Abstract
The skeletons of birds are universally described as lightweight as a result of selection for minimizing the energy required for flight. From a functional perspective, the weight (mass) of an animal relative to its lift-generating surfaces is a key determinant of the metabolic cost of flight. The evolution of birds has been characterized by many weight-saving adaptations that are reflected in bone shape, many of which strengthen and stiffen the skeleton. Although largely unstudied in birds, the material properties of bone tissue can also contribute to bone strength and stiffness. In this study, I calculated the density of the cranium, humerus and femur in passerine birds, rodents and bats by measuring bone mass and volume using helium displacement. I found that, on average, these bones are densest in birds, followed closely by bats. As bone density increases, so do bone stiffness and strength. Both of these optimization criteria are used in the design of strong and stiff, but lightweight, manmade airframes. By analogy, increased bone density in birds and bats may reflect adaptations for maximizing bone strength and stiffness while minimizing bone mass and volume. These data suggest that both bone shape and the material properties of bone tissue have played important roles in the evolution of flight. They also reconcile the conundrum of how bird skeletons can appear to be thin and delicate, yet contribute just as much to total body mass as do the skeletons of terrestrial mammals.
Keywords: birds, bats, bone density, bone strength
1. Introduction
In 1638, Galileo described bird bones as hollow and lightweight, and modern textbooks state as common knowledge that lightweight bones in birds are an adaptation that decreases the metabolic cost of flight (Galilei 1638; Fedducia 1996; Evans & Heiser 2004; Freeman 2005; Gill 2007). Bird skeletons exhibit many highly derived features that are unmistakably associated with flight, but how these features make bird skeletons lightweight is often not stated clearly. To describe a bird skeleton as ‘lightweight’ implies that it is light in weight relative to some other quantity. Unfortunately, the other quantity is rarely defined explicitly, and when it is, it is often defined in different ways by different researchers. This makes it difficult to explain, especially to the general public, exactly what is meant by the statement ‘bird skeletons are lightweight’.
One way in which bird skeletons are lightweight is with respect to the skeletons of their ancestors. The evolution of birds from their theropod predecessors was characterized by the gradual reduction, loss and fusion of many skeletal elements, and the expansion of pneumatized spaces within some bones (Buhler 1992; Fedducia 1996; Cubo & Casinos 2000; Dececchi & Larsson 2009). A recent analysis of Mesozoic birds documented a progressive reduction in overall body size and attributed it to selection for increasing the efficiency of flight within the lineage leading to modern birds (Hone et al. 2008). The same is true of bats, which have also experienced selection for small body size (Maurer et al. 2004). With the possible exception of some pterosaurs (Brower 2005), small body size (and by implication a small skeleton) is a common feature of flying vertebrates (Maina 2000).
A second way to define bird skeletons as lightweight has been to compare the weights and sizes of homologous skeletal elements between birds and mammals. Given a bird bone and a mammal bone of the same length, the bird bone is almost always lighter (Evans & Heiser 2004). Similarly, the bones of flighted birds are typically lighter than those of flightless and diving birds, where thicker-walled bones may serve as reinforcement or ballast (Currey & Alexander 1985; Evans & Heiser 2004; Habib & Ruff 2008). The relationship between the size and weight of bird and mammal bones has led some researchers to suggest that bird skeletons are lightweight with respect to body volume (Buhler 1992; Fedducia 1996; Evans & Heiser 2004). While it is true that birds appear to have larger volumes than mammals of similar weight, this is probably an illusion that can be traced to the fact that birds are infiltrated by extensive respiratory passages, and have feathers and enlarged lift-generating surfaces.
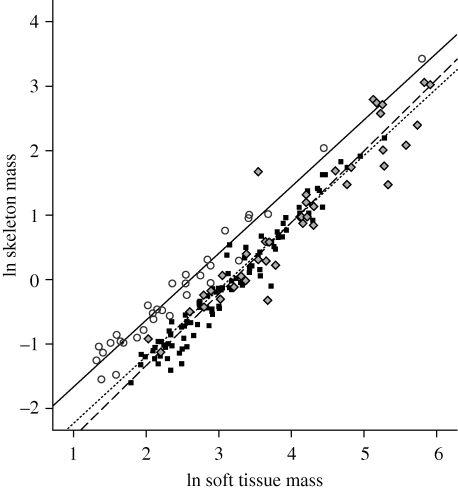
A third way to define lightweight is within the context of flight mechanics, where well-documented formulae illustrate that the cost of flight decreases with surface area and increases with body mass (Winter & von Helversen 1998; Videler 2005). Even with their wings folded against their bodies, birds have larger surface areas relative to body mass than, for example, rodents of similar size (compare data in Walsberg & King 1978; Reynolds 1997); this difference can only increase when birds' wings are unfurled. Many people, both the lay public and many biologists, also assume that given a bird and a mammal of the same total body weight, the bird's skeleton weighs less. This perception is reinforced by the delicate, fragile appearance of bird bones compared with those of most mammals. However, we know that bird skeletons contribute as much to total body mass as do the skeletons of terrestrial mammals (Prange et al. 1979). Similarly, new data collected for this study demonstrate that the same is true of skeletal mass relative to soft tissue mass (figure 1; data in the electronic supplementary material), suggesting that the weight of the skeletons and soft tissues of birds were equally affected by the reduction in body size that occurred during their evolution. This does not mean that adaptations for weight savings in the skeletons of birds do not exist, but it does highlight the contradiction between the delicate appearance of bird skeletons and their weight. One hypothesis for this incongruity is that bird skeletons are denser (i.e. have a higher mass per unit of volume) than mammal skeletons. If true, bird skeletons are not only heavier relative to their volume, but also stronger and stiffer.
Figure 1.
Regression of ln skeletal mass against ln soft tissue mass (= total body mass − skeletal mass) in passerine birds (n = 96; black squares, solid line), rodents (n = 39; grey diamonds, dashed line) and bats (n = 34; white circles, dotted line). Raw data is in the electronic supplementary material. To the mass of the mammal skeletons, 15 per cent was added to account for the fact that mammal bones are not pneumatized but contain marrow (Currey & Alexander 1985). Regression slopes do not differ significantly (p = 0.30). Comparison of skeletal mass using analysis of covariance demonstrate that the mass of bird skeletons is indistinguishable from that of rodents (p = 0.18). In contrast, bat skeletons are significantly heavier (p < 0.001) and thus comprise a larger proportion of total body mass. The 95 per cent confidence intervals for slopes of the rodent and bat regressions encompassed 1.0 (i.e. isometry; CIbats = 0.95–1.12; CIrodents = 0.91–1.16); the 95 per cent confidence interval for the bird regression indicated slight positive allometry (CIbirds = 1.06–1.16).
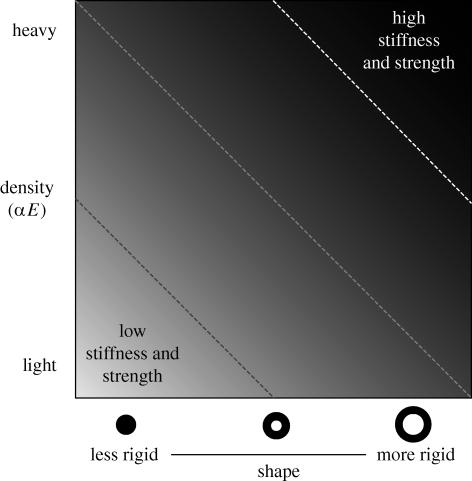
The skeletons of birds (and other flying vertebrates) need to be lightweight to minimize the metabolic cost of flight, and at the same time strong enough to withstand the forces encountered during flight. The same trade-off applies to manmade airframes. Aeronautical engineers satisfy these opposing demands in two ways: by designing load-bearing structures with shapes that confer strength, and by using materials that have high strength-to-weight and stiffness-to-weight ratios. The evolution of skeletal structures follows these principles as well; both the shape of a bone and the material properties of bone tissue contribute to a bone's stiffness and strength (figure 2).
Figure 2.
Conceptual diagram of the contribution of bone density and shape to bone stiffness and strength for a piece of bone of a given volume and length. Bone density is proportional to bone stiffness (E) and strength (yield stress). Dense bone is stiffer and stronger than less-dense bone, but it is also heavier. The overall shape of a bone affects its stiffness (but not necessarily its strength). For example, the shape axis illustrates sections though the shaft of a long bone that range from less rigid (solid cross-section) to more rigid (thin-walled, hollow cross-section). Diagonal lines within the figure represent isoclines of stiffness and strength; a given value of stiffness and strength can be achieved by different combinations of bone density and shape. If the weight of a bone is held constant, then stiffness can be optimized by adopting a more rigid shape. If shape is held constant and the volume of a bone is reduced, increased stiffness and strength can be achieved by increasing bone density.
It is well known that the shape of a bone is a significant factor in mediating its strength and stiffness (e.g. Currey 2002), and there is ample evidence that the shapes of many bird bones are associated with optimizing these qualities. For example, the round and thin-walled humeral shafts of birds are an optimal shape for resisting both torsion and bending (Alexander 1983), and fused skeletal elements have been interpreted as increasing force resistance (i.e. stiffness; Buhler 1992). In contrast to the many studies of bone shape in birds, the impact of the material properties of bone tissue on bone strength and stiffness has received little attention. Bone density reflects mineral content and is positively correlated with bone stiffness (Young's modulus, E, the ability to resist deformation) and strength (yield stress, the ability to resist fracture; Currey 2002). It is also negatively correlated with toughness (ductility). Importantly, stiffness and strength scale with positive allometry so that even a small bone can be strong and stiff if it is composed of dense bone tissue.
There is reason to suspect that bird bone as a tissue is dense, despite the fact that some bird bones are hollow and thin-walled. Many bird bones are composed primarily of cortical bone, which is less porous than other types of bone and may have a higher mineral content (Hodgkinson et al. 1989; Bonser 1995). In addition, as mentioned above, bird skeletons are not lightweight relative to total body or soft tissue mass, despite their delicate appearance. In this study I test the hypothesis that bird bones are dense and predict that, on average, bird bone is denser than the bone of mammals. If true, the skeletons of birds can be defined as lightweight with respect to strength and stiffness per unit of mass.
2. Material and methods
I measured the densities of dry crania (skulls without the dentaries), humeri and femora from 20 families of perching birds (Order Passeriformes), 11 families of rodents and 13 families of bats (species and data in the electronic supplementary material). These bones were selected because they are the largest skeletal elements within the feeding and locomotor systems. Moreover, both strength and stiffness are arguably important to the function of skeletal elements associated with feeding (the cranium) and locomotion (the humerus and femur; Currey 2002; Biewener 2005; Anderson et al. 2008). I sampled perching birds because they include more than half of all living birds and are a highly derived crown group within the subclass Neornithes (Livezey & Zusi 2007). I sampled rodents in order to compare bird skeletons with those of generalized terrestrial quadrupeds with high metabolic rates and a similar range of body sizes. Bats were sampled because they are the only other living vertebrates that are capable of powered flight and, like birds, are described as having lightweight skeletons (Hill & Smith 1984). Any similarities between birds and bats may signify common solutions to the challenges of powered flight. I limited the sample of species to those that weigh less than 400 g in order to generate datasets that cover a similar range of body sizes (see the electronic supplementary material).
To measure bone tissue density, dry crania, humeri and femora were first stored in sealed jars with desiccant for a minimum of 24 h. Bones were then weighed to the nearest 0.001 g to estimate mass, and volume was measured to the 0.001 cm3 via helium displacement using a gas pycnometer (Micromeritics AccuPyc 1330). Helium is a small molecule that fills spaces as small as bone canaliculi (Lievers et al. 2007), and therefore infiltrates both small (interstitial) and large (pneumatic) spaces within the bones. The density of each bone was calculated by dividing bone mass by bone volume. For each bony element and taxonomic group, density measurements that exceeded 2 s.d. of their respective mean were identified as outliers and removed; outliers comprised 0.02 per cent of the complete density dataset. The densities of each type of skeletal element were compared among groups using single classification analysis of variance and post hoc multiple comparisons using the Games and Howell method to adjust for unequal variances (Sokal & Rohlf 1995). Note that in most cases it was impossible to retrieve all three skeletal elements from the same individual without risking damage to the skeletal preparations. Therefore, density data for most species are based on the cranium from one individual and post-cranial elements from another.
As the pycnometer chamber was too small to accommodate complete skeletons, I could not determine the densities of all combined skeletal components from an individual in one measurement. Instead, I estimated total skeletal densities by calculating weighted means of skeletal element density for each group using the relative contributions of each element to skeletal mass as a weighting factor. Skeleton mass was measured by weighing complete, dry, degreased whole skeletons to the nearest 0.001 g (electronic supplementary material). Some skeletons had small fragments of dry connective tissue adhering to them, especially near the joints, but they appeared to occur with equal frequency in skeletons from all three groups. Complete skeleton mass, skeletal element mass and density datasets were available for 31 individual birds, allowing the calculation of 95 per cent confidence intervals around the weighted mean of skeletal density for this group. Complete datasets were available for only two individual bats and no individual rodents. For these groups, I calculated a single weighted mean of skeletal element density based on the average contribution of each skeletal element to average skeletal mass. To document the pattern of variation in skeletal element mass among birds, rodents and bats, I compared the mass of the three skeletal elements among groups again using single classification analysis of variance in conjunction with post hoc comparisons among means using a Bonferroni correction (Sokal & Rohlf 1995). All statistics were calculated using PASW Statistics, v. 17.0.2 (SPSS Inc., Chicago, IL, USA).
3. Results
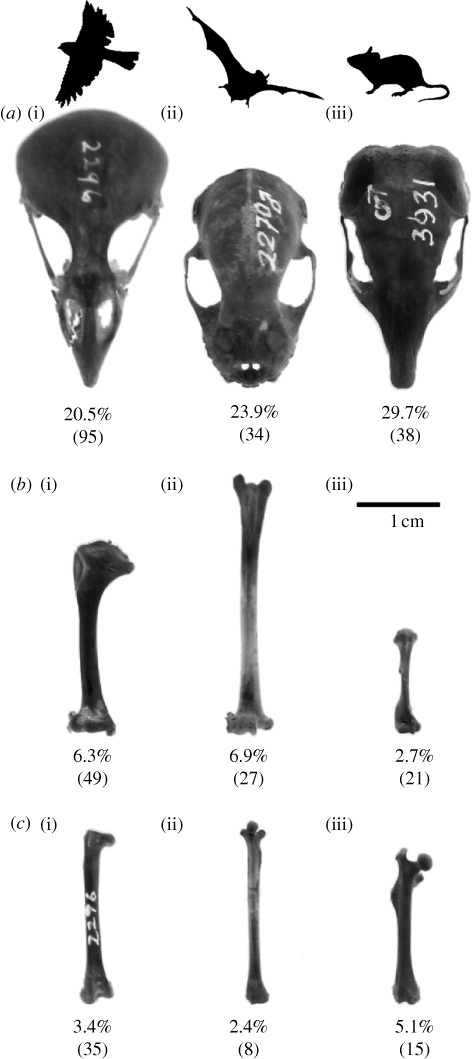
Figure 3 illustrates the skulls, humeri and femora of a representative passerine bird, a bat and a rodent, and the average contribution of those elements to total skeletal mass. While the skulls of these representative species are of roughly similar size, the humeri of the bird and the bat are, not unexpectedly, elongated relative to the rodent. The rodent's femur, on the other hand, is more robust. There were no significant differences between birds and bats in the relative contributions of the cranium, humerus and femur to total skeletal mass (pcranium = 0.55, phumerus = 0.82, pfemur = 0.26). Rodents had lighter humeri and heavier femora than both birds and bats (p ≤ 0.001). Rodent skulls were, on average, significantly heavier than those of birds (p = 0.001), but not bats (p = 0.18).
Figure 3.
The (a) crania, (b) humeri and (c) femora of (i) a passerine bird (Plocepasser mahali, 43 g, Order Passeriformes), (ii) a bat (Artibeus jamaicensis, 40 g, Order Chiroptera) and (iii) a rodent (Chaetodipus baileyi, 40 g, Order Rodentia) of similar body size. Percentages indicate the average contribution of each skeletal element to total skeletal mass; sample sizes are in parentheses.
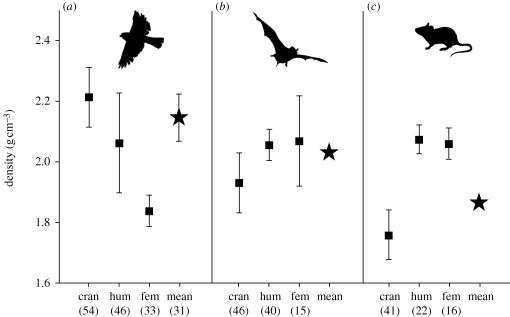
Visual inspection of the weighted means of skeletal element density suggests that bird skeletons are densest, the skeletons of bats are of intermediate density and rodent skeletons are the least dense (figure 4). The weighted mean for bats falls close to the 95 per cent confidence interval for birds; the weighted mean for rodents is much lower. These apparent differences in the average bone density are largely driven by the higher densities of the crania of birds and bats and the low density of the cranium in rodents. Cranial density differed significantly among all three groups (birds versus bats/rodents, p < 0.001; bats versus rodents p < 0.01). The femora of birds were significantly less dense than those of bats and rodents (pbirds vs bats < 0.008, pbirds vs rodents < 0.001), which did not differ from one another (p = 0.91). The density of the humerus did not vary among groups (p = 0.79).
Figure 4.
Means and 95 per cent confidence intervals for cranial (cran), humeral (hum) and femoral (fem) density in (a) birds, (b) bats and (c) rodents. Sample sizes in parentheses. Stars for bats and rodents indicate weighted means of bone density for each group. The mean of bone density for birds is based on 31 specimens for which density was available for all three skeletal elements, and is accompanied by 95 per cent confidence intervals.
4. Discussion
The similarities and differences among birds, bats and rodents in the contributions of their humeri, femora and crania to total skeletal mass reflect their divergent locomotor and feeding adaptations (figure 3). The wings of birds and bats are based on elongated forelimb elements, and this is clearly illustrated by their long and relatively heavy humeri. Likewise, the heavier femora of rodents reflect the important role of the hind limb in quadrupedal locomotion. Given their huge, ever-growing incisors, it is not surprising that rodents have relatively heavy crania. Birds and bats have lighter crania, which comprise similar proportions of skeletal mass. Compared with rodents, the lighter crania and heavier humeri of birds and bats are consistent with the location of the centre of gravity between the wings.
The bone density data reported here suggest that, on average, bird skeletons are stronger and stiffer relative to their weight than are the skeletons of small mammals, especially rodents (figure 4). In other words, bird skeletons have higher strength-to-weight and stiffness-to-weight ratios. This constitutes a novel and biomechanically informative definition of the term lightweight as it applies to bird skeletons.
Despite the higher average density of bird bones, there is a variation in bone density across skeletal elements within birds, bats and rodents. There is a fundamental trade-off between stiffness and toughness (Currey 2002); stiff bone is brittle and prone to fracture, while tough bone is more resilient. This trade-off associated with the material properties of bone tissue is often mediated by variation in bone shape (Currey 2002, 2003; figure 2). For example, long bones that are hollow and circular in cross-section are more resistant to torsion than are solid long bones with elliptical cross-sections, and this is true regardless of the material properties of the bone tissue. Bones with shapes that confer strength are typically stiffer (less ductile) than bones that are structurally weaker. In the context of this study, both bone density and bone shape are important sources of strength and stiffness for the crania, humeri and femora of birds and small mammals.
The density of the cranium drives differences in the average bone density among the birds, rodents and bats in this sample. The crania of both birds and bats are significantly denser than those of rodents. The exceptional density of bird crania is particularly impressive since, unlike mammals, birds do not have teeth. Given the association between bone density and material properties, the crania of birds and bats are probably stiffer and more brittle than those of rodents. Relative to rodents, bird and bat crania may be optimized for very specific functions, perhaps associated with feeding, at the expense of resistance to loads that are unpredictable in magnitude and/or direction. Similarly, the lower density, and therefore higher ductility, of rodent crania may be linked to their gnawing habits.
In contrast to their crania, the densities of the humeri of birds, rodents and bats are relatively constant. This indicates that specializations in shape are the primary means by which bird and bat humeri maintain the stiffness and strength needed to resist the loads that occur during flight (Swartz et al. 1992; Biewener & Dial 1995). The humeri of bats and flighted birds tend to be round in cross-section and thin-walled (Currey & Alexander 1985; Swartz 1997; De Margerie et al. 2005; Habib & Ruff 2008), a configuration that maximizes resistance to torsional loads (Alexander 1983; Swartz et al. 1992). The femora of birds are also known to experience torsion during bipedal locomotion (Carrano & Biewener 1999), and this is reflected in the round and sometimes thin walls of the femora in many species (Currey & Alexander 1985; De Margerie et al. 2005; Habib & Ruff 2008). Interestingly, bird femora are less dense than those of bats and rodents. For the perching birds sampled here, low density perhaps provides durability (via increased ductility) in the face of unpredictable loads encountered during launching, landing, perching on unsteady substrates and bipedal locomotion.
Despite regional variation in bone density, bird bones are, on average, denser than the bones of similarly sized mammals, and are therefore probably stiffer and stronger relative to their weight. This suggests that increased stiffness and strength per unit of mass of bone tissue is one of the many ways in which bird skeletons are lightweight. The higher average density of both bird and bat bones relative to the bones of rodents suggests that increased bone density is associated with flight. Perhaps increased bone density is related to the evolution of small body size in both bird and bat lineages (Maurer et al. 2004; Hone et al. 2008). Body size in living birds and bats approaches the theoretical limit predicted by the balance between the mechanical power required for flight and the capacity for metabolic output (Maina 2000). Figure 2 helps to demonstrate how selection for small bodies, and therefore absolutely small skeletons, could be related to increased bone density. For a bone of a given volume and length, bone stiffness and strength can be maintained via a trade-off between bone density and shape. However, bone density must increase in order to enhance bone strength and stiffness in absolutely smaller bones of the same shape. Rigorous testing of this idea will require detailed knowledge of bone size, shape and density among fossil taxa that span the transition to flight. It would also be of interest to look to pterosaurs for evidence of a similar trend.
High bone density contributes to high strength-to-weight and stiffness-to-weight ratios, and is one of the ways in which bird skeletons are lightweight. In addition, they are also absolutely smaller than those of their ancestors, support bodies that have high surface-area-to-mass ratios and exhibit gross morphological characteristics that confer strength and stiffness (thin-walled long bones with round cross-sections, fused elements, etc.). Importantly, this study helps to dispel the common misconception that bird skeletons are lightweight relative to body mass. Bird (and bat) skeletons have the appearance of being slender and delicate but are still relatively heavy because, on average, the bones are dense.
Acknowledgements
I thank E. Westwig, P. Hart, P. Sweet and curators in the Departments of Mammalogy and Ornithology at the American Museum of Natural History for access to specimens in their care; J. Tanner for creating figure silhouettes; J. Davis for comments on figure 2; and the many colleagues, students and reviewers who provided valuable critiques of this manuscript. This work was supported by grants from the National Science Foundation (IOB 0447616, DBI 0743460) and equipment provided by the David J. Klingener Endowment Fund in support of the Natural History Collections at the University of Massachusetts, Amherst.
References
- Alexander R. M.1983Animal mechanics Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- Anderson R., McBrayer L. D., Herrel A.2008Bite force in vertebrates: opportunities and caveats for use of a nonpareil whole-animal performance measure. Biol. J. Linn. Soc. 93, 709–720 (doi:10.1111/j.1095-8312.2007.00905.x) [Google Scholar]
- Biewener A. A.2005Biomechanical consequences of scaling. J. Exp. Zool. 208, 1665–1676 [DOI] [PubMed] [Google Scholar]
- Biewener A. A., Dial K. P.1995In vivo strain in the humerus of pigeons (Columba livia) during flight. J. Morph. 225, 61–75 (doi:10.1002/jmor.1052250106) [Google Scholar]
- Bonser R. H. C.1995Longitudinal variation in mechanical competence of bone along the avian humerus. J. Exp. Zool. 198, 209–212 [DOI] [PubMed] [Google Scholar]
- Brower J. C.2005A review of aerodynamic flight paradigms for large pterosaurs. GIS and Spatial Analysis 2, 1290–1295 [Google Scholar]
- Buhler P.1992Light bones in birds. LA Mus. Nat. Hist. Sci. Ser. 36, 385–394 [Google Scholar]
- Carrano M. T., Biewener A. A.1999Experimental alteration of limb posture in the chicken (Gallus gallus) and its bearing on the use of birds as analogs for dinosaur locomotion. J. Morph. 240, 237–249 (doi:10.1002/(SICI)1097-4687(199906)240:3<237::AID-JMOR3>3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- Cubo J., Casinos A.2000Incidence and mechanical significance of pneumatization in the long bones of birds. Zool. J. Linn. Soc. 130, 499–510 (doi:10.1111/j.1096-3642.2000.tb02198.x) [Google Scholar]
- Currey J. D.2002Bones: structure and mechanics Princeton, NJ: Princeton University Press [Google Scholar]
- Currey J. D.2003The many adaptations of bone. J. Biomech. 36, 1487–1495 (doi:10.1016/S0021-9290(03)00124-6) [DOI] [PubMed] [Google Scholar]
- Currey J. D., Alexander R. M.1985The thickness of the walls of tubular bones. J. Zool. 206, 453–468 [Google Scholar]
- Dececchi T. A., Larsson C. E.2009Patristic evolutionary rates suggest a punctuated pattern in forelimb evolution before and after the origin of birds. Paleobiology 35, 1–12 (doi:10.1666/07079.1) [Google Scholar]
- De Margerie E., Sanchez S., Cubo J., Castanet J.2005Torsional resistance as a principal component of the structural design of long bones: comparative multivariate evidence in birds. Anat. Rec. 282, 49–66 [DOI] [PubMed] [Google Scholar]
- Evans H. E., Heiser J. B.2004What's inside: anatomy and physiology. In The Cornell lab of ornithology's handbook of bird biology (eds Podulka S., Rohrbaugh R. W., Bonney R.). Ithaca, NY: Cornell Lab of Ornithology/Princeton, NJ: Princeton University Press [Google Scholar]
- Fedducia A.1996The origin and evolution of birds New Haven, CT: Yale University Press [Google Scholar]
- Freeman S.2005Biological science Upper Saddle River, NJ: Pearson Prentice Hall [Google Scholar]
- Galilei G.1638Dialogues concerning two new sciences New York, NY: Macmillan Company; [Transl. by H. Crew and A. de Salvio, 1933] [Google Scholar]
- Gill F. B.2007Ornithology New York, NY: W. H. Freeman & Co [Google Scholar]
- Habib M. B., Ruff C. B.2008The effect of locomotion on the structural characteristics of avian limb bones. Zool. J. Linn. Soc. 153, 601–624 (doi:10.1111/j.1096-3642.2008.00402.x) [Google Scholar]
- Hill J. E., Smith J. D.1984Bats: a natural history Dorchester, UK: Henry Ling Ltd [Google Scholar]
- Hodgkinson R., Currey J. D., Evans G. P.1989Hardness, an indicator of the mechanical competence of cancellous bone. J. Orthop. Res. 7, 754–758 (doi:10.1002/jor.1100070518) [DOI] [PubMed] [Google Scholar]
- Hone D. W. E., Dyke G. J., Haden M., Benton M. J.2008Body size evolution in Mesozoic birds. J. Evol. Biol. 21, 618–624 (doi:10.1111/j.1420-9101.2007.01483.x) [DOI] [PubMed] [Google Scholar]
- Lievers W. B., Lee V., Arsenault S. M., Waldman S. D., Pilkey A. K.2007Specimen size effect in the volumetric shrinkage of cancellous bone measured at two levels of dehydration. J. Biomech. 40, 1903–1909 (doi:10.1016/j.jbiomech.2006.09.002) [DOI] [PubMed] [Google Scholar]
- Livezey B. C., Zusi R. L.2007Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and discussion. Zool. J. Linn. Soc. 149, 1–95 (doi:10.1111/j.1096-3642.2006.00293.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina J. N.2000What it takes to fly: the structural and functional respiratory refinements in birds and bats. J. Exp. Zool. 203, 3045–3064 [DOI] [PubMed] [Google Scholar]
- Maurer B. A., et al. 2004Similarities in body size distributions of small-bodied flying vertebrates. Evol. Ecol. Res. 6, 783–797 [Google Scholar]
- Prange H. D., Anderson J. F., Rahn H.1979Scaling of skeletal mass to body mass in birds and mammals. Am. Nat. 113, 103–122 (doi:10.1086/283367) [Google Scholar]
- Reynolds P. S.1997Phylogenetic analysis of surface areas of mammals. J. Mammal 78, 859–868 (doi:10.2307/1382944) [Google Scholar]
- Sokal R. R., Rohlf F. J.1995Biometry: the principles and practice of statistics in biological research New York, NY: W. H. Freeman & Co [Google Scholar]
- Swartz S. M.1997Allometric patterning in the limb skeleton of bats: implications for the mechanics and energetics of powered flight. J. Morph. 234, 277–294 (doi:10.1002/(SICI)1097-4687(199712)234:3<277::AID-JMOR6>3.0.CO;2-6) [DOI] [PubMed] [Google Scholar]
- Swartz S. M., Bennett M. B., Carrier D. R.1992Wing bone stresses in free flying bats and the evolution of skeletal design for flight. Nature 359, 726–729 (doi:10.1038/359726a0) [DOI] [PubMed] [Google Scholar]
- Videler J. J.2005Avian flight New York, NY: Oxford University Press [Google Scholar]
- Walsberg G. E., King J. R.1978Relationship of external surface area of birds to skin surface area and body mass. J. Exp. Zool. 76, 185–189 [Google Scholar]
- Winter Y., von Helversen O.1998The energy cost of flight: do small bats fly more cheaply than birds? J. Comp. Physiol. 168, 105–111 [DOI] [PubMed] [Google Scholar]