Abstract
Biologists have long searched for mechanisms responsible for the increase in species richness with decreasing latitude. The strong correlation between species richness and climate is frequently interpreted as reflecting a causal link via processes linked to energy or evolutionary rates. Here, we investigate how the aggregation of clades, as dictated by phylogeny, can give rise to significant climate–richness gradients without gradients in diversification or environmental carrying capacity. The relationship between climate and species richness varies considerably between clades, regions and time periods in a global-scale phylogenetically informed analysis of all terrestrial mammal species. Many young clades show negative richness–temperature slopes (more species at cooler temperatures), with the ages of these clades coinciding with the expansion of temperate climate zones in the late Eocene. In carnivores, we find steeply positive richness–temperature slopes in clades with restricted distributions and tropical origins (e.g. cat clade), whereas widespread, temperate clades exhibit shallow, negative slopes (e.g. dog–bear clade). We show that the slope of the global climate–richness gradient in mammals is driven by aggregating Chiroptera (bats) with their Eutherian sister group. Our findings indicate that the evolutionary history should be accounted for as part of any search for causal links between environment and species richness.
Keywords: bats, mammals, evolutionary history, latitudinal diversity gradient, phylogenetic niche conservatism, phylogeny
1. Introduction
Explaining regional variation in the distribution of species richness has challenged ecologists and evolutionary biologists for almost 200 years (Hawkins 2001). For most major groups of animals and plants, species richness tends to be higher at lower latitudes (Hillebrand 2004), a pattern that has sparked a long-standing search for mechanistic explanations linking climate and richness. This search has yielded a steady accumulation of competing hypotheses (Rahbek & Graves 2001) and little consensus. Within species-rich groups, taxonomic richness typically correlates most strongly with measures of environmental energy and productivity (Currie et al. 2004). Ecological explanations for the climate–richness relationship (CRR) frequently focus on abiotic and biotic factors that enable species coexistence (Willig et al. 2003)—increasing environmental carrying capacity in tropical climates (i.e. where environmental carrying capacity reflects limitations on local species richness owing to resource availability). Evolutionary (including temporal) explanations have focused on either variation in rates of diversification or the amount of time available for speciation within a region (Mittelbach et al. 2007). Here, we question whether environmental limits to either coexistence or diversification rates are necessary to explain climate–richness correlations and the latitudinal gradient in species richness. We suggest that radiation of clades in different environments could explain the observed CRR in many higher taxa. First, we explore the phylogenetic pattern in species' environmental niche attributes across the tree-of-life for mammals and its implications for global patterns in species richness. Second, we evaluate whether phylogenetic conservatism in range location and/or environmental tolerances can account for the observed relationships between environment and species richness within higher clades.
Species' ranges may be constrained by both environmental (climatic) tolerances and non-climatic barriers to dispersal (e.g. mountain ranges and oceans). In the former case, species might disperse to a new habitat but fail to become established, whereas in the latter case species might never have the opportunity to reach the new habitat even though they have the attributes needed to persist there. We refer to the tendency for lineages to maintain similar environmental limits to their range extents over time as phylogenetic niche conservatism (PNC; Peterson et al. 1999; Wiens 2004). Several studies have found patterns of phylogenetic community structure along richness gradients consistent with niche conservatism. For example, less diverse temperate communities may be more phylogenetically derived, suggesting recent dispersal to temperate habitats (e.g. Stevens 2006). CRRs have been shown to vary among plant species that belong to higher taxa of different climatic origins (Harrison & Grace 2007; Ackerly 2009) and between basal and derived groups of birds (Hawkins et al. 2007)—suggesting a strong influence of the evolutionary history on diversity patterns. Further, Wiens et al. (2006, 2009) showed that the latitudinal diversity gradient in frogs is related to their longer time in the tropics and more recent dispersal to temperate habitats, suggesting conservatism in environmental tolerances was driving global richness patterns.
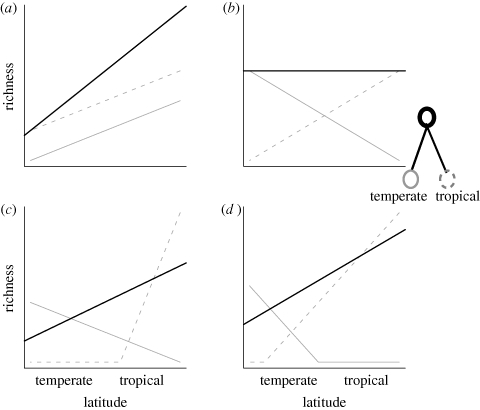
We explore the influence of PNC on richness gradients via four scenarios (figure 1). (A) Latitudinal gradients in carrying capacity and/or diversification rates: we would then expect consistent, positive temperature–richness slopes independent of clade identity or origin. (B) Strict PNC (niche conservatism in the absence of variation in diversification rates or carrying capacity): within each clade, the number of species will be highest within environments most closely resembling the clade's ancestral niche because of longer times for speciation and evolutionary constraints to range shifts (Wiens & Donoghue 2004; Donoghue 2008). Differences in CRR slopes would reflect the different climatic origins of clades assuming either that rates of niche evolution are slow relative to diversification or that niche shifts occur only rarely. For example, for two sister clades in which one originated in tropical environments and the other in temperate environments, we predict a positive (more species in tropics) slope for the clade with a tropical origin and a negative slope (fewer species in tropics) for the clade with a temperate origin. If the number of species in both clades is equal, the aggregated parent clade might not demonstrate any strong latitudinal gradient. (C) PNC with escape (variable niche conservatism in the absence of variation in diversification rates or carrying capacity): significant CRR slopes might emerge for higher clades even in the absence of gradients in environmental carrying capacity or diversification rate if the strength of PNC differs between daughter clades. Novel adaptations facilitating niche shifts, and persistence and diversification in new ecological zones (Ricklefs 2006), allow escape from PNC and might alter the slope of the CRR for the radiating clade (Diniz-Filho et al. 2007). Specifically, we predict that clades for which PNC is strong would tend to have steeper CRR slopes. This may be the case for tropical species, which tend to have narrow thermal tolerances (suggested by smaller elevational ranges; Ghalambor et al. 2006; McCain 2009). (D) PNC with stochastic variation in diversification rates: a significant CRR for the parent clade might also result from aggregating daughter clades differing in species richness, but, in contrast to scenario A, aggregate slopes may be positive or negative with equal probability because the differences in diversification rates are not related to the environment. We have here presented each scenario as independent; however, it should be recognized that they are not mutually exclusive; for example, strong niche conservatism in tropical clades does not exclude the possibility of faster tropical diversification or higher tropical carrying capacity.
Figure 1.
We explore the influence of PNC on diversity gradients via four scenarios for a clade with a temperate origin (solid grey line), a sister clade with a tropical origin (dashed grey line) and the parent clade (solid black line). If latitudinal gradients in carrying capacity or diversification gradients are governing the diversity gradient, we expect consistent, positive slopes (a). Under PNC, if the number of species in both clades is equal, the aggregated parent clade might not demonstrate any strong latitudinal gradient (b). A strong CRR for the parent clade may result from aggregating clades if the CRR slopes of daughter clades differ in steepness (c) or species richness (d). While we show scenarios corresponding to more species in the tropics, stronger conservatism and species richness may also occur in temperate areas, reversing the slope for the parent clade in (c) and (d).
We evaluate the influence of PNC on the CRR by contrasting slopes of the climate–richness gradient across nested (subset) mammal clades. While richness gradients in different taxonomic groups have been contrasted previously (Currie 1991; Kaufman 1995; Kaufman & Willig 1998; Stevens 2004), the recent species-level phylogeny with branch lengths (Bininda-Emonds et al. 2007) and distribution data (Grenyer et al. 2006) available for mammals provides an opportunity to evaluate the importance of phylogenetic history in shaping global richness gradients. Specifically, we assess whether CRR slopes for more inclusive (parent) clades simply reflect the CRR slopes of the nested (daughter) clades within them (e.g. scenario A), or whether they are an emergent property resulting from aggregating daughter clades with different slopes and/or number of species (e.g. scenarios B–D). Our approach of clade aggregation can be viewed as the reciprocal of the ‘deconstruction’ or ‘disaggregation’ of diversity patterns advocated by Huston (1994) and Marquet et al. (2004), with an explicit phylogenetic focus. We explore the CRR across all nested clades of terrestrial mammals for two key environmental variables: mean annual temperature and actual evaporation (AET). Although AET and temperature are strongly correlated, we might still expect clade-specific CRR slopes to be different between these two variables. Low temperatures can potentially constrain the geographical range limits of mammal species owing to limits on physiological function and the increased energy required for thermoregulation in cold environments (McNab 2002). AET, closely associated with plant productivity, reflects water-temperature balance and is a strong predictor of mammal richness (Currie 1991).
If PNC is important in generating significant CRRs, we predict that the diverse geographical origins of major mammalian lineages (Kemp 2005) will result in variable slopes (positive and negative) among nested clades (scenarios B–D versus scenario A). If PNC retains the ecological niches corresponding to different geographical origins, we predict that slopes will be steeper than corresponding slopes for randomly distributed species and expect few strongly negative slopes for clades older than the expansion of temperate habitats in the late Eocene because the climatic origin for most clades prior to then will be tropical (Janis 1993). In addition, we might expect a positive aggregate richness gradient if niche differences arose early and either tropical niches tend to be more conserved (scenario C) or a greater number of species happened to radiate from clades with more tropical origins (scenario D). These two scenarios are difficult to resolve except by comparing aggregate slopes for sister clades with equal richness.
We use the sister-clade comparison between Feliformia and Caniformia as an illustration of our approach. These two well-resolved clades share similar net diversification rates of terrestrial lineages, but different regions of occupancy and origin (as inferred from the fossil record—data that are only available for a few other clades), and therefore provide a useful test case. Feliformia (cats and cat-like carnivores; 111 species; Wilson & Reeder 1993) are thought to have initially radiated from South Central Asia (Collier & O'Brien 1985) and remain a predominantly tropical group. Caniformia (dogs and dog-like carnivores; 160 species, of which 126 are terrestrial; Wilson & Reeder 1993) diversified extensively outside of the tropics (Hunt 1996) and contain many species that are widespread in temperate areas (including foxes, skunks and raccoons), reflecting more labile environmental tolerances. Because Feliformia and Caniformia represent sister clades with similar terrestrial species richness, variation in their CRRs cannot therefore be explained by a simple relationship between climate and diversification (scenario A), nor can the aggregate slope be explained by differences in species numbers (scenario D). If PNC is important (scenarios B–C) in driving richness gradients within these two clades, we would predict that (i) positive (more species in tropics) slopes will predominate for the Feliformia clade owing to its tropical origin, whereas negative slopes will predominate for the Caniformia clade owing to its temperate origin (scenario B); and (ii) the Feliformia clade will have steeper slopes because its distribution is more tightly restricted, as reflected in narrower geographical limits, than the Caniformia clade, resulting in an overall positive CRR for the parent clade (scenario C).
2. Material and methods
Species distribution data were obtained from the database of mammalian geographical range maps collated by Sechrest (2003) and modified as described in Grenyer et al. (2006). Current distributions may be truncated owing to factors such as human land use, but the global gradient in mammal species richness appears little affected, and we do not believe this truncation should influence our broad-scale analysis. Geographic Information Systems (GIS) layers for mean annual temperature (dataset A03; http://www.ngdc.noaa.gov/ecosys/ged_toc.shtml) and AET (dataset GNV183; http://www.grid.unep.ch/data/) were derived from remote-sensing data at a resolution of 0.5° × 0.5°. Estimates of phylogenetic relationships and divergence times were obtained from Bininda-Emonds et al. (2007). This phylogeny is currently the most taxonomically comprehensive, with sampling 99 per cent complete at the species level (4510 mammal species out of 4554 recognized globally; Wilson & Reeder 1993), and best summarizes our current understanding of mammalian evolutionary relationships, although many relationships remain poorly resolved (46.7% resolved compared with a fully bifurcating tree). For unresolved nodes, we aggregate all directly descending daughter clades. We believe our methods and randomization procedures to be insensitive to tree resolution; however, it remains possible that there might be important signal within unresolved clades that we are unable to detect.
We calculate richness patterns across equal area (110 × 110 km) grid cells, approximately 1° at the equator. For each nested mammal clade, we extract species richness, mean annual temperature and AET across all occupied cells to derive the linear ordinary least squares regression slope of the relationship between temperature and richness. We use a linear rather than curvilinear regression to facilitate interpretation of the climate–richness slope. To evaluate potential for bias, we checked whether curvilinear regressions provided a substantially better fit to the data by comparing relative change in r2. For all mammals, including a quadratic term for the environmental variable does not alter the r2-value and does not substantially change the linear coefficient. The quadratic term is only marginally significant (p < 0.03) compared with the strong significance of the linear term (p < 1 × 10−15). Considering that autocorrelation will disturb the type I error, we find little support for inclusion of a quadratic term. Adding a quadratic term increases the r2-value by less than 1 per cent for the major radiations of rodents and bats. Although it provides a significant improvement in the r2-value for the weak CRRs of the Caniformia clade, the Feliformia clade fits a linear model equally well. We use current species distributions to estimate the minimum value of annual temperature and AET across the species' ranges within each clade as a proxy for their environmental tolerances.
We first examined variability in environmental limits and slope of the CRR among clades. We tested for phylogenetic signal in species distributions with a simple randomization procedure, which swapped the identity (and associated attributes, including location) of terminal taxa across the phylogenetic tree (i.e. randomizing species membership of clades). We compared the observed CRR slopes with the slopes from 1000 randomizations using a Wilcoxon sign-rank test. If the distributions of species within a clade were unconstrained, such that any species could become established in any environment, each clade would have a richness gradient similar to the overall global pattern (scenario A), differing only in the number of species, contrasting with expectations of PNC (scenarios B–D). To evaluate sensitivity of our results to the particular choice of environmental variable, we repeated each analysis, first with temperature and then with AET as the key environmental niche axis.
Second, we examined the changing slope of the CRR as nested clades with different evolutionary histories are aggregated by plotting CRR slope against clade age and species richness. We then used the morphological disparity metric (MDI) of Harmon et al. (2003) to characterize the relative variance in the minimum annual mean temperature across a species's range within versus between clades over the evolutionary history of a radiation. Although the minimum temperature observed within a species range does not actually evolve along the branches of the phylogeny, the physiological tolerances and general location in space of a species can presumably be inherited from its ancestors. Hence, environmental range attributes are predicted to exhibit phylogenetic signal if niche conservatism is widespread. The MDI metric is derived from the standardized mean pair-wise distance between species and therefore does not necessitate the reconstruction of ancestral states. Values of MDI near 0 indicate that most of the variation is partitioned between clades, whereas values near 1 indicate that most variation is among species within subclades. Because at the limits MDI must be 1 at the root and 0 at the tips, we compared observed MDI to expectations under a Brownian motion model of trait evolution (Garland et al. 1993; Harmon et al. 2003). In a scenario in which lineages shifted into novel environments early in the phylogeny and descendent species maintain conserved ancestral traits (niche conservatism), most variation in minimum temperature would be between clades (observed MDI less than or equal to null). In contrast, in a scenario in which niche conservatism is weak, we would expect more variation in minimum temperature to be captured among species within clades (observed MDI greater than or equal to null).
Last, we compared the strength of PNC in minimum annual temperature (see above) between two focal clades of Carnivora. We use two alternative metrics to quantify PNC. The first is Bloomberg's K, which indicates the amount of phylogenetic signal in the tip data relative to the expectation (K = 1) for a trait that evolved by Brownian motion along the specified topology and branch lengths (Blomberg et al. 2003). Higher K-values indicate that close relatives are more similar in their traits. For each clade, significance was assessed by comparing the variance of independent contrasts for 1000 randomized (tip-swapped) trees to the observed trees (Blomberg et al. 2003; implemented by phylosignal in R package picante). The second metric is Pagel's (1999) λ, which ranges between 0 (phylogenetic independence) and 1 (species traits covary in direct proportion to their shared evolutionary history). We evaluated whether λ differed significantly between Caniformia and Feliformia by likelihood ratio (LR) as −2 log(Λ), assuming a χ2 distribution and comparing the fit of the maximum likelihood (ML) estimates of λ so that, for example, the ML estimate of λ for Caniformia was evaluated against the fit of the ML estimate of λ from Feliformia. We restrict our analysis to clade topologies derived from the mammal supertree because this taxonomy matches the range data, and global diversity patterns have previously been explored using this dataset (e.g. Grenyer et al. 2006; Davies et al. 2008). Mammal taxonomy and phylogeny are in a state of flux and are likely to be so for many years. However, it is unlikely that alternative phylogenetic topologies would qualitatively alter our results.
3. Results
(a). All mammals
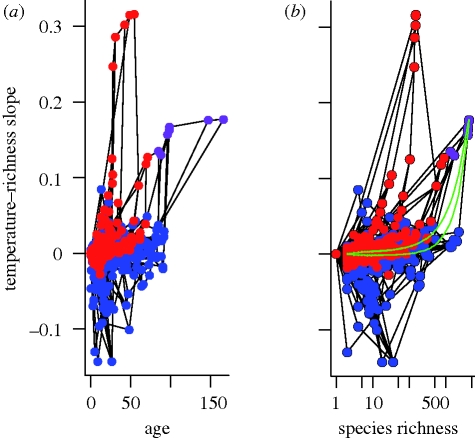
At odds with the apparent ubiquity of the latitudinal gradient in species richness and predictions from scenario A, we show that CRR slopes vary considerably between clades, with the majority of clades having near-zero slopes (figure 2). Across nodes, we find that the observed distribution of slopes includes more strongly positive and negative slopes than expected by chance (Wilcoxon sign-rank test, p < 0.004). Our randomizations demonstrate that negative temperature–richness gradients would be extremely rare if there were no phylogenetic constraints to species climatic distributions (figure 2b). The age of the oldest clades with strongly negative slopes (figure 2a) is approximately coincident with the expansion of temperate habitats in the late Eocene (Janis 1993). Our results suggest that the global diversity gradient for mammals cannot be explained solely by latitudinal gradients in carrying capacity or diversification rates (scenario A).
Figure 2.
The slope of the temperature–richness relationship for clades as a function of (a) clade age and (b) species richness. Bat nodes are depicted in red, nodes without bats are depicted in blue and their ancestral nodes are depicted in purple. Ancestry is depicted with lines. In (b), the green lines indicate the central 95 per cent of 1000 tip-swapped randomizations. The figures essentially show the mammal phylogenetic tree on its side with the ancestral clade depicted at the right (as the oldest clade with the most species) and the branches depicted on a y-axis (temperature–richness slope). The lineage lines link clades on the left that are a subset of the clades on the right.
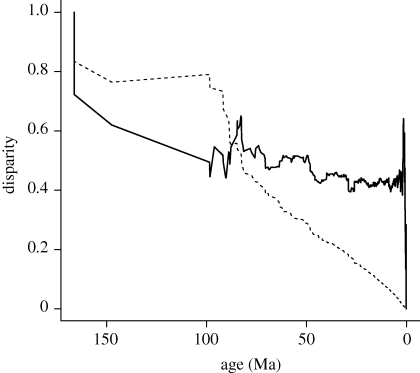
To explore how the aggregate richness gradient emerges, we first characterize the phylogenetic distribution of niche differences using Harmon et al.'s (2003) MDI metric (figure 3). We show that most variation in thermal distribution (minimum mean annual temperature recorded across a species's geographical range) is between clades towards the root of the tree, consistent with early niche divergence and phylogenetic conservatism. Towards the tips of the tree, most variation is among species within clades rather than between clades, perhaps indicative of a more recent relaxation of niche constraints within the bounds defined by higher clade membership. The transition notably occurs approximately at the stem age for bats (85 Ma). A plot of the proportion of nodes within 5 Myr age bins that depart from random expectations shown in figure 2 illustrates how the climate–richness slopes change through time (figure S1 in the electronic supplementary material). At approximately 50 Ma, during the warm Eocene period with its rapid diversification (Bininda-Emonds et al. 2007), including the radiation of bats in the tropics (e.g. the tropical and sub-tropical Phyllostomidae; Jones et al. 2005), the majority of nodes (approx. 80%) have slopes that are higher than expected. From 40 Ma to present, CRR slopes for clades increasingly fall within null expectations from our randomizations. These trends support a model of early divergence and subsequent phylogenetic conservatism of ecological niches that has been relaxed within more recent time periods.
Figure 3.
The trait disparity value at a given point in time (actual data, solid line; expected from phylogenetic simulations, dashed line) is the ratio of the average disparity of subclades for which ancestral lineages were present at that time relative to the disparity of all mammals. Age (Ma) is calibrated in millions of years before the present. Toward the tips, a large proportion of the variation in thermal environments occurs within mammal clades (high relative disparity). Towards the root, most variation in thermal environment is between mammal clades (low relative disparity).
Our results provide strong evidence for PNC and suggest that one explanation for the predominance of positive CRR slopes among higher clades (greater than 50 Myr old) might reflect an increase in the origination rate of tropical nested clades within the warm Eocene period (consistent with scenario B). We assume here that clades that originated during times when the geographical expanse of the tropics was large are more likely to have tropical ancestry. However, stronger tropical conservatism (scenario C) and/or faster tropical diversification (scenario D) might additionally shape the aggregate CRR.
(b). Carnivora
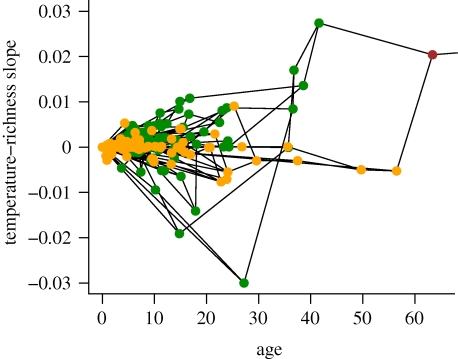
The CRR slopes for clades within Carnivora are variable, with many negative slopes, also consistent with the influence of strict PNC (scenario B). As predicted by their estimated regions of origin, the predominantly tropical Feliformia clade exhibits predominantly positive CRR slopes, whereas the predominantly temperate Caniformia clade exhibits many negative slopes (figure 4). As predicted by scenario C (PNC with stronger tropical conservatism), Feliformia exhibit greater phylogenetic conservation of their thermal niche (K = 0.46, p < 0.001; λ = 0.15, p < 10−15 for the clade, p < 10−15 from the LR test with λ from Caniformia) than the more widespread Caniformia (K = 0.11, p = 0.1; λ = 10−8, p = 1 for the clade, p < 10−15 from the LR test with λ from Feliformia). Accordingly, Feliformia clades exhibit steeper slopes, with the strongest positive CRR in clades with a stem age around 40 Ma, coinciding with the Eocene thermal maximum. Although the CRR slopes for Feliformia appear to exhibit greater variation than those for Caniformia, Feliformia clades with negative slopes contain few species (e.g. the clade with most negative slope contains only three species). In contrast, Caniformia exhibits shallow, often negative CRR slopes, even within species-rich clades. Critically, aggregating the largely temperate Caniformia with the more geographically restricted and tropical Feliformia clade generates an overall strongly positive aggregate CRR slope, although all but one nested subclade demonstrate shallower CRR slopes, and the aggregate slope for Caniformia is negative. Because these sister clades have similar species richness, variation in diversification rates (scenario D) cannot explain the aggregate slope; rather, we suggest PNC with escape (scenario C) in the Caniformia clade best explains the observed CRR for Carnivora.
Figure 4.
The carnivore subset of the slopes of the temperature–richness relationship as a function of clade age (figure 2a). Cat (subfamily Feliformia) nodes are depicted in green, dog and bear (subfamily Caniformia) nodes are depicted in orange and the ancestral carnivore node is depicted in brown. Ancestry is depicted with lines.
(c). Bats
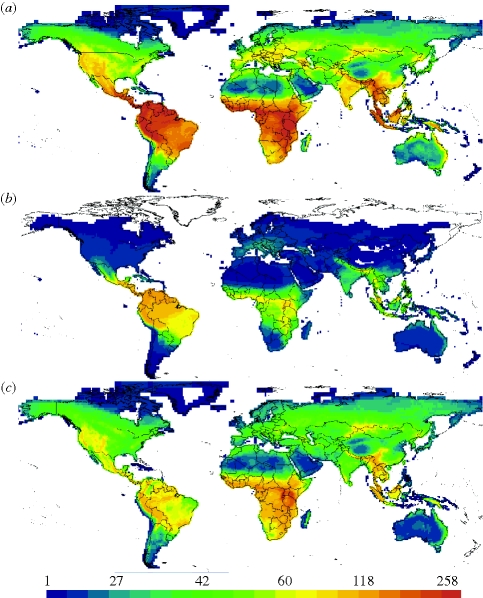
Our analysis illustrates the importance of bats (which account for approximately a quarter of all mammal species) in shaping the global richness gradient (figure 5). The global temperature–richness slope for all species excluding bats is shallow (slope + CI = 0.07 + 0.003, p < 10−15, r2 = 0.15) and the CRR slope for non-bat clades never exceeds 0.1. The equivalent gradient for bats is more than four times as steep at 0.32 (p < 10−15, r2 = 0.32), and the aggregate richness gradient for all terrestrial mammals is 0.18 (slope + CI = 0.18 + 0.01, p < 10−15, r2 = 0.21). Bats represent a highly successful radiation, providing some support for faster diversification (scenario D), although, depending upon tree resolution, diversification rates for the clade might not be unusual (Helgen 2003). We also find significant tropical conservatism for bats (K = 0.17, p < 0.001; λ = 0.07, p < 10−15), consistent with their strong influence on the overall gradient (scenario C). The bat richness gradients contrast with those of the other major mammalian group—the predominantly temperate and widespread Rodentia (Kemp 2005), representing 44 per cent of mammals. Rodents have many nodes with near-zero slopes, as well as several nodes with the most negative slopes of any mammalian clade (figure S2 in the electronic supplementary material). Our examination of the AET–richness relationship confirms that the global CRR emerges from aggregating clades with disparate slopes and is largely driven by the New World bat clades, which demonstrate strongly positive slopes (figure S3 in the electronic supplementary material), strong niche conservatism and perhaps rapid diversification.
Figure 5.
Species richness maps for (a) all the species, (b) bats only and (c) all species other than bats, showing how bats influence the overall latitudinal diversity gradient. Data are depicted as 20 quantiles based on the data for all mammals with warm colours indicating higher richness.
4. Discussion
We show that the relationship between climate and species richness varies considerably between clades, regions and time periods. A large body of work has sought to derive mechanistic links between climate and richness from the correlations between species richness and various environmental variables (Willig et al. 2003). Differences in CRRs between regions and between taxa have been interpreted as reflecting differences in ecological mechanisms driving richness patterns (reviewed in Hawkins et al. 2003). We suggest that, in contrast to more traditional explanations, this variability is expected under a scenario in which environmental niches are evolutionarily conserved and clades differ in their geographical and climatic origins. In addition, our analyses support contentions that global climate–richness gradients can emerge in the absence of gradients in diversification or environmental carrying capacity (see Wiens & Donoghue 2004). While our findings do not preclude a causal link between climate and richness, they suggest that the evolutionary origins and lability of clades should be considered when interpreting correlative patterns.
Gradients in energy or evolutionary rates are predicted to produce positive slopes across clades via their effect on environmental carrying capacity or diversification. However, we find that many clades show negative slopes (i.e. higher richness in cooler climates). In addition, we show that broad environmental tolerances of clades were determined early in the radiation of mammals (greater than 80 Ma). The Eocene warming may have increased the proportion of new clades with tropical origins because of the expansion of the tropical biome, enhancing the overall trend for positive CRRs globally. With temperatures cooling following the Eocene thermal maximum (approx. 50 Ma), we start to see an increasing frequency of clades with negative temperature–richness slopes. Within more recent time periods (less than 40 Ma), clade richness gradients differ little from null expectations, perhaps reflecting the geographical radiation of species within the broad environmental niches defined by their higher clade membership.
Positive slopes can emerge from the aggregation of nested clades with disparate slopes. Aggregate slopes will be influenced by variation in species richness between daughter clades and in the environments they occupy, as well as strength of PNC. Nonetheless, steep slopes were observed across clades varying in both species richness and environmental range limits. Critically, even aggregating clades with negative climate–richness gradients can generate an overall positive gradient if the more tropical clade has greater species richness or a more restricted distribution. Our results are consistent with PNC plus periodic niche shifts over the evolutionary history of mammals. Differences in phylogenetic constraints, because of either differences in dispersal limitations or the evolutionary lability of environmental tolerances, may be reflected in the steepness of CRR slopes among clades, while occasional niche shifts might reverse their direction. The evolutionary history of the Feliformia and Caniformia clades of carnivores highlights the influence of phylogenetic constraints and clade origins on the shape of the CRR. The overall positive climate–richness gradient for Carnivora emerges from aggregating the steep and positive CRR slope for Feliformia with the shallow negative CRR slope for Caniformia. Further, we show that the aggregate global richness gradient for all mammals is also shaped by differences in phylogenetic constraints among constituent clades and, in particular, the tropical conservatism of the New World bats (see figure S3 in the electronic supplementary material; see also Fleming 1973; Kaufman & Willig 1998; Stevens 2004, 2006).
The species-rich noctilionoid clade diversified extensively in the Neotropics by the end of early Oligocene (approx. 30 Ma; Simmons 2005). A close link between temperature and energetic costs for bats (Stevens 2006; McCain 2009) may account for their largely tropical distribution and, as a consequence, the steep climate–richness gradient for the clade. As species shifted to novel diets (e.g. nectar and fruit feeding in the New World leaf-nosed bats, Phyllostomidae), their metabolic rates probably increased, allowing more precise thermoregulation but making a shift to temperate regions energetically prohibitive (McNab 1982; Stevens 2006). It appears that few bat lineages were able to overcome the energetic constraints on moving beyond the tropics, resulting in the climate–richness gradient observed today. However, Vespertilionidae (54 Ma), which radiated in temperate North America following several distinct dispersal events (Stevens 2004), represent an excellent example of the occurrence of occasional niche shifts. These bats exhibit reproductive and physiological adaptations to temperate environments (Stevens 2004) and provide an exception to the strong positive latitudinal gradients observed more generally among New World bat families.
Our results emphasize the importance of employing appropriate phylogenetic models when interpreting ecological patterns. The use and abuse of null models in community ecology was the subject of heated debate in the 1980s (Harvey et al. 1983), and the increasing availability of detailed phylogenetic data offered a simple and robust alternative evolutionary model for community structure (Harvey 1996; Webb et al. 2002). However, methods for incorporating phylogenetic information into predictive models of species richness and distribution are only just emerging (e.g. Rangel & Diniz-Filho 2005; Rangel et al. 2007; Freckleton & Jetz 2009; Wiens et al. 2009). We suggest such approaches will provide new insights into the mechanisms and drivers of current biodiversity gradients.
Acknowledgements
This work was conducted as part of a working group on The Role of Niche Conservatism in Producing Biodiversity Gradients, supported by the National Center for Ecological Analysis and Synthesis (NCEAS), a centre funded by NSF (grant no. DEB-0553768), the University of California, Santa Barbara and the State of California, and the National Evolutionary Synthesis Center, a centre funded by the National Science Foundation (grant no. EF-0423641). L.B.B., T.J.D., D.D.A. and N.J.B.K. conceived and designed the experiments. L.B.B. and T.J.D. analysed the data. L.B.B., T.J.D. and S.P.H. wrote the paper. Other authors participated in discussions that led to the paper and assisted with editing the manuscript. Additional support was provided to T.J.D. as an NCEAS Postdoctoral Associate. Thanks to A. Diniz-Filho, R. Holt, A. Hurlbert, K. Roy and anonymous reviewers for helpful comments and discussions.
References
- Ackerly D. D.2009Evolution, origin and age of lineages in the Californian and Mediterranean floras. J. Biogeogr. 36, 1221–1233 (doi:10.1111/j.1365-2699.2009.02097.x) [Google Scholar]
- Bininda-Emonds O. R. P., et al. 2007The delayed rise of present-day mammals. Nature 446, 507–512 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- Blomberg S. P., Garland T., Ives A. R.2003Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745 [DOI] [PubMed] [Google Scholar]
- Collier G. E., O'Brien S. J.1985A molecular phylogeny of the Felidae: immunological distance. Evolution 39, 473–487 (doi:10.2307/2408647) [DOI] [PubMed] [Google Scholar]
- Currie D. J.1991Energy and large-scale patterns of animal- and plant-species richness. Am. Nat. 137, 27–49 (doi:10.1086/285144) [Google Scholar]
- Currie D. J., et al. 2004Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 (doi:10.1111/j.1461-0248.2004.00671.x) [Google Scholar]
- Davies T. J., et al. 2008Phylogenetic trees and the future of mammalian biodiversity. Proc. Natl Acad. Sci. USA 105, 11 556 (doi:10.1073/pnas.0801917105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz-Filho J. A. F., Rangel T., Bini L. M., Hawkins B. A.2007Macroevolutionary dynamics in environmental space and the latitudinal diversity gradient in New World birds. Proc. R. Soc. B 274, 43–52 (doi:10.1098/rspb.2006.3712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M. J.2008A phylogenetic perspective on the distribution of plant diversity. Proc. Natl Acad. Sci. USA 105, 11 549–11 555 (doi:10.1073/pnas.0801962105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T. H.1973Numbers of mammal species in North and Central American forest communities. Ecology 54, 555–563 (doi:10.2307/1935340) [Google Scholar]
- Freckleton R. P., Jetz W.2009Space versus phylogeny: disentangling phylogenetic and spatial signals in comparative data. Proc. R. Soc. B 276, 21–30 (doi:10.1098/rspb.2008.0905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T., Jr, Dickerman A. W., Janis C. M., Jones J. A.1993Phylogenetic analysis of covariance by computer simulation. Syst. Biol. 42, 265–292 [Google Scholar]
- Ghalambor C. K., Huey R. B., Martin P. R., Tewksbury J. J., Wang G.2006Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Int. Comp. Biol. 46, 5–17 (doi:10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- Grenyer R., et al. 2006Global distribution and conservation of rare and threatened vertebrates. Nature 444, 93–96 (doi:10.1038/nature05237) [DOI] [PubMed] [Google Scholar]
- Harmon L. J., Schulte J. A., Larson A., Losos J. B.2003Tempo and mode of evolutionary radiation in iguanian lizards. Science 301, 961–964 (doi:10.1126/science.1084786) [DOI] [PubMed] [Google Scholar]
- Harrison S., Grace J. B.2007Biogeographic affinity helps explain productivity–richness relationships at regional and local scales. Am. Nat. 170, 5–15 (doi:10.1086/519010) [DOI] [PubMed] [Google Scholar]
- Harvey P. H.1996Phylogenies for ecologists. J. Anim. Ecol. 65, 255–263 (doi:10.2307/5872) [Google Scholar]
- Harvey P. H., Colwell R. K., Silvertown J. W., May R. M.1983Null models in ecology. Annu. Rev. Ecol. Syst. 14, 189–211 (doi:10.1146/annurev.es.14.110183.001201) [Google Scholar]
- Hawkins B. A.2001Ecology's oldest pattern? Trends Ecol. Evol. 16, 470 (doi:10.1016/S0169-5347(01)02197-8) [Google Scholar]
- Hawkins B. A., et al. 2003Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (doi:10.1890/03-8006) [Google Scholar]
- Hawkins B. A., Diniz-Filho J. A. F., Jaramillo C. A., Soeller S. A.2007Climate, niche conservatism, and the global bird diversity gradient. Am. Nat. 170, 16–27 (doi:10.1086/519009) [DOI] [PubMed] [Google Scholar]
- Helgen K. M.2003Major mammalian clades: a review under consideration of molecular and palaeontological evidence. Mamm. Biol. 68, 1–15 (doi:10.1078/1616-5047-1610057) [Google Scholar]
- Hillebrand H.2004On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (doi:10.1086/381004) [DOI] [PubMed] [Google Scholar]
- Hunt R. M., Jr1996Biogeography of the order Carnivora. In Carnivore behavior, ecology, and evolution (ed. Gittleman J. L.), pp. 485–541 Ithaca, NY: Cornell University Press [Google Scholar]
- Huston M. A.1994Biological diversity: the coexistence of species on changing landscapes. Cambridge, UK: Cambridge University Press [Google Scholar]
- Janis C. M.1993Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annu. Rev. Ecol. Syst. 24, 467–500 (doi:10.1146/annurev.es.24.110193.002343) [Google Scholar]
- Jones K. E., Bininda-Emonds O. R. P., Gittleman J. L.2005Bats, clocks, and rocks: diversification patterns in Chiroptera. Evolution 59, 2243–2255 [PubMed] [Google Scholar]
- Kaufman D. M.1995Diversity of New World mammals: universality of the latitudinal gradients of species and bauplans. J. Mamm. 76, 322–334 (doi:10.2307/1382344) [Google Scholar]
- Kaufman D. M., Willig M. R.1998Latitudinal patterns of mammalian species richness in the New World: the effects of sampling method and faunal group. J. Biogeogr. 25, 795–805 (doi:10.1046/j.1365-2699.1998.2540795.x) [Google Scholar]
- Kemp T.2005The origin and evolution of mammals Oxford, UK: Oxford University Press [Google Scholar]
- Marquet P. A., Fernández M., Navarrete S. A., Valdovinos C.2004Diversity emerging: toward a deconstruction of biodiversity patterns. In Frontiers of biogeography: new directions in the geography of nature, pp. 192–209 Sunderland, MA: Sinauer Associates [Google Scholar]
- McCain C. M.2009Vertebrate range sizes indicate that mountains may be ‘higher’ in the tropics. Ecol. Lett. 12, 550–560 (doi:10.1111/j.1461-0248.2009.01308.x) [DOI] [PubMed] [Google Scholar]
- McNab B. K.1982Evolutionary alternatives in the physiological ecology of bats. In Ecology of bats (ed. Kunz T. H.), pp. 151–200 New York, NY: Plenum Publishing Corporation [Google Scholar]
- McNab B. K.2002The physiological ecology of vertebrates: a view from energetics. Ithaca, NY: Cornell University Press [Google Scholar]
- Mittelbach G. G., et al. 2007Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (doi:10.1111/j.1461-0248.2007.01020.x) [DOI] [PubMed] [Google Scholar]
- Pagel M.1999Inferring the historical patterns of biological evolution. Nature 401, 877–884 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- Peterson A. T., Soberón J., Sanchez-Cordero V.1999Conservatism of ecological niches in evolutionary time. Science 285, 419–433 (doi:10.1126/science.285.5431.1265) [DOI] [PubMed] [Google Scholar]
- Rahbek C., Graves G. R.2001Multiscale assessment of patterns of avian species richness. Proc. Natl Acad. Sci. USA 98, 4534–4539 (doi:10.1073/pnas.071034898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel T., Diniz-Filho J. A. F.2005An evolutionary tolerance model explaining spatial patterns in species richness under environmental gradients and geometric constraints. Ecography 28, 253–263 (doi:10.1111/j.0906-7590.2005.04038.x) [Google Scholar]
- Rangel T., Diniz-Filho J. A. F., Colwell R. K.2007Species richness and evolutionary niche dynamics: a spatial pattern-oriented simulation experiment. Am. Nat. 170, 602–616 (doi:10.1086/521315) [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E.2006Evolutionary diversification and the origin of the diversity–environment relationship. Ecology 87, 3–13 (doi:10.1890/0012-9658(2006)87[3:EDATOO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Sechrest W. W.2003Global diversity, endemism, and conservation of mammals. Thesis, University of Virginia, Charlottesville, VA [Google Scholar]
- Simmons N. B.2005Order Chiroptera. In Mammal species of the world: a taxonomic and geographic reference (eds Wilson D. E., Reeder D. A. M.), pp. 312–529 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Stevens R. D.2004Untangling latitudinal richness gradients at higher taxonomic levels: familial perspectives on the diversity of New World bat communities. J. Biogeogr. 31, 665–674 (doi:10.1111/j.1365-2699.2003.01042.x) [Google Scholar]
- Stevens R. D.2006Historical processes enhance patterns of diversity along latitudinal gradients. Proc. R. Soc. B 273, 2283–2289 (doi:10.1098/rspb.2006.3596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C. O., Ackerly D. D., McPeek M. A., Donoghue M. J.2002Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475–505 (doi:10.1146/annurev.ecolsys.33.010802.150448) [Google Scholar]
- Wiens J. J.2004Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution 58, 193–197 [DOI] [PubMed] [Google Scholar]
- Wiens J. J., Donoghue M. J.2004Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644 (doi:10.1016/j.tree.2004.09.011) [DOI] [PubMed] [Google Scholar]
- Wiens J. J., Graham C. H., Moen D. S., Smith S. A., Reeder T. W.2006Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am. Nat. 168, 579–596 (doi:10.1086/507882) [DOI] [PubMed] [Google Scholar]
- Wiens J. J., Sukumaran J., Pyron R. A., Brown R. M.2009Evolutionary and biogeographic origins of high tropical diversity in Old World frogs (Ranidae). Evolution 63, 1217–1231 (doi:10.1111/j.1558-5646.2009.00610.x) [DOI] [PubMed] [Google Scholar]
- Willig M. R., Kaufman D. M., Stevens R. D.2003Latitudinal gradients of biodiversity: pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 34, 273–309 (doi:10.1146/annurev.ecolsys.34.012103.144032) [Google Scholar]
- Wilson D. E., Reeder D. A. M.1993Mammal species of the world: a taxonomic and geographic reference. Washington, DC: Smithsonian Institution Press [Google Scholar]