Abstract
Human behaviour is often based on social learning, a mechanism that has been documented also in a variety of other vertebrates. However, social learning as a means of problem-solving may be optimal only under specific conditions, and both theoretical work and laboratory experiments highlight the importance of a potential model's identity. Here we present the results from a social learning experiment on six wild vervet monkey groups, where models were either a dominant female or a dominant male. We presented ‘artificial fruit’ boxes that had doors on opposite, differently coloured ends for access to food. One option was blocked during the demonstration phase, creating consistent demonstrations of one possible solution. Following demonstrations we found a significantly higher participation rate and same-door manipulation in groups with female models compared to groups with male models. These differences appeared to be owing to selective attention of bystanders to female model behaviour rather than owing to female tolerance. Our results demonstrate the favoured role of dominant females as a source for ‘directed’ social learning in a species with female philopatry. Our findings imply that migration does not necessarily lead to an exchange of socially acquired information within populations, potentially causing highly localized traditions.
Keywords: social learning, tradition, selective attention, vervet monkeys, artificial fruit, field experiment
1. Introduction
Efficient social learning plays an essential role in human life as it provides the basis for traditions and culture (Plotkin 2007). As a consequence, studying the roots of culture in other animals has been a key research topic for decades (Whiten 2009). Theoretical studies on social learning rules suggest that individuals should be selective when deciding both when to learn socially and who to choose as a model (Boyd & Richerson 1985; de Waal 2001; Henrich & Gil-White 2001; Giraldeau et al. 2002; Laland 2004; Mesoudi 2008). A few empirical studies have identified rules for choosing models (Nicol & Pope 1999; Schwab et al. 2008), revealing typically that successful individuals are likely to induce social learning. For example, laboratory experiments demonstrated that nine-spined sticklebacks preferably copied foraging patches of larger individuals (Duffy et al. 2009). However, sticklebacks are also able to compare their own foraging success with the success of others and choose foraging locations accordingly (Kendal et al. 2009). This latter result implies that these fish are flexible with respect to the question ‘who is a good model?’ and are thus able to choose the best option in each situation.
For primates, living in stable social groups with hierarchical structures and certain levels of kin relationships (Smuts et al. 1987), it has been argued that certain individuals are predisposed to be models for other group members, independent of their suitability in a given situation (de Waal 2001). The ‘social model hypothesis’—also known as bonding and identification-based observational learning (BIOL)—predicts that primates living in structured social groups are most likely to learn from social models such as knowledgeable, older, high ranking members of the same group and species (de Waal 2001). In addition, the hypothesis predicts that social learning in this taxonomic group is linked to conformity. Young and subordinate individuals want to behave like old and dominant individuals do. Therefore, individuals may copy the behaviour of models even if their behaviour is unsuitable for the current situation, and fail to copy the behaviour of other group members even when that would be favourable (de Waal 2001). The hypothesis could explain why the use of humans as models often yields negative results for social learning in non-human primates, despite the models' perfect knowledge for the tasks in question (Call & Tomasello 1996). In contrast, the use of female conspecifics as models has allowed the demonstration of the development of arbitrary traditions in captive chimpanzees (Whiten et al. 2005). Nevertheless, there are no demonstrations that an individual's identity (that is, its relatedness to other group members and/or its social status) affects the likelihood that others will copy its behaviour.
In this paper we report tests of the social model hypothesis in a field experiment on six vervet monkey groups. We used a standard experimental design in laboratory studies on primates: a baited box, called an ‘artificial fruit’ (Whiten et al. 1996). These artificial fruits can be opened in two different ways, but one option is blocked during the demonstration phase so that models consistently open the box in one way (figure 1). During the experiment, subjects could potentially open the box in both ways. Therefore, a significant repetition of the models' behaviour demonstrates social learning. We had three groups where the dominant female acted as model and three groups where a dominant male acted as model. In vervet monkeys, females are the philopatric sex, while males migrate at sexual maturity (Dunbar & Thelma 2001). Therefore, we could investigate a more refined aspect of the social model hypothesis, namely that members of the philopatric sex might elicit more social learning than members of the migrating sex. In that case, we predicted that female models would be more likely to attract group members to the task and more likely to induce social learning than male models. In contrast, if dominance per se is a key factor to induce social learning, we predicted that groups with male models would learn as well as groups with female models.
Figure 1.
(a) A vervet monkey manipulating the pull door, marked with wooden colour, and (b) a vervet monkey manipulating the slide door, marked with black colour.
In our experiment, any effect of the sex of the model on the likelihood of social learning could not be explained by differences in relevant knowledge, but two alternative explanations would remain. First, members of one sex could be more aggressive, keeping group members away and therefore precluding efficient social learning. Second, group members might pay selectively more attention to the actions of models of one sex, therefore being more likely to learn from members of this sex. To distinguish between these alternatives, we noted the number of bystanders during the demonstrations, whether they looked at the model during the moment of box opening and the number of aggressive actions initiated by the model during the demonstrations. We predicted that if tolerance is the key to successful social learning, models of the less aggressive sex would elicit a greater number of bystanders. Likewise, we predicted that if the effect of the sex of the model is caused by selective attention, models of the sex that elicits more successful social learning would receive more attention during the task.
2. Material and methods
(a). Study site and population
Experiments were conducted between 2006 and 2008 on six neighbouring groups of habituated wild vervet monkeys (Chlorocebus aethiops) at Loskop Dam Nature Reserve, South Africa. The reserve, situated 250 km northeast of Johannesburg, covers 25 000 ha. Vervet monkeys live in stable family groups, which varied from 13 to 23 individuals during our experiments. Groups are typically composed of an alpha male, a few subordinate males and several matrilines (i.e. females and their offspring). Females remain in their natal group all their life, while males migrate to another group when they are sexually mature, usually at around 4 years of age. Our six study groups—Picnic, Nooitgedacht, Blesbokvlakte, Donga, Bay and Fishing Camp (named after sites on the park map)—live in contiguous home ranges along a tourist road that allows easy access to each group. Group compositions are summarized in table 1.
Table 1.
The composition of the study groups. Males are scored as adults once they have migrated, while females are scored as adults once they have given birth. Group members that did not fulfil these criteria were scored as juveniles if they were at least one year old, and as infants if they were younger.
| group | adult male | adult female | juvenile | infant | total |
|---|---|---|---|---|---|
| Bay | 4 | 5 | 7 | 0 | 16 |
| Picnic | 2 | 4 | 6 | 3 | 15 |
| Blesbokvlakte | 2 | 3 | 5 | 3 | 13 |
| Donga | 4 | 6 | 6 | 4 | 20 |
| Nooitgedacht | 3 | 5 | 6 | 3 | 21 |
| Fishing Camp | 3 | 5 | 12 | 3 | 23 |
All groups had been exposed to the presence of human researchers for at least 1 year before they were tested. All individuals were recognized by their faces, and a recognition file with portrait pictures and specific individual features (scars, etc.) was constructed for each group. Two of the six groups were in regular contact with tourists: the Fishing Camp group and the Picnic group. The latter and the Donga group had been used for experiments before (Fruteau et al. 2009).
(b). Experimental design
We used an established laboratory design, the artificial fruit (Whiten et al. 1996), to test for the presence of social learning. Our artificial fruits were wooden boxes with two Plexiglas doors on opposite ends (figure 1), with one-eighth of an apple inside. One door could be opened by pulling a knob (electronic supplementary material, movie S1), while the other door could be opened by sliding it to the left side holding a knob (electronic supplementary material, movie S2). One door was locked during the demonstration phase. Observers could potentially identify the door that the model used because the knobs were placed at different locations on the respective doors and because the two sides of the box differed in colour: one half was wooden while the other half was black.
As we worked with wild groups we could not choose a model and train it in isolation from the other group members. Therefore, we started by simply offering a baited open box to the group, which was invariably soon monopolized by a dominant individual. In subsequent trials we made sure that this dominant was in proximity to the box so that it would continue to prevent other group members from gaining personal experience. During the initial demonstration phase, a model learned to open the box in one particular way because the alternative method was prevented. This led to consistent behavioural demonstrations of how to open the box in the presence of the subjects. The demonstration phase continued until the dominant had performed 25 successive successful trials, which consisted of approaching, manipulating and opening the correct door without prior touching of the blocked door. We conducted one session consisting of eight demonstration trials per day to keep the models motivated. Human experimenters sat about 5 m away from the box during trials, waited for the dominant to eat the piece of fruit, and then walked up to the box to bait it again. Our six models needed between 5 and 15 sessions spread over 11–63 days to complete the demonstration.
Monopolizing individuals were female for three models (Bay, Blesbokvlakte and Picnic groups) and male for three models: twice the alpha male (Donga and Nooitgedacht groups) and once the fully grown son of the alpha female (Fishing Camp group). We assigned one pull door (Picnic) and one slide door (Bay) task to female models and to male models, respectively (Nooitgedacht = pull, Donga = slide). A coin toss determined that the third female model (Blesbokvlakte) be confronted with a pull-door task, and then we assigned the slide door to the third male model (Fishing Camp) in order to have an even number of models on each type of door.
(c). Data collection
During the experiments we used two means to prevent the model from monopolizing the box, so that other group members could access it as well: we either offered four dispersed boxes simultaneously or we targeted isolated individuals and placed the box close to them. Now the boxes could be opened from both sides (in two different ways). We noted who participated and whether participants manipulated the same door as the model. All trials were filmed with a digital video camera. The data could be coded unambiguously: an individual participated if it touched the box, and location of first manipulation could be identified because of the colour coding of the two halves.
To investigate how male or female models affect the behaviour of other group members, we collected information on the number of bystanders, the frequency with which models behaved aggressively towards bystanders and whether bystanders looked at the models during the opening of the artificial fruit. We defined bystanders as individuals within 5 m of the artificial fruit. Data on the number of bystanders were collected each time the model opened the box.
(d). Data analyses
For the analyses on social learning, we calculated participation rate as the percentage of individuals that touched a box once during the experimental phase. Of all the individuals that touched the box we counted the number of individuals per group that touched the same door as the model. For the statistical analyses, we excluded group members that had gained access to the box during the demonstration phase, either before the model consistently monopolized the box or if the individual was tolerated by the model during the demonstrations. Such early experiences might have modified behaviour independently of the models' demonstrations. Also, individuals younger than one year were not counted for group size as they never participated in the experiments.
To investigate how male or female models affect the behaviour of other group members during demonstrations, we calculated for each trial the ratio of bystanders divided by group size. These values were then used to compare the six study groups with respect to attendance of demonstrations. We also compared the total number of different bystanders between groups with male or female models. To complete this last analysis, we checked the number of different bystanders in each group that attended the demonstrations at least once. We also calculated one value per day for the frequency of aggression shown by the models. We divided the number of the models' aggressive acts by the mean number of bystanders and by the total duration of one demonstration session (as aggression was noted for the entire duration of an experimental session rather than just when a box was baited). Finally, we calculated for each bystander the frequency of looking at the model during the opening of the box. We analysed the data once for all group members and once excluding the offspring of dominant female models to test the potential effect of matriline membership.
(e). Statistical analyses
We conducted both χ2 tests that treated each experimental individual as an independent data point and generalized linear binomial models (using the lme4 package under the R CRAN 2009 interface; Bates & Sarkar 2007) with group identity as a nested variable to control for potential dependencies between members of the same group. The similarity of the results indicates the robustness of our conclusions. We conducted two-level nested design ANOVA using SPSS 16.0 for all the non-binomial datasets.
3. Results
(a). Female models promote more social learning than male models do
Individuals without any prior experience were more likely to participate in the experimental phase if the model was a female rather than a male (χ2 tests: n = 64 potential participants, χ2 = 15, d.f. = 1, p < 0.001; figure 2). This difference persisted in a nested generalized linear binomial model controlling for potential group effects (GLM model using Laplace: n = 64, z = −3.846, p < 0.001).
Figure 2.
Percentage of individuals belonging to six different groups (Bay, Picnic and Blesbokvlakte with female models; Donga, Nooitgedacht and Fishing Camp with male models) that participated in the experiment. Numbers in white represent sample sizes for each group.
Individuals manipulated the same side as the model significantly more often than expected by chance (χ2 test: n = 35, χ2 = 4.1, d.f. = 1, p < 0.05). Separate analyses for male and female models revealed that individuals manipulated the same side if a female was the model (χ2 test: n = 23, χ2 = 8.5, d.f. = 1, p < 0.01), while side choice was not significantly different from random with male models (χ2 test: n = 12, χ2 = 0.1, d.f. = 1, ns). The difference between males and females was significant (χ2 test: n = 35, χ2 = 4.4, d.f. = 1, p < 0.05; figure 3). The effect of model sex persisted in a nested generalized linear binomial model controlling for potential group effects (GLM model using Laplace: n = 35, z = −2.358, p = 0.018). The key results remained when we removed all data on members of the female models' matrilines to exclude the potentially confounding effects of mother–offspring relationships on our dataset. In these control analyses, we still found that females elicited higher levels of participation than males (χ2 test: n = 63, χ2 = 11.6, d.f. = 1, p < 0.001) and that individuals more often manipulated the same side that the female model had used than expected by chance (χ2 test: n = 34, χ2 = 4.5, d.f. = 1, p < 0.05).
Figure 3.
Number of individuals in six different groups that manipulated the box either on the same side as the model (black parts) or on the opposite side (white parts).
(b). Causes of differences in social learning depending on the sex of the model
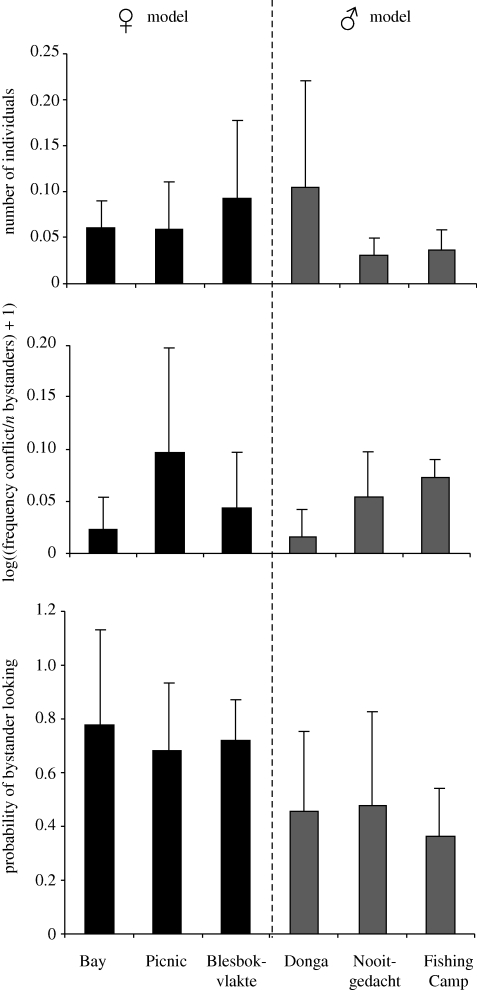
There was no significant difference of attendance in groups with female or male models (two-level nested-design ANOVA: n = 36, F = 0.288, p = 0.619; figure 4a). This lack of significant difference persisted when we checked how many group members were at least once a bystander (GLM model using Laplace: n = 104, z = 0.707, p = 0.489). In addition, male and female models did not differ significantly with respect to the frequency of aggressive acts towards nearby individuals during the experiments (two-level nested-design ANOVA: n = 31, F = 1.029, p = 0.365; figure 4b). In contrast, we found that individuals within 5 m of the box were more likely to look at female models at the moment of box opening than at male models (two-level nested-design ANOVA: n = 32, F = 9.935, p = 0.008; figure 4c). Excluding the offspring of dominant female models to control for effects of matriline membership did not alter the results (attendance per trial—two-level nested-design ANOVA: n = 36, F = 0.003, p = 0.962; n different individuals attending—GLM model using Laplace: n = 97, z = 0.055, p = 0.956; frequency of aggressive acts—two-level nested-design ANOVA: n = 31, F = 0.025, p = 0.882; look at model—two-level nested-design ANOVA: n = 30, F = 22.090, p < 0.001).
Figure 4.
(a) Number of individuals within 5 m of the box (bystanders) per trial corrected for group size in six different groups. (b) The log-transformed frequency of aggression performed by the model towards bystanders per experimental session, and (c) the mean probability for each bystander that it looks at the model at the moment of box opening. For all three results, means ((a) per trial, (b) per experimental session, (c) of individual bystander means) and s.d. are shown. Results for groups with female models (Bay, Picnic and Blesbokvlakte) are shown in black bars, while results for groups with male models (Donga, Nooitgedacht and Fishing Camp) are shown in grey bars.
4. Discussion
The aim of our experiment was to test whether wild vervet monkeys learn preferentially from male or female models, and if so what causes such differential social learning. In addressing these questions, we also tested whether wild vervet monkeys learn socially at all in a task that allowed the demonstration of social learning in other primate species under laboratory conditions.
(a). Bystanders pay more attention to female models than to male models
The most important conclusion from our experiment is that in vervet monkeys bystanders seem to use only core members of the social group as role models for the spread of novel foraging behaviours under natural conditions. Theoreticians have pointed out that individuals should be selective about who they observe when gathering information and speculated about optimal social learning rules (Boyd & Richerson 1985; de Waal 2001; Henrich & Gil-White 2001; Giraldeau et al. 2002; Laland 2004; Mesoudi 2008). The hypothesis that individuals should copy successful group members has repeatedly received experimental support (Nicol & Pope 1999; Duffy et al. 2009; Kendal et al. 2009). In contrast to these laboratory studies, wild vervet monkeys appeared to ignore success per se: male models induced less stimulus enhancement (participation in the experiment) than female models did, and they did not induce local enhancement in other group members despite being successful at the task of opening the box and being successful in general as indicated by their dominance.
The social learning rule demonstrated by the vervets may have evolved because females, as members of the philopatric sex, might have both more detailed knowledge about the distribution of food resources in their territory and closer ties with most other group members (Smuts et al. 1987; Dunbar 1988). If this was the case, they may often be better than immigrants as sources for social learning, at least in the context of foraging. Based on our findings we hypothesize that in species in which members of one sex form the core of stable groups, the migration of members of the other sex leads to proper exchange of genetic adaptations but much less to the exchange of socially acquired adaptive information. Our hypothesis leads to the testable prediction that naturally occurring traditions based on social learning may not only be readily identified in comparisons between populations (Whiten et al. 1999; van Schaik et al. 2003) but also in comparisons between sympatric or even neighbouring groups. Such idiosyncratic group traditions should then be expressed primarily by members of the philopatric sex and the offspring.
(b). Female models elicit social learning because of selective attention by group members
We had two hypotheses that could have explained why female models elicit more social learning in group members than male models do. The data do not support the idea that male models are more aggressive towards bystanders than female models are. Therefore, the hypothesis that variation in the models' tolerance may either allow or hinder social learning in bystanders is not supported. In contrast, we found clear evidence in favour of the hypothesis that group members pay selective attention to female models. Experiments on common marmosets demonstrate that animals are often limited with respect to the duration for which they can direct their attention to a specific observation task (Range & Huber 2007): individuals paid longer attention to models of the opposite sex. In ravens, individuals show more attention towards affiliated group members (Scheid et al. 2007), a rule that explains our results also. This is because though the differences between male and female models persisted when we removed all offspring of the female models from the analyses, most other group members will still be both more related to and more familiar with female models than with male models. Our results are in line with a comparative study on keas, dogs and humans that supports the notion that selective attention according to identity of models and situation should be incorporated in studies on social learning to better understand variation in results (Range et al. 2008).
(c). Methodological considerations
One important notion is that while we had planned to obtain equal numbers of male and female models for our six groups, we naturally obtained three males and two females by chance and only had to specifically attract the dominant female of the Blesbokvlakte group to replace a juvenile as model. Ideally, the models would have been preselected by us based on random choice. Thus, we cannot exclude the possibility that some unknown variable that correlates with model sex may have influenced our results. Our treatment groups did not vary systematically with respect to levels of habituation, access to human facilities, group size, number of males in the group or territory size. As we did not find any effect of group identity within each model sex class, we can conclude that these variables cannot explain our results. Thus, the sex of the model indeed seems to be the key variable for the observed differences between groups.
(d). Experimental evidence for social learning in wild primates
To our knowledge, our study provides the first experimental evidence that wild primates learn socially from a model. Such evidence is paramount in laboratory studies on primates and other vertebrate taxa (Laland & Plotkin 1990; Gajdon et al. 2004; Whiten et al. 2005; Dindo et al. 2008). Under field conditions, experimental evidence for social learning has been provided only for other vertebrate taxa (Helfman & Schultz 1984; Lefebvre 1986; Warner 1988; Langen 1996; Thornton & Malapert 2008). For primates, indirect evidence exists based on the documentation of naturally occurring diffusion of novel behaviours within a group (Itani & Nishimura 1973) or on the identification of major behavioural differences between populations that do not seem to be well explained by any ecological differences between sites (Whiten et al. 1999; van Schaik et al. 2003). Therefore, there is a clear need for more experimental field studies on learning mechanisms.
Our results provide evidence for both stimulus enhancement and local enhancement. Female models attracted more group members to the task than male models did, and monkeys with female models apparently not only learned that an object may be of interest but also where to manipulate the object. Evidence for more complex social learning mechanisms like production imitation (Hoppitt & Laland 2008) are still lacking for field studies. In fact wild keas failed in a social learning task where captive ones had succeeded (Gajdon et al. 2004). Another important future direction would be to offer artificial fruits for an extended period of time and monitor similarities between members of the same group. The persistence of different opening methods in different groups would demonstrate the establishment of arbitrary traditions for which until now there has been no clear-cut evidence from the small number of field experiments (Thornton & Malapert 2008; Pesendorfer et al. 2009).
5. Conclusions
It has been noted that the fact that the overwhelming majority of social learning studies have been completed in captivity limits the validity of the field as a whole (Whiten & Mesoudi 2008). Our study joins a very few others (Helfman & Schultz 1984; Lefebvre 1986; Warner 1988; Langen 1996; Thornton & Malapert 2008; Pesendorfer et al. 2009) in demonstrating that it is possible to conduct field experiments in order to bridge the gap of knowledge on decision rules for social learning and the establishment of traditions in wild animals. With more studies of this kind, we will be able to establish the conditions under which animals may learn socially, what mechanisms they use and what circumstances lead to the formation of traditions. With such new evidence, we will soon be able to properly reflect on what specific aspects of our cultural transmission capacities are shared with other species.
Acknowledgements
The project was funded by the Swiss Science Foundation (grant to R.B.) and Fonds Marguerite Wübrich and A. Mathey-Dupraz from the University of Neuchâtel.
We thank Mpumalanga parks board for permission to work at Loskop Dam Nature Reserve and their help in the field. We thank L. Brown, L. Barrett, P. Henzi, R. Noë and T. de Beer for their logistical support. We are grateful to M. van de Waal for the creation of the experimental boxes. We also thank C. Borgeaud, Y. Bouquet, A. Piller, M. Spinelli, E. Tournier, V. Tournier and A. Vail for their great help with the field experiments. We thank N. Alvarez for his help with the statistics, and A. Whiten, S. Reader, N. Raihani and two anonymous referees for discussion and comments on the manuscript.
References
- Bates D., Sarkar D.2007lme4: linear mixed-effects models using S4 classes. R package version 0.9975-12. http://CRAN.R-project.org/ [Google Scholar]
- Boyd R., Richerson P. J.1985Culture and the evolutionary process Chicago, IL: University of Chicago Press [Google Scholar]
- Call J., Tomasello M.1996The effect of humans on the cognitive development of apes. In Reaching into thought: the minds of the great apes (eds Russon A. E., Bard K. A., Parker S. T.), pp. 371–403 Cambridge, UK: Cambridge University Press [Google Scholar]
- de Waal F. B. M.2001The ape and the sushi master: cultural reflections of a primatologist New York, NY: Basic Books [Google Scholar]
- Dindo M., Thierry B., Whiten A.2008Social diffusion of novel foraging methods in brown capuchin monkeys (Cebus apella). Proc. R. Soc. B. 275, 187–193 (doi:10.1098/rspb.2007.1318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy G. A., Pike T. W., Laland K. N.2009Size-dependent directed social learning in nine-spined sticklebacks. Anim. Behav. 78, 371–375 (doi:10.1016/j.anbehav.2009.05.015) [Google Scholar]
- Dunbar R. I. M.1988Primate social systems Ithaca, NY: Cornell University Press [Google Scholar]
- Dunbar R. I. M., Thelma E.2001Guenons, macaques, and baboons. In The new encyclopedia of mammals (ed. MacDonald D.), pp. 356–375 Oxford, UK: Oxford University Pres [Google Scholar]
- Fruteau C., Voelkl B., van Damme E., Noe R.2009Supply and demand determine the market value of food providers in wild vervet monkeys. Proc. Natl Acad. Sci. USA 106, 12 007–12 012 (doi:10.1073/pnas.0812280106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdon G., Fijn N., Huber L.2004Testing social learning in a wild mountain parrot, the Kea (Nestor notabilis). Learn. Behav. 32, 62–71 [DOI] [PubMed] [Google Scholar]
- Giraldeau L.-A., Valone T. J., Templeton J. J.2002Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B. 357, 1559–1566 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman G. S., Schultz E. T.1984Social transmission of behavioural traditions in a coral reef fish. Anim. Behav. 32, 379–384 (doi:10.1016/S0003-3472(84)80272-9) [Google Scholar]
- Henrich J., Gil-White F. J.2001The evolution of prestige: freely conferred deference as a mechanism for enhancing the benefits of cultural transmission. Evol. Hum. Behav. 22, 165–196 (doi:10.1016/S1090-5138(00)00071-4) [DOI] [PubMed] [Google Scholar]
- Hoppitt W., Laland K. N.2008Social processes influencing learning in animals: a review of the evidence. Adv. Study Behav. 38, 105–165 (doi:10.1016/S0065-3454(08)00003-X) [Google Scholar]
- Itani J., Nishimura A.1973The study of infrahuman culture in Japan: a review. In Precultural behaviour (ed. Menzel E. W., Jr), pp. 26–50 Basel, Switzerland: Karger [Google Scholar]
- Kendal J. R., Rendell L., Pike T. W., Laland K. N.2009Nine-spined sticklebacks deploy a hill-climbing social learning strategy. Behav. Ecol. 20, 238–244 (doi:10.1093/beheco/arp016) [Google Scholar]
- Laland K. N.2004Social learning strategies. Learn. Behav. 32, 4–14 [DOI] [PubMed] [Google Scholar]
- Laland K. N., Plotkin H. C.1990Social learning and social transmission of foraging information in Norway rats (Rattus norvegicus). Anim. Learn. Behav. 18, 246–251 [Google Scholar]
- Langen T. A.1996Social learning of a novel foraging skill by white-throated magpie-jays (Calocitta formosa, Corvidae): a field experiment. Ethology 102, 157–166 [Google Scholar]
- Lefebvre L.1986Cultural diffusion of a novel food-finding behaviour in urban pigeons: an experimental field test. Ethology 71, 295–304 [Google Scholar]
- Mesoudi A.2008An experimental simulation of the ‘copy successful individuals’ cultural learning strategy: adaptive landscapes, producer–scrounger dynamics and informational access costs. Evol. Hum. Behav. 29, 350–363 (doi:10.1016/j.evolhumbehav.2008.04.005) [Google Scholar]
- Nicol C. J., Pope S. J.1999The effects of demonstrator social status and prior foraging success on social learning in laying hens. Anim. Behav. 57, 163–171 (doi:10.1006/anbe.1998.0920) [DOI] [PubMed] [Google Scholar]
- Pesendorfer M. B., Gunhold T., Schiel N., Souto A., Huber L., Range F.2009The maintenance of traditions in marmosets: individual habit, not social conformity? A field experiment. PLoS ONE 4, e4472 (doi:10.1371/journal.pone.0004472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin H.2007The power of culture. In The Oxford handbook of evolutionary psychology (eds Dunbar R. I. M., Barrett L.), pp. 11–19 Oxford, UK: Oxford University Press [Google Scholar]
- Range F., Huber L.2007Attention in common marmosets: implication for social learning experiments. Anim. Behav. 73, 1033–1041 (doi:10.1016/j.anbehav.2006.07.015) [Google Scholar]
- Range F., Horn L., Burgnyar T., Gadjon K. G., Huber L.2008Social attention in keas, dogs and human children. Anim. Cogn. 12, 181–192 (doi:10.1007/s10071-008-0181-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid C., Range F., Bugnyar T.2007When, what and whom to watch? Quantifying attention in ravens (Corvus corax) and jackdaws (Corvus monedula). J. Comp. Psychol. 121, 380–386 (doi:10.1037/0735-7036.121.4.380) [DOI] [PubMed] [Google Scholar]
- Schwab C., Bugnyar T., Kotrschal K.2008Preferential learning from non-affiliated individuals in jackdaws (Corvus monedula). Behav. Proc. 79, 148–155 (doi:10.1016/j.beproc.2008.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuts B. B., Cheney D. L., Seyfarth R. M., Wrangham R. W., Struhsaker T. T. (eds) 1987Primate societies Chicago, IL: Chicago University Press [Google Scholar]
- Thornton A., Malapert A.2008The rise and fall of an arbitrary tradition: an experiment with wild meerkats. Proc. R. Soc. B 276, 1269–1276 (doi:10.1098/rspb.2008.1794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik C. P., Ancrenaz M., Borgen G., Galdikas B., Knott C. D., Singleton I., Suzuki A., Utami S. S., Merrill M.2003Orangutan cultures and the evolution of material culture. Science 299, 102–105 (doi:10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- Warner R. R.1988Traditionality of mating site preferences in a coral reef fish. Nature 335, 719–721 (doi:10.1038/335719a0) [Google Scholar]
- Whiten A.2009The identification and differentiation of culture in chimpanzees and other animals: from natural history to diffusion experiments. In The question of animal culture (eds Laland K. N., Galef B. G., Jr), pp. 99–124 Cambridge, MA: Harvard University Press [Google Scholar]
- Whiten A., Mesoudi A.2008Establishing an experimental science of culture: animal social diffusion experiments. Phil. Trans. R. Soc. B. 363, 3477–3488 (doi:10.1098/rstb.008.0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A., Custance D. M., Gomez J.-C., Teixidor P., Bard K. A.1996Imitative learning of artificial fruit processing in children (Homo sapiens) and chimpanzees (Pan troglodytes). J. Comp. Psychol. 110, 3–14 (doi:10.1037/0735-7036.110.1.3) [DOI] [PubMed] [Google Scholar]
- Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V., Sugiyama Y., Tutin C. E. G., Wrangham R. W., Boesch C.1999Cultures in chimpanzees. Nature 399, 682–685 (doi:10.1038/21415) [DOI] [PubMed] [Google Scholar]
- Whiten A., Horner V., de Waal F. B. M.2005Conformity to cultural norms of tool use in Chimpanzees. Nature 437, 737–740 (doi:10.1038/nature04047) [DOI] [PubMed] [Google Scholar]