Abstract
In contrast to the patagial membranes of gliding vertebrates, the aerodynamic surfaces used by falling wingless ants to direct their aerial descent are unknown. We conducted ablation experiments to assess the relative contributions of the hindlegs, midlegs and gaster to gliding success in workers of the Neotropical arboreal ant Cephalotes atratus (Hymenoptera: Formicidae). Removal of hindlegs significantly reduced the success rate of directed aerial descent as well as the glide index for successful flights. Removal of the gaster alone did not significantly alter performance relative to controls. Equilibrium glide angles during successful targeting to vertical columns were statistically equivalent between control ants and ants with either the gaster or the hindlegs removed. High-speed video recordings suggested possible use of bilaterally asymmetric motions of the hindlegs to effect body rotations about the vertical axis during targeting manoeuvre. Overall, the control of gliding flight was remarkably robust to dramatic anatomical perturbations, suggesting effective control mechanisms in the face of adverse initial conditions (e.g. falling upside down), variable targeting decisions and turbulent wind gusts during flight.
Keywords: aerodynamics, appendages, arboreal, behaviour, Formicidae, tropics
1. Introduction
The recently described phenomenon of directed aerial descent in some arboreal ants represents a highly specialized form of arthropod locomotion (Yanoviak et al. 2005). When dropped from the tree canopy, wingless worker ants first tumble downwards, effect a dorsoventral righting reflex and then glide at a steep angle towards and subsequently land upon the target tree trunk. These ants typically glide backwards, and use visual cues to target landing sites (Yanoviak & Dudley 2006). The behaviour characterizes multiple ant species and genera in both the Old and New World tropics (Yanoviak et al. 2005, 2008a), and enables avoidance of the ground by otherwise canopy-inhabiting species following either intentional jumps or inadvertent falls (Haemig 1997; Longino & Colwell 1997; Yanoviak & Dudley 2006). From the perspective of ant ecology, such locomotor capacity may enhance colonization of angiosperm canopies and trophic diversification onto associated plant and insect exudates (Davidson et al. 2003; Wilson & Hölldobler 2005).
The manoeuvre used by arboreal ants to implement directed aerial descent are of double biomechanical interest given that backwards flight is otherwise undocumented in volant taxa, and that incipient aerial behaviours may precede the evolutionary acquisition of wings in insects (Yanoviak et al. 2009). In particular, the use of both axial and appendicular structures to carry out manoeuvre poses questions of aerodynamic control that are of more general interest to the origins of flapping flight within an arboreal context (Dudley et al. 2007). Although ants are highly derived within the Hymenoptera and cannot elucidate the phylogenetic origin of insect wings, the hindlegs of many canopy ants are relatively elongated (90–190% of body length, whereas midlegs for the same ants range 65–140% of body length; S. P. Yanoviak & M. Kaspari 2009, unpublished data), which in conjunction with flattened tarsi (electronic supplementary material, figure S1) may enhance the rotational moment arm associated with distally generated aerodynamic forces. In volant pterygotes, both abdominal and leg-initiated steering is well documented, but typically supplements control torques generated by the flapping wings (Dudley 2000).
Here, we use ablations of the hindlegs, midlegs, and gaster (i.e. the postpetiolar abdomen) to determine the influence of these structures on gliding performance in the ant species Cephalotes atratus, the principal taxon used in prior studies of this behaviour (Yanoviak et al. 2005; Yanoviak & Dudley 2006). We also assess consequences of leg and gaster ablation for body kinematics during equilibrium glides, and use high-speed video to describe hindleg orientations that may serve in steering of the body. Because removal of one or both hindlegs, or of midlegs, leaves at least four remaining functional legs, we hypothesized that gliding performance would be impaired but not fully eliminated given this anatomical deficit. By contrast, because of the large mass of the gaster and its substantial contributions to moments of inertia about each of the three orthogonal body axes, together with its contribution to the aerodynamic profile, backwards gliding abilities might well be expected to disappear fully following gaster removal.
2. Material and methods
Experiments were conducted on Barro Colorado Island (BCI), Panama (9.16° N, 79.85° W), in November 2005 and May 2007, and at the Amazon Conservatory for Tropical Studies (ACTS) field station 67 km northeast of Iquitos, Peru (3.25° S, 72.90° W), in June 2009 (see Leigh et al. 1982; Vásquez 1997; Madigosky & Vatnick 2000 for additional information about these sites). Worker Cephalotes atratus were collected from four separate colonies, and were dropped from four different trees (field drops) as well as outdoors from the BCI laboratory balcony (laboratory drops). All drop heights permitted clear views of ant trajectories and, with the exception of those from the balcony, exceeded 10 m. Ants were restrained individually within vials coated internally with fluon and then were dropped directly from those vials. For field drops, individuals were visually tracked during falling to determine whether they landed either on the tree trunk or on the ground. Ants for which the glide outcome could not be determined (e.g. the landing point was outside the observer's field of view) were excluded from subsequent analysis. As in previous studies of this behaviour (Yanoviak et al. 2005; Yanoviak & Dudley 2006) the number of such ‘lost’ ants was less than 5 per cent of the total and was evenly distributed among treatments. Ants were dropped at different lateral distances from the circumference of the nominally vertical trunk axis (mean: 1.3 m; range: 0.8–1.7 m). Vertical distances travelled during a successful glide were either measured with a laser distance meter or visually estimated from a string suspended immediately adjacent to the trunk and marked with flagging at 0.5 m intervals (Yanoviak et al. 2005). A glide index was calculated as the ratio of the net horizontal to net vertical distance transited for each ant that successfully landed on the tree trunk. All drop tests were conducted under near windless conditions, as assessed visually via conspicuous movement of vegetation.
In field drops, either the gaster, one hindleg, both hindlegs, both midlegs or the gaster and both hindlegs were removed by pinching the coxa-trochanter junction or the postpetiole-gaster junction, respectively, with fine forceps (figure 1). The ant was then dropped within 1 min of the ablation. Unmanipulated ants and those missing their hindlegs survive within vials for more than 24 h (n = 9 in each case), whereas those with ablated gasters survive approximately 12 h (n = 9). Because of the difficulty of accurately weighing individual ants within a tree crown, we measured head width of each ant using digital callipers; this measure is tightly correlated with body mass (Yanoviak et al. 2005). Estimated body mass and glide index were compared among treatments with ANOVA and post hoc Tukey tests (SAS Institute 2002). Normality was determined with Kolmogorov–Smirnov tests (SAS Institute 2002), and data were log-transformed before analysis to correct non-normality when necessary (Sokal & Rohlf 1995). Morphological measurements were also taken on 21 C. atratus workers collected on BCI from the same colony used for the laboratory drops, and included body mass, gaster mass, the lengths of the right fore-, mid-, and hindlegs, and the lengths of the gaster and of the total body along its longitudinal axis.
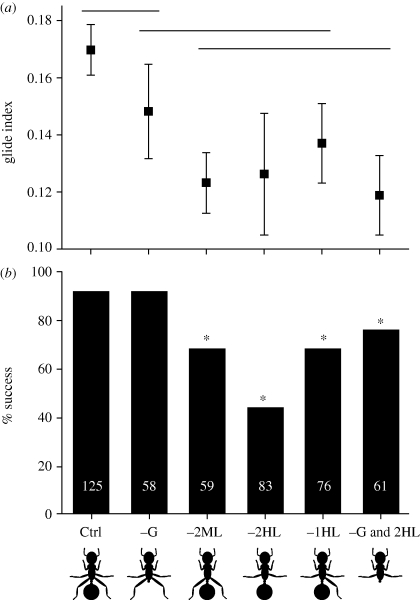
Figure 1.
Average (±95% CI) values of the glide index (a; horizontal distance per unit vertical drop distance) and per cent trunk landing success (b) for control drops of C. atratus workers (Ctrl) and for five experimental manipulations: missing gaster (−G), missing both midlegs (−2ML), missing both hindlegs (−2HL), missing one hindleg (−1HL) or missing gaster and hindlegs (−G and 2HL). Sample sizes in (a) from left to right: 95, 37, 40, 26, 52 and 46 individual ants. Horizontal lines in (a) indicate a lack of significant difference among means. Numbers superimposed on bars in (b) indicate total number of dropped ants. * = treatment success rate differs from control (G > 12.1, d.f. = 1, p < 0.001).
In laboratory drops, equilibrium glides of ants during directed aerial descent were filmed using a tripod-mounted consumer video camera (Canon ZR10; 30 frames s−1) mounted such that the optical axis was parallel to the targeting surface and perpendicular to the predicted glide trajectory (Yanoviak et al. 2005). Individual ants were dropped at a height of approximately 8 m in front of a vertically aligned white fabric column (width 36 cm); the final 3 m of descent were filmed for ants that successfully landed on the column. Video editing software (NIH ImageJ) was used to obtain positional coordinates of the moving ant centroid as a function of time. Equilibrium gliding was defined as an interval during which instantaneous estimates of translational velocity (calculated using the program Quicksand; Walker 1998) varied by no more than 5 per cent of the mean value over the time period in question. Glide angle was estimated from the slope of a linear regression of positional coordinates for the same film sequence. Equilibrium glide velocities and angles were obtained for a total of five ants missing both of their hindlegs and seven ants missing their gaster; initial body masses of these two samples did not differ (t = 0.46, d.f. = 10, p = 0.66). Each experimental treatment was compared using unpaired t-tests with control data from Yanoviak et al. (2005), which were obtained using the same experimental configuration.
Video recordings were also made from above falling ants in laboratory drops using a portable high-speed camera (Troubleshooter, Fastec Imaging) operated at 125 frames s−1 with a 1.6 ms exposure duration. The camera's optical axis was oriented vertically downwards and focused on a region approximately 2 m beneath the point of ant release to obtain images of descending flight subsequent to aerial righting, but during the targeting stage when ants orient their gaster and hindlegs towards the target tree trunk. Because of the higher filming speed and presence of only ambient sunshine for lighting, the lens aperture was opened fully to enable sufficient contrast of falling ants, at cost of the depth of field which was typically less than 50 cm. Unmanipulated ants were repeatedly dropped until suitable orientation behaviours were recorded with adequate focus in the filmed spatial volume. Consecutive video frames for sequences of interest were imported into NIH ImageJ, which was then used to measure the angular orientation of the longitudinal body axis towards the target column's centre as projected horizontally for each frame. This angle γ equals 0° when the ant's anteroposterior axis is targeted (backwards) directly at the column (electronic supplementary material, figure S2). Linear regressions were used to assess average change in γ over the period of these rotations. The two projected angles between this axis and the dorsolaterally extended right and left hindlegs (θleft and θright, respectively) were also measured (electronic supplementary material, figure S2). The horizontally projected body length was measured in each frame and then normalized by the maximum value obtained for each sequence. All high-speed angular data as well as lengths were smoothed using a three-point moving average. Paired t-tests were used to compare θleft and θright for all frames of a given rotational sequence.
3. Results
In field drops, unmanipulated ants were highly successful in landing on their target tree trunk (approx. 91%), and this success rate did not change significantly following gaster ablation (figure 1). (When ‘lost’ ants were conservatively included in the dataset as ground landings, the overall success rate was 87 per cent, but the general statistical results were unchanged.) Any manipulation involving either the midlegs or hindlegs significantly reduced both the success rate of directed aerial descent and the glide index for successful glides (F5,290 = 14.05, p < 0.001; figure 1). Glide index decreased with increasing body size for the hindleg removal treatment (F1,24 = 4.34, p = 0.048), but not for the controls or other treatments (p > 0.10 in all cases). Average body size for all dropped ants did not differ among treatments (F5,456 = 0.19, p = 0.97).
Whereas gaster removal is fatal and unlikely under most natural circumstances (but see Yanoviak et al. (2008b)), C. atratus workers do exhibit partial or complete appendicular ablations in nature. Approximately 4 per cent of 250 C. atratus workers surveyed at ACTS were missing all or part of an appendage, 89 per cent of which were either the hindlegs or midlegs.
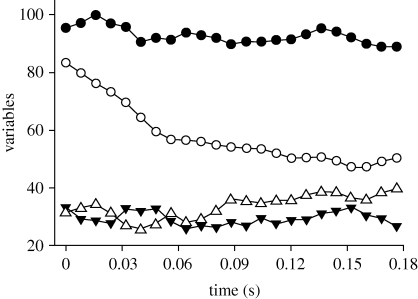
In laboratory drops, equilibrium glide angles did not differ between controls and either ants with no gaster or those with both hindlegs ablated, but glide velocities were significantly lower in individuals with no gaster, a result consistent with their much reduced body mass (table 1). High-speed videos of targeting manoeuvre in four individual ants revealed substantial body rotations in yaw with only small within-sequence variation in the horizontally projected body length (maximally 8.5–17.6%) derived from changes in body angle (i.e. foreshortening; see figure 2 and table 2; electronic supplementary material, figure S3). Each of the four ants also demonstrated a systematic decline in projected body length during the manoeuvre (consistent with downwards translation away from the camera and concomitantly reduced apparent length), but the magnitude of this reduction as averaged over the sequence via linear regression was always less than 7 per cent (range: 1.9–6.5%). These rotational manoeuvre occurred in less than 200 ms (table 2), with rotational velocities in yaw (averaged over the entire manoeuvre) on the order of hundreds of degrees per second (range: 187–714° s−1).
Table 1.
Average (± s.d.) post-ablation mass (m), body length (L), equilibrium glide angle (α), equilibrium glide velocity (V) and Reynolds number (Re) for experimental ants in laboratory drops and for the unmanipulated ants (control; n = 6) of Yanoviak et al. (2005) obtained with the same experimental configuration. Unpaired t-tests compare C. atratus workers lacking either gasters (−G; n = 7) or both hindlegs (−2HL; n = 5) with data for controls.
| variable | control | −G | t | p | −2HL | t | p |
|---|---|---|---|---|---|---|---|
| m (mg) | 47.8 (16.1) | 15.1 (3.9) | 5.3 | <0.0003 | 25.5 (5.2) | 2.9 | 0.02 |
| L (mm) | 11.6 (1.4) | 6.3 (0.5)a | 9.5 | <0.0001 | 10.9 (1.1) | 0.9 | 0.39 |
| α (°) | 75.0 (2.2) | 73.2 (7.9) | 0.5 | 0.61 | 75.3 (5.6) | 0.02 | 0.99 |
| V (m s−1) | 4.3 (0.4) | 2.9 (0.4) | 6.3 | <0.0001 | 3.8 (0.5) | 1.9 | 0.09 |
| Re | 3334 (469) | 1210 (183)b | 11.1 | <0.0001 | 2790 (379) | 2.2 | 0.57 |
aSummed head and thoracic lengths.
bBased on ablated body length.
Figure 2.
Normalized body length as projected horizontally (filled circle, 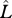 ), longitudinal body orientation (open circle, γ) during rotation in yaw towards target column, and the horizontally projected angles between the longitudinal body axis and the left and right legs (filled triangle, θleft; open triangle, θright) for ant no. 66.
), longitudinal body orientation (open circle, γ) during rotation in yaw towards target column, and the horizontally projected angles between the longitudinal body axis and the left and right legs (filled triangle, θleft; open triangle, θright) for ant no. 66.
Table 2.
Ant identity code (ID), overall change in body orientation (γ) during rotation in yaw towards the target column over time interval Δt, and the mean values (range) for the horizontally projected angles between the longitudinal body axis and the left and right legs (θleft and θright, respectively). See text for statistical results.
| ant ID | Δγ(°) | Δt (s) | θleft (°) | θright (°) |
|---|---|---|---|---|
| 66 | 33.0 | 0.176 | 29.4 (25.9–33.2) | 33.5 (25.4–39.6) |
| 72 | 35.9 | 0.112 | 26.2 (23.4–31.8) | 31.7 (28.3–37.8) |
| 74 | 119.9 | 0.168 | 28.2 (18.6–36.5) | 48.7 (41.7–56.7) |
| 94 | 58.3 | 0.152 | 35.3 (25.8–46.3) | 41.3 (27.9–49.2) |
In all four analysed laboratory drop sequences, the projected angle of the right leg relative to the longitudinal body axis was significantly greater than that of the left leg (paired t-test, p < 0.001 in each case) as the ant rotated to its right towards the target column (figure 2; electronic supplementary material, figure S3). We also qualitatively observed landing manoeuvre in some video sequences whereby ants appeared to roll immediately prior to impact, and also to pitch substantially nose-down such that all six legs contacted the substrate nearly simultaneously. Substantial impact forces and opposing recoil of the ant's body were also evident. During field drops, ants occasionally would bounce off a tree trunk in a landing attempt, and then fall further prior to resuming directed gliding and successfully landing on the same trunk.
For the 21 measured C. atratus workers, gaster mass averaged 24.7 per cent of total body mass, and the mass of the hindleg pair averaged 9.8 per cent of body mass. Gaster length for the same sample averaged 37.7 per cent of total body length, whereas hindleg length averaged 92.5 per cent of body length. Relative lengths of the mid- and forelegs were substantially smaller, averaging 76.2 and 58.3 per cent of body length, respectively.
4. Discussion
The phenomenon of directed aerial descent in ants involves an initial jump or fall, aerial dorsoventral righting if necessary, targeting of and translation to a tree trunk, and finally a landing manoeuvre. Various abiotic and biotic factors may motivate the initiation of aerial descent. Strong storms and heavy rains of the rainforest can displace ant workers while foraging at leaves and flowers. Attacks by predators, including various birds (e.g. woodcreepers), arboreal lizards and mammals can induce jumping in arboreal ants, as may either looming objects or alarm pheromones (Weber 1957; Yanoviak & Dudley 2006). Initial body orientation may be at arbitrary angles relative to vertical during the initial stages of falling, and aerial righting is routinely observed in the field. Following this behaviour and further acceleration under gravity, the ant initiates targeting through body rotation in yaw (figure 2). Once aerodynamic equilibrium is attained at a nominally constant translational velocity (Yanoviak et al. 2005), ants glide backwards with the body axis aligned approximately with the flight trajectory, and with the hindlegs extended laterally and dorsally. Substantially different tasks in aerodynamic control thus characterize the composite aerial behaviour termed directed aerial descent.
In aggregate, these tasks are remarkably insensitive to structural deficits. The removal of the gaster impairs neither success rate of gliding nor the glide index for successful glides to a tree trunk. Similarly, ants missing either their gaster or one or both hindlegs exhibit equilibrium glide angles similar to those for unmanipulated ants (approx. 75°; Yanoviak et al. 2005). Gaster removal involves a considerable reduction in total mass (approx. 25%) and substantial changes in the relative position of centre of body mass along the anteroposterior axis. Aerodynamic torques about the body axis will correspondingly be altered, mandating active compensatory pitching moments if stable gliding is to be attained. By contrast, removal of the hindlegs, either by themselves or in concert with gaster ablation, clearly compromises flight performance: success rates drop by a factor of two, and the glide index for successful glides is substantially reduced. Part of this reduction may derive from a decreased ability to engage in aerial righting during the initial period of falling and accelerating to an equilibrium velocity. Although the hindlegs constitute only a small percentage of total body mass, their substantial length (relative to the other leg pairs and to the body), in combination with dorsolateral deployment and backwards translation of the body, provides effective steering surfaces.
The observed changes in projected angles between the hindlegs and the body axis can be interpreted in two ways that are not mutually exclusive. If, during a turn, the ants change the angle of their body axis in both roll and yaw, such a movement would result in the observed changes in projected leg angles: for dorsolaterally extended appendages, a roll to the right will result in the projected angle increasing for the right hindleg and decreasing for the left. Alternatively, if the ants turn only in yaw, then the observed asymmetries in projected leg angles would be qualitatively consistent with their use as a control surface for manoeuvre. As characterizes appendicular steering in insect flight generally (reviewed in Dudley 2000), increased lateral displacement of one appendage would yield increased aerodynamic drag on the same side of the body. Given the high relative length of the hindlegs in C. atratus, associated torque could then rotate the body in the same direction. The hindlegs are also elevated dorsally relative to the longitudinal body axis, and given the possibility of aerodynamic interaction between ipsilateral legs, it is not possible to exclude additional mechanisms of directional control. For example, as in winged insects (e.g. Nolen & Hoy 1984; Rowell 1989), asymmetric positioning of the abdomen and legs also influences aerodynamic torque production. Only three-dimensional analysis of manoeuvring ants can assess the relative importance of the legs, abdomen, and even possibly head motions during steering. Both large-amplitude body rotations and small-scale trajectory corrections during glides in turbulent air would, however, be necessary to effect the sophisticated course control seen in these ants.
Substantial numbers of ants are known to fall from the tropical forest canopy to the ground (Haemig 1997; Longino & Colwell 1997). Damage is unlikely for such small insects, and indirect advantages to directed aerial descent must otherwise be sought. Vertical stratification of ant species in tropical rainforests is pronounced, with little or no overlap between taxa nesting in the canopy and those on the ground (Yanoviak & Kaspari 2000). As a consequence, ants that drop vertically from the canopy, either intentionally or inadvertently, are confronted with a novel biotic environment upon landing. Once on the ground, the chance that these individuals will find their host tree while successfully evading heterospecific ants or other predators is low (Yanoviak 2010). Given its high success rate, the behaviour of directed aerial descent increases dramatically the chance that falling individuals are not lost to the colony. As in other arboreal ants, the nests of C. atratus are typically located within major tree branches, and workers rarely forage on the ground in forest settings. Successful glides to the main tree trunk or branches may be frequent in this species, although sampling of the interspecific intensity of ant fallout at different heights in the forest would be necessary to establish the ecological frequency of inadvertent or intentional falling.
Alternative methods of directed aerial descent can be identified in canopy ants and other arboreal arthropods. Individuals of at least one genus of ants (Camponotus) glide to tree trunks headfirst in both West African and Neotropical rainforests (Yanoviak 2010), as do arboreal apterygote archaeognathans that are ancestral to the winged insects. In this latter case, caudal filaments are used in ruddering and control of body orientation, as indicated by reduced gliding success following their ablation (Yanoviak et al. 2009). Finally, ants in the genera Dolichoderus and Pachycondyla spin about the coronal axis following aerial righting (S. Yanoviak 2006, personal observation), which probably slows their rate of descent and may increase the likelihood of intercepting vegetation during lateral wind-driven displacements of the trajectory.
A diversity of aerial behaviours thus characterizes certain arboreal arthropods, and selection for effectively remaining within the canopy habitat is probably strong. These selective pressures may also have motivated antecedents to flapping flight in what are now fully volant lineages (Dudley et al. 2007). For example, directed aerial descent in the ancestrally wingless archaeognathans suggests that insects engaged in controlled aerial behaviours prior to the origin of wings (Yanoviak et al. 2009). The evolution of gliding and directed descent can thus be biomechanically and conceptually decoupled from the otherwise important (and, for insects, unresolved) anatomical question of wing origins. Flight in the sense of aerial manoeuvre and steep glides may be widespread among arboreal animals (both vertebrate and arthropod), and both its taxonomic diversity and associated aerodynamic mechanisms merit further investigation.
Acknowledgements
We thank O. Acevedo, P. Bucur, P. Jensen and S. Madigosky for logistical support, and A. Courtemanch for assistance in the field. The Smithsonian Tropical Research Institute, the Panamanian Autoridad Nacional del Ambiente (ANAM), and the Peruvian Instituto Nacional de Recursos Naturales (INRENA) provided research permits. The Amazon Conservatory for Tropical Studies and Amazon Explorama Lodges facilitated access to field sites in Peru. Y. Zeng and A. Jusufi provided helpful comments on the manuscript. This research was supported in part by grants from the National Science Foundation (IOS-0837866 to R.D. and IOS-0843120 to S.Y.) and from the National Geographic Society (CRE 7896-05).
References
- Davidson D. W., Cook S. C., Snelling R. R., Chua T. H.2003Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972 (doi:10.1126/science.1082074) [DOI] [PubMed] [Google Scholar]
- Dudley R.2000The biomechanics of insect flight: form, function, evolution Princeton, NJ: Princeton University Press [Google Scholar]
- Dudley R., Byrnes G., Yanoviak S. P., Borrell B. J., Brown R., McGuire J. A.2007Gliding and the functional origins of flight: biomechanical novelty or necessity? Ann. Rev. Ecol. Evol. Syst. 38, 179–201 (doi:10.1146/annurev.ecolsys.37.091305.110014) [Google Scholar]
- Haemig P. D.1997Effects of birds on the intensity of ant rain: a terrestrial form of invertebrate drift. Anim. Behav. 54, 89–97 (doi:10.1006/anbe.1996.0428) [DOI] [PubMed] [Google Scholar]
- Leigh E. G., Rand A. S., Windsor D. M.1982The ecology of a tropical forest. Washington, DC: Smithsonian Institution Press [Google Scholar]
- Longino J. T., Colwell R. K.1997Biodiversity assessment using structured inventory: capturing the ant fauna of a tropical rain forest. Ecol. Appl. 7, 1263–1277 (doi:10.1890/1051-0761(1997)007[1263:BAUSIC]2.0.CO;2) [Google Scholar]
- Madigosky S. R., Vatnick I.2000Microclimatic characteristics of a primary tropical Amazonian rain forest, ACEER, Iquitos, Peru. Selbyana 21, 165–172 [Google Scholar]
- Nolen T. G., Hoy R. R.1984Initiation of behavior by single neurons: the role of behavioral context. Science 226, 992–994 (doi:10.1126/science.6505681) [DOI] [PubMed] [Google Scholar]
- Rowell C. H. F.1989Descending interneurones of the locust reporting deviation from flight course: what is their role in steering? J. Exp. Biol. 146, 177–194 [Google Scholar]
- SAS Institute 2002SAS/STAT user's guide, version 9 Cary, NC: SAS Institute [Google Scholar]
- Sokal R. R., Rohlf F. J.1995Biometry, 3rd edn.New York, NY: W.H. Freeman [Google Scholar]
- Vásquez M. R.1997Flórula de las reservas biológicas de Iquitos, Perú. Monographs in systematic botany, vol. 63. St Louis, MS: Missouri Botanical Garden [Google Scholar]
- Walker J. A.1998Estimating velocities and accelerations of animal locomotion: a simulation experiment comparing numerical differentiation algorithms. J. Exp. Biol. 201, 981–995 [Google Scholar]
- Weber N. A.1957The nest of an anomalous colony of the arboreal ant Cephalotes atratus. Psyche 64, 60–69 (doi:10.1155/1957/71981) [Google Scholar]
- Wilson E. O., Hölldobler B.2005The rise of the ants: a phylogenetic and ecological explanation. Proc. Natl. Acad. Sci. USA 102, 7411–7414 (doi:10.1073/pnas.0502264102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanoviak S. P.2010Gliding ants of the tropical forest canopy. In Ant ecology (eds Lach L., Parr C., Abbott K.), pp. 223–224 Oxford, UK: Oxford University Press [Google Scholar]
- Yanoviak S. P., Dudley R.2006The role of visual cues in directed aerial descent of Cephalotes atratus workers (Hymenoptera: Formicidae). J. Exp. Biol. 209, 1777–1783 (doi:10.1242/jeb.02170) [DOI] [PubMed] [Google Scholar]
- Yanoviak S. P., Kaspari M.2000Community structure and the habitat templet: ants in the tropical forest canopy and litter. Oikos 89, 259–266 (doi:10.1034/j.1600-0706.2000.890206.x) [Google Scholar]
- Yanoviak S. P., Dudley R., Kaspari M.2005Directed aerial descent in canopy ants. Nature 433, 624–626 (doi:10.1038/nature03254) [DOI] [PubMed] [Google Scholar]
- Yanoviak S. P., Fisher B. L., Alonso A.2008aDirected aerial descent behavior in African canopy ants (Hymenoptera: Formicidae). J. Insect Behav. 21, 164–171 (doi:10.1007/s10905-008-9116-5) [Google Scholar]
- Yanoviak S. P., Kaspari M., Dudley R., Poinar G., Jr2008bParasite-induced fruit mimicry in a tropical canopy ant. Am. Nat. 171, 536–544 (doi:10.1086/528968) [DOI] [PubMed] [Google Scholar]
- Yanoviak S. P., Kaspari M., Dudley R.2009Gliding hexapods and the origins of insect aerial behaviour. Biol. Lett. 5, 510–512 (doi:10.1098/rsbl.2009.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]