM (MP Biomedicals, Salon, OH, USA) diluted 1 : 500 in PBS was used for day 5 samples and alkaline phosphatase-conjugated anti-Syrian hamster IgG (Rockland, Gilbertsville, PA, USA) was diluted 1 : 500 in PBS and used for day 10 samples.
Following incubation, plates were again washed with PBS-T, and 150 µl of the enzyme substrate p-nitro-phenyl phosphate (Sigma, St Louis, MO, USA; 1 mg ml−1 in diethanolamine substrate buffer) was added to each well. Absorbance was measured (Bio-Rad Benchmark Plate Reader, Hercules, CA, USA) at 405 nm. The mean for each sample was calculated and expressed as a percentage of the positive control mean (% plate positive).
(g). Serum leptin
Insulin is known to influence serum leptin levels (Saladin et al. 1995); therefore, we measured circulating leptin concentrations in the present study. Serum leptin concentrations were determined in day 10 serum samples (1 : 2 dilution with assay buffer) using an ELISA from a commercially prepared murine kit (EZML-82K, Millipore, St Charles, MO, USA). This assay was previously validated for use with Siberian hamsters (French et al. 2009). All procedures were followed as directed by the manufacturer's instructions. The antiserum used was highly specific for leptin; cross-reactivity with related hormones is less than 0.05 per cent and the sensitivity of the assay is 0.05 ng ml. Intra-assay variability was consistently low and coefficients of variance were less than 10 per cent for all samples.
(h). Bactericidal assay
As a functional assessment of an animal's ability to eliminate a bacterial infection, we used an ex vivo bactericidal assay, based on a modification (French et al. 2009) of a previously published protocol (Matson et al. 2006). This assay quantifies the relative number of Escherichia coli colony forming units (CFUs) that grow after incubation with serum. Differences in CFU presumably represent differences in the ability of serum proteins (i.e. complement) to kill bacterial colonies. Briefly, E. coli (ATCC #8739, Microbiologics, St Cloud, MN, USA) (1 pellet = 107 CFU) was added to 40 ml 1 M sterile PBS warmed to 35–37°C, which was vortexed to create a bacterial stock solution and activated by incubation for 30 min at 37°C. Serum samples were diluted 1 : 40 in glutamine-enriched CO2-independent media (Invitrogen Corp., Carlsbad, CA, USA). This dilution was validated for serum with a dose–response curve prior to the experiment. The stock bacteria solution (500 000 CFU ml−1) was diluted with sterile 1 M PBS to create a 50 000 CFU ml−1 working solution. To obtain estimates of bacterial numbers (i.e. positive control), the working solution was diluted 1 : 10 with glutamine-enriched CO2-independent media. For each sample, the working solution was added at a 1 : 10 ratio to the diluted serum sample. The bacteria/serum cocktails were incubated for 30 min at 37°C. All samples were vortexed and 50 µl was added to Petri plates in duplicate and spread with a flame-sterilized spreader. All plates were stored upside down overnight at 37°C. Following incubation, bacteria colonies were counted on each plate, and duplicates were averaged. The mean value for each sample was expressed as a per cent of bacteria killed relative to the control plates, in which no killing occurred.
(i). Necropsies and tissue collection
At the conclusion of the study, hamsters were euthanized via an overdose of ketamine cocktail (20 mg ml−1 ketamine and 4 mg ml−1 xylazine in 0.9% saline solution; Nelson & Demas 1996). Inguinal white adipose tissue (IWAT), retroperitoneal WAT (RWAT), epididymal WAT (EWAT) and paired testes were collected, cleaned of connective tissues and weighed to the nearest 0.01 g.
(j). Statistical analyses
All data were analysed with Minitab 15 (Mintab Inc., State College, PA, USA) using a two-way (photoperiod × treatment) analysis of variance (ANOVA), except for food consumption data, which were analysed by a repeated measures ANOVA using JMP 7.0 (SAS, Cary, NC, USA). Data for repeated measures analysis were taken from the 9-day period that began following the first administration of full-dose insulin. A composite adipose tissue score was calculated for each hamster by summing the individual WAT pad masses (Zysling & Demas 2007).
All pair-wise comparisons of means were conducted using Tukey's honestly significant difference post hoc comparisons. Differences between group means were considered significant at p < 0.05. Datasets were analysed for normality using the Anderson–Darling test. Bactericidal assay data did not meet requirements for normality and were transformed by taking the arcsine of the square root of each data point, a standard transformation for percentages.
3. Results
(a). Body and tissue masses and food intake
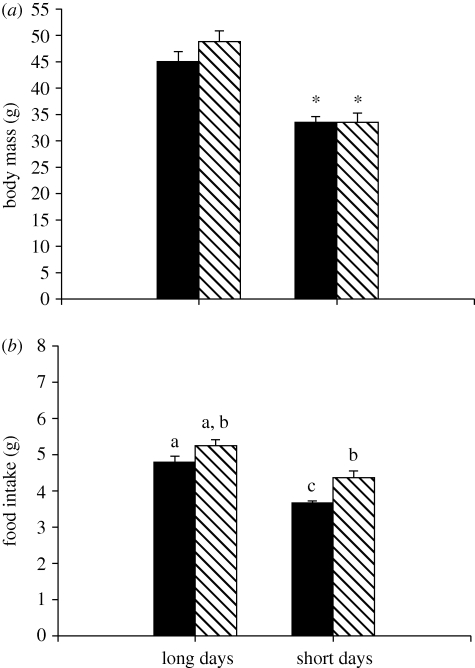
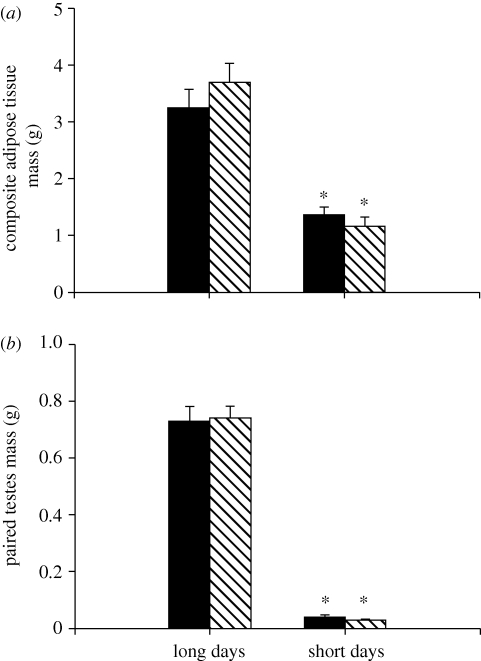
Short-day hamsters had significantly lower final body masses than long-day animals (F1,32 = 65.24, p < 0.001; figure 1a); however, insulin treatment had no significant effect on body mass (F1,32 = 0.63, p > 0.05). In addition, short-day animals had significantly smaller IWAT (F1,32 = 65.99, p < 0.001), RWAT (F1,32 = 52.87, p < 0.001) and EWAT pad masses (F1,32 = 65.81, p < 0.001), as well as lower composite adipose tissue scores (F1,32 = 77.46, p < 0.001; figure 2a) compared with long-day animals. Insulin treatment had no effect on any IWAT pad masses or composite adipose tissue scores (p > 0.05 in all cases). Short-day hamsters had significantly regressed paired testes when compared with long-days animals (F1,32 = 494.05, p < 0.001; figure 2b). Insulin treatment had no effect on paired testes mass (F1,32 = 0.01, p > 0.05).
Figure 1.
Mean (±s.e.m.) final body masses (a) and food intake (b) in Siberian hamsters housed in either long or short days and administered exogenous insulin (striped bar) or vehicle (filled bar) control. Significant differences in food intake are indicated by different letters, whereas significant differences in final body masses are indicated by an asterisk (*) if p < 0.05.
Figure 2.
Mean (±s.e.m.) composite white adipose tissue masses (a) and paired testes masses (b) in Siberian hamsters housed in either long or short days and administered exogenous insulin (striped bar) or vehicle (filled bar) control. Significant differences between pair-wise means are indicated by an asterisk (*) if p < 0.05.
(b). Food intake
During the initial baseline (i.e. pre-insulin treatment) period, short-day hamsters consumed significantly less food than long-day animals (F1,34 = 6.34; p < 0.001; figure 1b). During the experimental treatment period, both photoperiod (F1,30 = 21.43, p < 0.001; figure 1b) and insulin treatment (F1,30 = 9.89, p < 0.001) significantly affected food consumption following KLH exposure; short-day animals consumed less food than long-day animals, and animals receiving insulin injections consumed more when compared with vehicle-injected animals. There was no significant interaction between the two treatments (F1,30 = 0.31, p > 0.05).
(c). Blood glucose levels
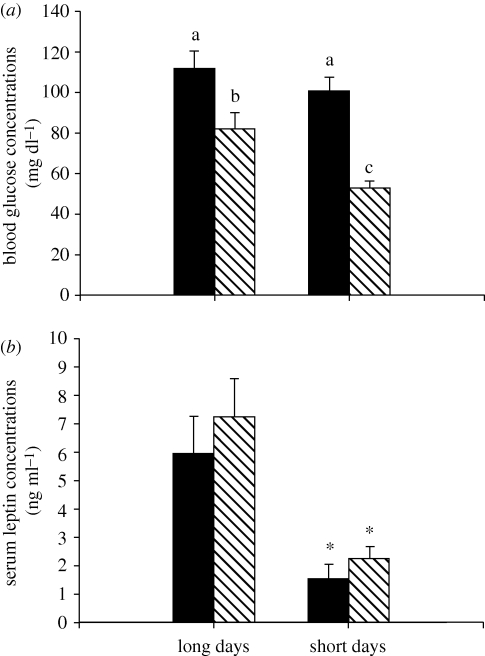
Blood glucose levels were significantly affected by photoperiod treatment (F1,32 = 8.08, p = 0.008; figure 3a); blood glucose levels were significantly lower in short-day hamsters compared with long-day hamsters. Insulin-treated short-day hamsters had significantly lower blood glucose levels when compared with insulin-treated long-day hamsters (p = 0.029); however, there was no difference between long- and short-day hamsters treated with saline (p > 0.05). There was also a significant main effect of hormone treatment on glucose with lower blood glucose levels in hamsters receiving insulin when compared with hamsters injected with saline (F1,32 = 29.82, p < 0.001; figure 3a). Specifically, blood glucose levels were lower in long-day hamsters treated with insulin when compared with saline-injected long-day hamsters (p = 0.027).
Figure 3.
Mean (±s.e.m.) blood glucose (a) and serum leptin concentrations (b) in Siberian hamsters housed in either long or short days and administered exogenous insulin (striped bar) or vehicle (filled bar) control. Significant differences in blood glucose are indicated by different letters, whereas significant differences in serum leptin concentration are indicated by an asterisk (*) if p < 0.05.
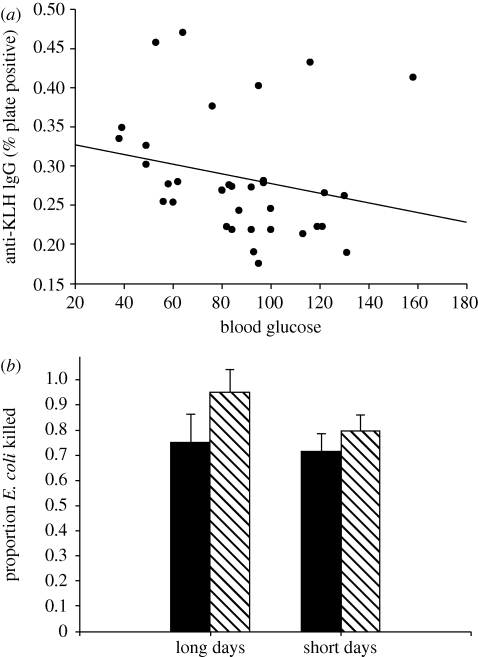
(d). Immune responses
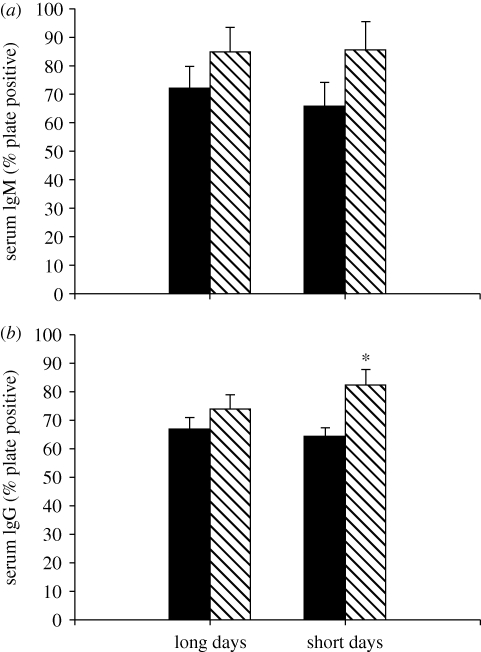
Insulin-treated hamsters displayed significantly elevated IgG levels when compared with animals injected with saline (F1,32 = 7.67, p = 0.010; figure 4b). Specifically, short-day animals treated with insulin showed significantly greater IgG responses with compared with short-day saline-treated animals (p = 0.044). A similar, but non-significant trend, was found for serum anti-KLH IgM levels (F1,31 = 3.28, p = 0.081; figure 4a). Blood glucose levels were negatively correlated with anti-KLH IgG (figure 5a) and anti-KLH IgM per cent plate positive values (R2 = 0.187, p = 0.012; R2 = 0.140, p = 0.035). There was no effect of photoperiod on either serum anti-KLH IgG levels (p > 0.05), or serum anti-KLH IgM levels (p > 0.05). There was no significant interaction between photoperiod and insulin treatment on either serum anti-KLH IgG levels (p > 0.05), or serum anti-KLH IgM levels (p > 0.05). Neither photoperiod (F1,65 = 1.30, p > 0.05) nor insulin treatment (F1,65 = 2.77, p > 0.05) had a significant effect on serum bacterial killing ability (figure 5).
Figure 4.
Mean (±s.e.m.) serum anti-KLH IgM (a) and IgG (b) levels in Siberian hamsters housed in either long or short days and administered exogenous insulin (striped bar) or vehicle (filled bar) control. Significant differences between pair-wise means are indicated by an asterisk (*) if p < 0.05.
Figure 5.
(a) Pearson product-moment correlation between blood glucose levels (day 10 sample) and IgG (R2 = 0.187, p = 0.012). Mean (±s.e.m.) bacterial killing ability (b) in Siberian hamsters housed in either long or short days and given exogenous administration of insulin (striped bar) or vehicle (filled bar) control.
(e). Serum leptin concentrations
Serum leptin concentrations were significantly lower in short-day animals when compared with long-day animals (F1,26 = 23.47, p < 0.001; figure 3b); however, there were no significant effects of insulin treatment (F1,26 = 1.12, p > 0.05), or an interaction between insulin and photoperiod treatments (F1,26 = 0.10, p > 0.05; figure 3b). All other pair-wise comparisons were not statistically significant (p > 0.05 in all cases). Serum leptin levels were correlated with adipose tissue composite scores (R2 = 0.435, p < 0.001), but not day 10 blood glucose levels (R2 = 0.009, p > 0.05) or anti-KLH IgG plate positive values (R2 = 0.001, p > 0.05).
4. Discussion
In the current study, we investigated a potential role for the pancreatic peptide insulin in mediating seasonal energetic trade-offs with immunity in Siberian hamsters. Specifically, we asked: (i) whether exogenous administration of insulin alters humoural and innate immune responses and (ii) whether this effect is photoperiod-dependent. As expected, short-day animals displayed gonadal regression and marked reductions in total body fat relative to long-day hamsters. Exogenous insulin increased KLH-specific IgG production, but only in short-day hamsters; insulin did not affect humoural immunity in long-day animals. Anti-KLH IgM followed a similar, non-significant, trend. In contrast, insulin had no effect on innate immunity (i.e. bacterial killing ability) regardless of photoperiodic condition. Serum leptin was reduced in short-day animals, but was not altered in response to insulin treatment. The findings from the present study support the idea that insulin can influence immune responses in hamsters and the effects of insulin on immunity can vary according to photoperiodic status. In addition, these results suggest that insulin acts as a peripheral signal of energy availability and probably mediates, at least in part, energetic trade-offs between immunity and other physiological systems (e.g. reproduction).
Blood glucose levels were significantly lower 1 h after insulin injection, suggesting that hamsters are physiologically responsive to hormone. Short-day hamsters appear to be more sensitive to the insulin signal as blood glucose levels in short-day animals were significantly lower than long-day animals given insulin. In contrast, there was no significant difference in glucose levels between long-day and short-day control hamsters. A similar pattern was observed with food intake across experimental treatments. Consistent with previous findings in this species (Bartness et al. 1995), long-day insulin-treated hamsters consumed significantly more food than all other groups. In contrast, an increase in humoural immune response was observed in insulin-treated short-day hamsters, but not long-day hamsters. Thus, insulin-induced immunoenhancement was not probably a direct result of increased food intake. These findings suggest that insulin-induced immunoenhancement is not merely a product of abundant energy, but is also dependent on the photoperiodic state of the animal. Previous work in this and other species suggested that short-, but not long-day conditions can buffer immune suppression from an energetic stressor, suggesting long-day hamsters may be more sensitive to energy changes (Demas et al. 1997b; Zysling et al. 2006). However, where energy abundance is concerned, this may not be the case. Under stressful winter conditions, an animal may stand to profit from upregulating immunity if it receives a signal of immediate energy.
Changes in insulin levels alter a wide range of metabolic processes, which may alter immune responses. For example, insulin can influence circulating levels of leptin (Saladin et al. 1995; Warne et al. 2009) and can alter the metabolic actions of adipocytes (Muller et al. 1997). Leptin, in turn, can affect humoural immune responses in a variety of mammalian species, including hamsters (Lord et al. 1998; Drazen et al. 2001). Thus, the effects of insulin on immunity may be indirect, via changes in leptin levels. Serum leptin concentrations were determined in the present study to assess whether the observed effects on immunity are caused by altered leptin levels. Leptin levels differed according to photoperiod treatment and, consistent with previous studies, were significantly lower in short-day hamsters (Drazen et al. 2000). Insulin treatment, however, had no effect on serum leptin levels. Because leptin concentrations were lower in short-day hamsters and insulin increased antibody responses in these animals, leptin is not probably playing a role in the photoperiod-dependent effects of insulin on humoural immunity.
Although metabolic factors other than leptin cannot be ruled out, insulin may act directly on peripheral immune tissues to enhance immune responses in short-day hamsters. Like most tissues, peripheral lymphoid tissue possesses insulin receptors (Gavin et al. 1973). Interestingly, the insulin receptor is expressed on resting neutrophils, monocytes and B lymphocytes, but not T lymphocytes; activation of T cells, however, results in marked upregulation of insulin receptors on these cells (Viardot et al. 2007). Consistent with this idea, lymphocyte proliferation and activation is altered by in vitro insulin administration (Viardot et al. 2007), suggesting that insulin can act directly on lymphocytes to modulate acquired immune responses.
Alternatively, insulin may act indirectly, via the central nervous system, to affect immunity. Such a mechanism has been suggested as part of the effects of leptin on humoural immunity (Demas 2002). Previous research has demonstrated that central insulin receptor expression is reduced under short-day conditions in the arcuate nucleus (ARC) and short-day animals are presumably less sensitive to the insulin signal (Tups et al. 2006). Similar findings of short-day decreases in insulin receptors have been reported in Japanese quail in the infundibular nucleus, a structure homologous to the mammalian ARC (Anraku et al. 2007). In contrast to previous findings, short-day hamsters in the present study appear more sensitive to exogenous insulin (e.g. display largest decrease in blood glucose levels and increase in immunoglobulin response), suggesting possible upregulation of insulin receptors, either centrally or in peripheral tissues (e.g. lymphoid tissues). According to a tissue-specific analysis of the insulin receptor expression in the mouse transcriptome, the insulin receptor is a ubiquitous housekeeping gene: there is no significant difference between expression in the hypothalamus compared with lymphoid organs (Su et al. 2002). To our knowledge, no studies have examined photoperiodic changes in insulin receptors on lymphoid tissues in this or other seasonally breeding species.
Previous studies in Siberian hamsters have reported a photoperiod-dependent decrease in immune responses. Specifically, short-day hamsters have a lower antibody response than long-day animals (Drazen et al. 2001; Demas et al. 2002). In the current study, the expected decrease in immune response in short-day hamsters, however, was not observed; short-day levels of anti-KLH immunoglobulins did not differ between long- and short-day hamsters. This is somewhat surprising given that short-day hamsters exhibited all other typical photoperiod-induced characteristics including decreased body mass, food intake, adipose tissue mass and gonadal regression (Bartness 1996). Although it is not known why immunity was not altered in short-day hamsters, it is possible that daily injections and handling acted as repeated stressors, potentially altering immune responses and masking expected photoperiodic changes in immunity. Male hamsters typically have elevated glucocorticoid levels in short days and exhibited greater immune responses to restraint stress (Bilbo & Nelson 2003). Unlike the effects on antibody levels, exogenous insulin had no effect on innate immunity as assessed by bacterial killing ability. Innate immune responses tend to remain uniform. Innate responses use intrinsic microbial molecules, pathogen-associated molecular patterns (PAMPs), to bind foreign molecules (Medzhitov & Janeway 2002). Alteration in the ability to recognize PAMPs is commonly deleterious to pathogen recognition (Beutler 2004). Therefore, we might not expect to see a difference in innate immunity even under conditions where a signal of additional energy is involved.
Regardless of the precise mechanisms of action, the present results support the idea that insulin alters humoural immune responses, either directly or indirectly, in Siberian hamsters. Further research is required to determine the specific mechanisms mediating this effect. Taken together, these results suggest that insulin serves as an important peripheral signal linking energy availability and immunity. Exogenous insulin can enhance immune responses in short-day animals and, like leptin, can enhance energy availability owing to increased food intake. These findings support the idea that reduced energy availability can lead to changes in immunity which, in turn, can affect disease susceptibility. In addition, these results suggest an important role for insulin in regulating energetic trade-offs among competing physiological systems and provide an important step towards understanding the neuroendocrine mechanisms regulating seasonal changes in mammalian immunity.
Acknowledgements
This work was reviewed and approved by the Institutional Animal Care and Use Committee at Indiana University. This work was supported by a SICB Grant-in-Aid and NSF International Research Fellowship to T.J.G., NIH Training Grant HD 049336-04 Common Themes in Reproductive Diversity Training grant, NIH to S.S.F. and T.J.G., a fellowship from the Center for the Integrative Study of Animal Behaviour (CISAB) to E.M.C., and a Faculty Research Support (FRSP) grant and NSF grant IOB-0543798 to G.E.D.
References
- Anraku T., et al. 2007Photoperiodic changes in hypothalamic insulin receptor gene expression are regulated by gonadal testosterone. Brain Res. 1163, 86–90 (doi:10.1016/j.brainres.2007.06.028) [DOI] [PubMed] [Google Scholar]
- Bartness T. J.1996Photoperiod, sex, gonadal steroids, and housing density affect body fat in hamsters. Physiol. Behav. 60, 517–529 (doi:10.1016/S0031-9384(96)80027-8) [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Clein M. R.1994Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 266, R1111–R1117 [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Demas G. E.2004Comparative studies of food intake. In Handbook of neurobiology, vol. 10, Neurobiology of food and fluid intake (eds Stricker E. M., Woods S.), pp. 423–467 New York, NY: Kluwer Press [Google Scholar]
- Bartness T. J., McGriff W. R., Maharaj M. P.1991Effects of diabetes and insulin on photoperiodic responses in Siberian hamsters. Physiol. Behav. 49, 613–620 (doi:10.1016/0031-9384(91)90287-X) [DOI] [PubMed] [Google Scholar]
- Bartness T. J., Morley J. E., Levine A. S.1995Effects of food deprivation and metabolic fuel utilization on the photoperiodic control of food intake in Siberian hamsters. Physiol. Behav. 57, 61–68 (doi:10.1016/0031-9384(94)00203-H) [DOI] [PubMed] [Google Scholar]
- Benoit S. C., Clegg D. J., Seeley R. J., Woods S. C.2004Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 59, 267–285 (doi:10.1210/rp.59.1.267) [DOI] [PubMed] [Google Scholar]
- Beutler B.2004Innate immunity: an overview. Mol. Immunol. 40, 845–859 (doi:10.1016/j.molimm.2003.10.005) [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Nelson R. J.2001Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R207–R213 [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Nelson R. J.2003Sex differences in photoperiodic and stress-induced enhancement of immune function in Siberian hamsters. Brain Behav. Immun. 17, 462–472 (doi:10.1016/S0889-1591(03)00063-1) [DOI] [PubMed] [Google Scholar]
- Bilbo S. D., Drazen D. L., Quan N., He L., Nelson R. J.2002Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc. R. Soc. Lond. B 269, 447–454 (doi:10.1098/rspb.2001.1915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson F. H., Heideman P. E.1994Seasonal regulation of reproduction in mammals. In The physiology of reproduction, vol. 2, 2nd edn (eds Knobil E., Neill J. D.), pp. 541–584 New York, NY: Raven Press [Google Scholar]
- Demas G. E.2002Splenic denervation blocks leptin-induced enhancement of humoral immunity in Siberian hamsters (Phodopus sungorus). Neuroendocrinology 76, 178–184 (doi:10.1159/000064527) [DOI] [PubMed] [Google Scholar]
- Demas G. E.2004The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm. Behav. 45, 173–180 (doi:10.1016/j.yhbeh.2003.11.002) [DOI] [PubMed] [Google Scholar]
- Demas G. E., Bartness T. J.1999Effects of food deprivation and metabolic fuel utilization on food hoarding by jirds (Meriones shawi). Physiol. Behav. 67, 243–248 (doi:10.1016/S0031-9384(99)00066-9) [DOI] [PubMed] [Google Scholar]
- Demas G. E., Nelson R. J.1998aExogenous melatonin enhances cell-mediated, but not humoral, immune function in adult male deer mice (Peromyscus maniculatus). J. Biol. Rhythms 13, 245–252 (doi:10.1177/074873098129000084) [DOI] [PubMed] [Google Scholar]
- Demas G. E., Nelson R. J.1998bShort-day enhancement of immune function is independent of steroid hormones in deer mice (Peromyscus maniculatus). J. Comp. Physiol. B 168, 419–426 [DOI] [PubMed] [Google Scholar]
- Demas G. E., Sakaria S.2005Leptin regulates energetic tradeoffs between body fat and humoural immunity. Proc. R. Soc. B 272, 1845–1850 (doi:10.1098/rspb.2005.3126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas G. E., Chefer V., Talan M. I., Nelson R. J.1997aMetabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 42, R1631–R1637 [DOI] [PubMed] [Google Scholar]
- Demas G. E., DeVries A. C., Nelson R. J.1997bEffects of photoperiod and 2-deoxy-d-glucose-induced metabolic stress on immune function in female deer mice (Peromyscus maniculatus). Am. J. Physiol. 272, R1762–R1767 [DOI] [PubMed] [Google Scholar]
- Demas G. E., Drazen D. L., Jasnow A. M., Bartness T. J., Nelson R. J.2002Sympathoadrenal system differentially affects photoperiodic changes in humoral immunity of Siberian hamsters (Phodopus sungorus). J. Neuroendocrinol. 14, 29–35 (doi:10.1046/j.0007-1331.2001.00736.x) [DOI] [PubMed] [Google Scholar]
- Dixon F. J., Jacot-Guillarmod H., McConahey P. J.1966The antibody responses of rabbits and rats to hemocyanin. J. Immunol. 97, 350–355 [PubMed] [Google Scholar]
- Drazen D. L., Kriegsfeld L. J., Schneider J. E., Nelson R. J.2000Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1401–R1407 [DOI] [PubMed] [Google Scholar]
- Drazen D. L., Demas G. E., Nelson R. J.2001Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus). Endocrinology 142, 2768–2775 (doi:10.1210/en.142.7.2768) [DOI] [PubMed] [Google Scholar]
- French S. S., Greives T. J., Zysling D. A., Chester E. M., Demas G. E.2009Leptin increases maternal investment. Proc. R. Soc. B 276, 4003–4011 (doi:10.1098/rspb.2009.1199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin J. R., III, Gorden P., Roth J., Archer J. A., Buell D. N.1973Characteristics of the human lymphocyte insulin receptor. J. Biol. Chem. 248, 2202–2207 [PubMed] [Google Scholar]
- Greives T. J., Mason A. O., Scotti M.-A. L., Levine J., Ketterson E. D., Kriegsfeld L. J., Demas G. E.2007Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology 148, 1158–1166 (doi:10.1210/en.2006-1249) [DOI] [PubMed] [Google Scholar]
- Greives T. J., Kriegsfeld L. J., Demas G. E.2008Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus). Gen. Comp. Endocrinol. 156, 552–558 (doi:10.1016/j.ygcen.2008.02.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey S. G., Prendergast B. J., Nelson R. J.2003Photoperiod and stress affect wound healing in Siberian hamsters. Physiol. Behav. 78, 205–211 (doi:10.1016/S0031-9384(02)00967-8) [DOI] [PubMed] [Google Scholar]
- Klein-Schneegans A. S., Gaveriaux C., Fonteneau P., Loor F.1989Indirect double sandwich ELISA for the specific and quantitative measurement of mouse IgM, IgA and IgG subclasses. J. Immunol. Methods 119, 117–125 [DOI] [PubMed] [Google Scholar]
- Knutson V.1991Cellular trafficking and processing of the insulin receptor. FASEB J. 5, 2130–2138 [DOI] [PubMed] [Google Scholar]
- Lochmiller R. L., Deerenberg C.2000Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98 (doi:10.1034/j.1600-0706.2000.880110.x) [Google Scholar]
- Lord G. M., Matarese G., Howard J. K., Baker R. J., Bloom S. R., Lechler R. I.1998Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394, 897–901 (doi:10.1038/29795) [DOI] [PubMed] [Google Scholar]
- Martin L. B., Weil Z. M., Nelson R. J.2008Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc. B 363, 321–339 (doi:10.1098/rstb.2007.2142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson K. D., Tieleman B. I., Klasing K. C.2006Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Physiol. Biochem. Zool. 79, 556–564 (doi:10.1086/501057) [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C. A., Jr2002Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 (doi:10.1126/science.1068883) [DOI] [PubMed] [Google Scholar]
- Muller G., Ertl J., Gerl M., Preibisch G.1997Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J. Biol. Chem. 272, 10 585–10 593 [DOI] [PubMed] [Google Scholar]
- Nelson R. J., Demas G. E.1996Seasonal changes in immune function. Q. Rev. Biol. 71, 511–548 (doi:10.1086/419555) [DOI] [PubMed] [Google Scholar]
- Puchalski W., Lynch G. R.1986Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J. Comp. Physiol. A 159, 7–11 (doi:10.1007/BF00612490) [DOI] [PubMed] [Google Scholar]
- Saladin R., De Vos P., Guerre-Millo M., Leturque A., Girard J., Staels B., Auwerx J.1995Transient increase in obese gene expression after food intake or insulin administration. Nature 377, 527–529 (doi:10.1038/377527a0) [DOI] [PubMed] [Google Scholar]
- Schwartz M. W., Kahn S. E.1999Insulin resistance and obesity. Nature 402, 860–861 (doi:10.1038/47209) [DOI] [PubMed] [Google Scholar]
- Su A. I., et al. 2002Large-scale analysis of the human and mouse transcriptomes. Proc. Natl Acad. Sci. USA 99, 4465–4470 (doi:10.1073/pnas.012025199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tups A., Helwig M., Stohr S., Barrett P., Mercer J. G., Klingenspor M.2006Photoperiodic regulation of insulin receptor mRNA and intracellular insulin signaling in the arcuate nucleus of the Siberian hamster, Phodopus sungorus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R643–R650 [DOI] [PubMed] [Google Scholar]
- Viardot A., Grey S. T., Mackay F., Chisholm D.2007Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology 148, 346–353 (doi:10.1210/en.2006-0686) [DOI] [PubMed] [Google Scholar]
- Warne J. P., Akana S. F., Ginsberg A. B., Horneman H. F., Pecoraro N. C., Dallman M. F.2009Disengaging insulin from corticosterone: roles of each on energy intake and disposition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1366–R1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil Z. M., Bowers S. L., Nelson R. J.2007Photoperiod alters affective responses in collared lemmings. Behav. Brain Res. 179, 305–309 (doi:10.1016/j.bbr.2007.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolowczuk I., Verwaerde C., Viltart O., Delanoye A., Delacre M., Pot B., Grangette C.2008Feeding our immune system: impact on metabolism. Clin. Dev. Immunol. 2008, 639 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon S. M., Teasley L. A., Fagoaga O. R., Nguyen H. C., Truong H. N., Nehlsen-Cannarella S. L.1999Role of photoperiod and the pineal gland in T cell-dependent humoral immune reactivity in the Siberian hamster. J. Pineal Res. 27, 243–248 (doi:10.1111/j.1600-079X.1999.tb00622.x) [DOI] [PubMed] [Google Scholar]
- Zysling D., Demas G.2007Metabolic stress suppresses humoral immune function in long-day, but not short-day, Siberian hamsters (Phodopus sungorus). J. Comp. Physiol. B 177, 339–347 [DOI] [PubMed] [Google Scholar]
- Zysling D. A., Greives T. J., Breuner C. W., Casto J. M., Dernas G. E., Ketterson E. D.2006Behavioral and physiological responses to experimentally elevated testosterone in female dark-eyed juncos (Junco hyemalis carolinensis). Horm. Behav. 50, 200–207 (doi:10.1016/j.yhbeh.2006.03.004) [DOI] [PubMed] [Google Scholar]