Abstract
Although humans usually prefer mates that resemble themselves, mating preferences can vary with context. Stress has been shown to alter mating preferences in animals, but the effects of stress on human mating preferences are unknown. Here, we investigated whether stress alters men's preference for self-resembling mates. Participants first underwent a cold-pressor test (stress induction) or a control procedure. Then, participants viewed either neutral pictures or pictures of erotic female nudes whose facial characteristics were computer-modified to resemble either the participant or another participant, or were not modified, while startle eyeblink responses were elicited by noise probes. Erotic pictures were rated as being pleasant, and reduced startle magnitude compared with neutral pictures. In the control group, startle magnitude was smaller during foreground presentation of photographs of self-resembling female nudes compared with other-resembling female nudes and non-manipulated female nudes, indicating a higher approach motivation to self-resembling mates. In the stress group, startle magnitude was larger during foreground presentation of self-resembling female nudes compared with other-resembling female nudes and non-manipulated female nudes, indicating a higher approach motivation to dissimilar mates. Our findings show that stress affects human mating preferences: unstressed individuals showed the expected preference for similar mates, but stressed individuals seem to prefer dissimilar mates.
Keywords: mate choice, facial self-resemblance, stress, cold-pressor test, startle
1. Introduction
Mating is a central aspect of human and animal life, and many studies have investigated how humans and animals choose their mating partners. In humans, one of the most frequently investigated topics is the preference for similar versus dissimilar mates. Studies have shown that human romantic partners tend to resemble each other in many traits, including facial characteristics (Griffths & Kunz 1973; Zajonc et al. 1987; Bereczkei et al. 2002, 2004). Recently, we also showed a preference for similar mates in an experimental manipulation of facial resemblance (Lass-Hennemann et al. submitted). Overall, experimental evidence indicates a preference for self-resembling mates, even though there is one study that showed a preference for dissimilar mates specifically for short-term relationships (DeBruine 2005).
However, mating preferences are also context-dependent, and one factor that has repeatedly been found to alter mate choice in animals is stress. Stress is an adaptive response that is typically thought to enhance the probability of survival in the face of threat, but it is becoming apparent that stress is also associated with sexual behaviour and mate choice. For example, corticosterone (a hormone released in response to stress) has been shown to reduce male odour preferences in female mice (Kavaliers & Ossenkopp 2001). Furthermore, research has shown that stress alters the mating preference of stalk-eyed flies, which show less preference for more attractive mates and a willingness to mate with less attractive mates than do non-stressed animals (Lopez 1999; Hingle et al. 2001). These studies seem to indicate that stressed animals lose their ‘normal’ mating preferences.
However, the effects of stress on human mating preferences have not yet been investigated. From the animal studies described above, one could expect that humans also simply lose their preference for similar mates under stress. However, life-history theory (Stearns 1992) predicts that the optimal reproductive strategy for individuals in stressful environments is to maximize current reproduction to minimize the chances of lineage extinction. Having short-term relationships instead of long-term relationships is a way of increasing current reproduction and it has been shown that individuals who experience psychosocial stress have more short-term relationships than do individuals without a history of psychosocial stress (Koehler & Chisholm 2009). As described above, Debruine (2005) found a preference for dissimilar mates in the context of a short-term relationship. Therefore, one might also argue that stress not only decreases mating preference for similar mates, but also increases mating preference for dissimilar mates.
To investigate the effects of stress on men's preferences for similar versus dissimilar mates, we used a set of erotic pictures of female nudes that were computer-modified to resemble either the participant or another participant. We employed two independent methods to measure men's sexual attraction to the different erotic picture categories, subjective valence and arousal ratings of the erotic pictures, and affective startle response modulation. Affective startle modulation is a well validated and widely used method for assessing affective valence in the laboratory (Bradley et al. 1999). Numerous studies have shown that the startle reflex (elicited by a brief burst of noise) is facilitated when people view aversive pictures and inhibited when people view pleasant (especially erotic) pictures (Bradley et al. 2001). Viewing of aversive pictures leads to an activation of the defensive system and, therefore leads to an augmentation of the congruent defensive startle reflex. Viewing of pleasant pictures, on the other hand, engages the appetitive/approach system and leads to an inhibition of the non-congruent defensive startle reflex. Thus, affective startle modulation is not a direct measure of the physiological mechanisms underlying emotion, but rather an indirect measure of the activity of neurobiological structures involved in the processing of approach and withdrawal motivation. The inhibition of the startle reflex while viewing pleasant pictures reflects the activity of neurobiological circuits involved in approach motivation.
Affective startle modulation has been shown to generalize to a variety of foreground stimuli other than pictures that modify the emotional state of the participants (Bradley et al. 1999), and eliciting the startle response during foreground presentation of different stimuli serves as a validated measure of the affective valence of the presented stimuli. In particular, the startle modulation paradigm has been frequently used to analyse affective valence of and approach motivation to erotic pictures and film segments (Koukounas & Over 2000; Koukounas & McCabe 2001; Prause et al. 2008; Lass-Hennemann et al. 2009, submitted). Therefore, affective startle modulation appears to be an appropriate measure to evaluate how stress influences the attractiveness of, and approach motivation to, photographs of erotic female nudes. We used the affective startle modulation paradigm in addition to subjective ratings, because in previous studies it has served as a more sensitive measure for approach and withdrawal motivation than subjective ratings (Levenston et al. 2000; Lass-Hennemann et al. 2009, submitted). Subjective ratings are a voluntarily controllable measure, one that may be confounded by person variables such as social desirability and demand characteristics, while the startle response is a pre-attentive measure of approach motivation that is not easily controllable by the participant.
In our study, participants first underwent a stressful procedure or a non-stressful control procedure. Then, participants viewed pictures of erotic female nudes whose facial characteristics were either computer-modified to resemble the participant, or made to resemble another person, or were not manipulated, as well as viewing neutral pictures.
Based on previous research (Lass-Hennemann et al. submitted), we predicted that non-stressed participants would show a preference for similar mates, as indicated by decreased startle magnitude in the presence of pictures of self-resembling erotic female nudes. We predicted that stressed participants would not show the usual preference for similar mates, but instead a preference for dissimilar mates, as indicated by an inhibition of startle magnitude (indicating approach motivation) in the presence of pictures of other-resembling female nudes and non-manipulated female nudes.
2. Material and methods
(a). Participants
Participants were 50 male heterosexual students at the University of Trier, Germany, who responded to notices offering 25€ for taking part in two different experiments. Participation was limited to heterosexual Caucasian students without beards, piercings or tattoos in the facial region. Furthermore, only participants with normal or corrected to normal vision and no history of hearing problems were included in the study. Participation was also limited to healthy non-smokers with body mass index in the normal range of between 20 and 25 kg m2. Exclusion criteria were determined by a telephone screening interview that the respondent completed before being invited to take part. We also required participants to refrain from physical exercise, alcohol, caffeinated drinks and meals within 3 h prior to each of the two experimental sessions. The research was approved by the responsible local ethics committee, and all participants gave their written informed consent.
(b). Materials and design
Experimental material consisted of 40 pictures, 30 of which showed erotic female nudes with a completely visible face (i.e. no hair covering parts of the face), a direct gaze at the observer and a neutral facial expression. The other 10 pictures were neutral pictures selected from the International Affective Picture System1 (household objects) (Lang et al. 1999).
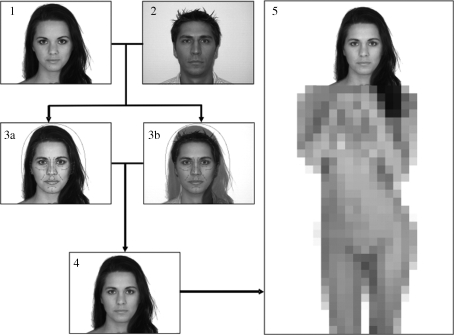
We used computer imaging techniques to manipulate facial resemblance between pictures of the face of each participant and the faces of the pictures of the erotic female nudes. The pictures of the erotic female nudes formed the basis for the morphing procedure, in which the face of the participant was morphed into the face of the erotic female nude (figure 1). Templates were created that specified the contours and certain landmarks on each face. The morphing routine itself comprises two processes: a shape morph averages the distance between the features of both faces that were specified in the template and a colour morph averages the colours of each pixel. These processes result in two different output images, and the colour morph was used as a layer on top of the shape morph. To ensure that the resulting female nude pictures still looked attractive and feminine, transparency was added to the colour morph layer to a 30 per cent degree. This means that the morphed picture shared 50 per cent of the shape of the participants’ face, but only 30 per cent of the colour information. By doing this, a composite face was created which was in favour of the women's features, i.e. the face was still primarily female and fitted to the body of the erotic female nude, even though it had a subtle resemblance to the participant. For further details on the underlying technology, see Tiddeman et al. (2001). No participants reported detecting the nature of the experiment, suggesting that the morphing did not result in conscious recognition of their own face by any participant.
Figure 1.
Image editing procedure: the nude woman's detailed face (1) was morphed with the portrait picture of the participant (2). The morphing software produces two output images, a shape-only morph (3a) and a combined shape-colour morph (3b). In a second step, the shape-colour morph is used as a semi-transparent layer on top of the shape-only morph. All artefacts of the morphing procedure are eliminated. The resulting image (4) was photo-mounted on the woman's body in a last step (5). The resulting image was used as a stimulus (the image was not masked in the experiment).
The 30 pictures of the erotic female nudes were randomly assigned to three subsets that consisted of 10 erotic female nudes each. For each participant, one of the three subsets was morphed with his own face and formed the ‘self-resembling’ erotic female nude picture set, so that 10 pictures had a subtle resemblance to the participant. Ten other photos of erotic female nudes were morphed with the face of a different participant and formed the ‘other-resembling’ erotic female nude picture set. The remaining 10 female nude pictures remained in their original state and were used as the ‘non-manipulated’ erotic female nude picture set. For the assignment of the picture sets to the participants, we employed a cross-over design: pairs of two participants were presented with the same version of the picture sets, so that the picture set that formed the self-resembling erotic female nude picture set for participant A formed the other-resembling erotic female nude picture set for participant B and vice versa. That is, every morphed picture set functioned once as the self-resembling erotic female nude picture set and once as the other-resembling erotic female nude picture set.
We included the non-manipulated erotic female nude pictures as a control condition to test whether morphed erotic female nude pictures led to a comparable startle inhibition as non-manipulated erotic female nude pictures. Furthermore, we included neutral pictures as a control to illustrate the effect of affective startle modulation. Pictures were displayed on an LCD monitor and picture onset was virtually instantaneous.
(c). Cover story
To be able to morph the participants’ faces into the faces of the erotic female nudes, we had to obtain a standardized photograph of each participant's face. For the nature of the experiment, it was necessary that participants did not know about the true purpose of the experiment. To assure this, we used the following cover story: participants were told that for a payment of 25€ they could participate in two unrelated studies in our laboratory: the experimenter told participants that the first study was to establish a new emotional face database that would be used for psychophysiological research in our laboratory. The second study would analyse the influence of acute pain on emotional and physiological reactions to erotic pictures. Furthermore, the experimenter explained that both studies were run together for economic reasons.
(d). Procedure
(i). Photograph acquisition
If the participant met the criteria in the telephonic interview, he was invited to our laboratory to take the photographs of his face. After arriving at the laboratory, the experimenter asked the participant to sign an informed consent in which the participant approved the scientific usage of the photographs of his face.
The experimenter instructed the participant to look into the camera with a neutral facial expression. Then, the experimenter took the portrait photographs with a digital camera from a distance of 80 cm in a fully lit room.
(ii). Startle pre-test
Directly after the photographs were taken, participants underwent a brief startle paradigm. The experimenter explained to the participants that the reaction to startle probes was the main dependent measure in the second study and that some people do not respond to these startle probes. Furthermore, he explained that only those people who showed a reliable startle reaction to the stimuli would be able to participate in the second study. The experimenter attached electrodes for electromyographic (EMG) measurement of the left musculus orbicularis oculi below the left eye of the participant with an inter-electrode distance of 1.5 cm (centre to centre). A third electrode taped on the forehead served as a reference. Electrode placement and skin preparation followed published guidelines (Blumenthal et al. 2005). Ten startle probes (105 db broadband noise, 50 ms, instantaneous rise time) were presented to each participant and the EMG responses to the startle probes were recorded.
After finishing the startle pre-test, an appointment for the second part of the study was made with the participants. Six participants showed less than 70 per cent measurable startle responses and were therefore not invited to the second part of the study. Every participant received 5€; for participation and left the laboratory.
(iii). Electrode placement and pre-measurement
Participants arrived for the second appointment about one week after the pictures were taken. During this week, participants’ pictures were morphed with one subset of the erotic female nude pictures.
Experimental sessions were run between 14.00 and 17.00 to control for diurnal cycle of cortisol. When participants arrived at our laboratory, they were asked to sit in a comfortable chair approximately 80 cm in front of a computer screen with a visual angle of 25°. Then, the experimenter attached electrodes for EMG measurement (as described above in §2d(ii)) and electrodes for electrocardiographic (ECG) measurement according to a standard lead II configuration. Furthermore, to measure continuous blood pressure, a cuff was placed around the middle finger of the left hand of each participant. After the electrode placement, ECG and blood pressure were recorded for 3 min (pre-measurement). After the pre-measurement, a saliva sample was collected using Salivette tubes (Sarstedt). The participant first placed a cotton swab provided with each Salivette tube in his mouth and gently chewed on it for about a minute. The swab was then placed back in the Salivette tube. Tubes were stored at room temperature until completion of the experimental session and were then kept at −20°C until analysis.
(iv). Assignment to stress or non-stress conditions
After we gathered the saliva sample, we assigned participants randomly to either a cold water stress condition or a warm water non-stress condition.
(v). Stress condition: cold-pressor test
The experimenter then informed participants assigned to the stress condition that they would be immersing their hand in ice water for as long as they could tolerate, that the procedure was potentially painful and that their performance would be videotaped so that the researchers could analyse their facial expressions. The cold-pressor (ice-water) test is a frequently used and well-validated laboratory pain stressor (for a review, see Lovallo 1975) and has been shown to activate both biological stress systems: the hypothalamo–pituitary–adrenal axis and the autonomic nervous system (Schwabe et al. 2008). Then, the experimenter asked participants to immerse their right hand up to the wrist into a cold water bath maintained at 0–4°C, while keeping the computer screen in view to see additional instructions. A female experimenter watched the participants during the cold water stress task. After 3 min, the computer screen told participants to remove their hand from the water. All participants kept their hand in the ice water for the full 3 min.
(vi). Non-stress condition: warm water test
The experimenter asked participants in the non-stress condition to place their right hand including the wrist into a tub of warm water, which was maintained at normal body temperature (35–37°C), and to keep the computer screen in view. The experimenter then left the room. After 3 min, the computer screen told participants to remove their hand from the water. All participants kept their hand in the warm water for the full 3 min.
(e). Ratings of stress, pain and unpleasantness
Immediately after the participants took their hand out of the cold or warm water, the computer screen prompted them to rate separately on scales ranging from 0 (‘not at all’) to 100 (‘very much’) in 10-point increments, first how ‘stressful’ the previous hand immersion had been, then how ‘unpleasant’ it had been and then how ‘painful’ it had been.
(f). Post-measurement
Immediately after the stress, unpleasantness and pain ratings, another saliva sample was collected and participants’ heart rate and blood pressure were measured again for 3 min (post-measurement).
(g). Affective startle modulation paradigm
After the post-measurement, participants were instructed via computer screen that a series of pictures would be displayed on the computer screen and that each picture should be viewed for its entire duration. Participants were also asked to relax, to neither move nor speak and to avoid long periods of eye closure. Finally, they were told that brief noises would be delivered via headphones. Six startle probes presented before the experimental session served as habituation trials. Then, the previously described pictures (self-resembling erotic female nudes, other-resembling erotic female nudes, non-manipulated erotic female nudes and neutral pictures) were displayed on the computer screen in a counterbalanced order for each participant. One slide of each of the four picture categories was presented once in a randomized order before a new cycle with another randomized sequence began, such that the same category could never be presented in more than two consecutive slides. Each picture was shown for 6 s and an acoustic startle probe was presented between 3 and 5 s after picture onset in eight (of 10) pictures per category, for a total of 40 picture trials. A black screen was shown for 4 s in every inter-picture interval. After completing the affective startle modulation paradigm (about 15 mins after the stress manipulation), the computer screen prompted participants to collect another saliva sample.
(i). Pleasure and arousal ratings
After collection of the second saliva sample, all pictures were presented again, one at a time, and the participants were asked to evaluate each picture for perceived pleasure and arousal using self-assessment-Manikin ratings ranging from one to nine (one indicates very low pleasure and arousal, and nine very high pleasure and arousal) (Bradley & Lang 1994).
After completing the ratings (about 30 min after the stress manipulation), participants provided another saliva sample. Then, the experimenter led participants to a nearby room and instructed them to collect additional saliva samples at 45 and 60 min after the stress or non-stress (cold or warm water) procedure. After the last saliva sample was collected, participants filled out a small questionnaire about their opinion about the purpose of the experiment: They were asked if they found anything special about the pictures of the erotic female nudes. None of the participants correctly detected that the pictures were digitally altered. After completing the questionnaire, the participants received 20€ and were thanked for their participation.
(ii). Debriefing
We did not inform participants about the true purpose of the experiment until we finished the data acquisition of all participants, because we wanted to avoid participants speaking about the experiment with other potential participants. After we finished data acquisition, we contacted each participant and explained the purpose of the experiment. Participants were encouraged to contact the experimenter at any time if they had further questions.
(h). Physiological recordings and data analysis
(i). Startle magnitude data
The startle response was assessed as peak EMG activity of the left orbicularis oculi, and was recorded with a Biopac MP 150 system and an EMG 100C amplifier at a sampling rate of 1000 Hz, with a notch filter of 50 Hz and a band-pass filter of 28–500 Hz. The raw signal was rectified and integrated online with a time constant of 10 ms. A semi-automated PC programme was used to analyse EMG data. The algorithm identified response peaks in the time interval of 20–150 ms after stimulus onset and baseline was set to 50 ms prior to stimulus onset. EMG data of all participants were manually confirmed with respect to non-response (no visible startle response) and/or artefacts (i.e. voluntary or spontaneous eyeblinks coinciding with the startle stimulus, trials with excessive background noise, multiple peaks).
The startle response was defined as the difference between a stable baseline (50 ms before stimulus onset) and the maximum magnitude of the EMG 20–150 ms after startle stimulus onset. If a response was not visible within the typical response latency range of a particular participant response magnitude was set to zero. Zero response magnitude data were included in the averaging procedure, with startle response magnitude as the output measure. There are wide inter-individual differences in the absolute size of the startle magnitude that are often unrelated to the experimental phenomena of interest. To test whether this was the case for our experiment, we compared startle magnitude for the stress and the control group using neutral pictures as the reference category. Startle magnitude for neutral pictures differed non-significantly between the two groups. To eliminate the impact of these differences, we standardized the raw data with intra-individual T-scores using all blinks of each participant as the standard distribution, as recommended by Blumenthal et al. (2005).
(ii). Cardiovascular data
The ECG signal was high-pass filtered (Biopac ECG100; HPF: 0.5 Hz) and stored to disc (1 kHz), and heart rate was derived from the ECG measure. Beat detection and artefact control was performed offline with WinCPRS (Absolute Aliens, Oy, Turku, Finland).
Continuous blood pressure was recorded using the Finapres System (Ohmeda, Englewood, CO, USA). Beat-to-beat systolic and diastolic blood pressure were determined offline by WinCPRS software. Owing to technical problems during data acquisition, the cardiovascular data of one participant in the warm water non-stress group and the cardiovascular data of two participants in the cold water stress group were missing.
(iii). Saliva
After thawing the saliva samples for biochemical analysis, the fraction of free cortisol in saliva was determined using a time-resolved immunoassay with fluorometric detection, as described in detail elsewhere (Dressendorfer & Kirschbaum 1992). The saliva of one participant in the warm water non-stress condition was missing.
(i). Statistical analysis
Data were analysed by repeated measures or mixed design analysis of variance (ANOVA) as appropriate, with the alpha level set at p < 0.05. Significant effects were further analysed by Bonferroni correction adjusted post hoc tests. Effect sizes are reported as partial η2.
3. Results
(a). Manipulation checks
In order to analyse the effects of stress on men's preference for similar mates, it was important to show that the cold water stress procedure actually stressed participants in contrast to the warm water non-stress procedure. This requirement was met, as follows.
(i). Stress induction: cortisol response
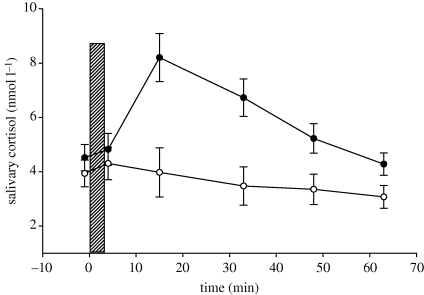
The results for cortisol, which indicate activity of the hypothalamo–pituitary–adrenal axis, supported the effectiveness of the stress manipulation. A mixed 2 (stress condition: cold water stress, warm water non-stress) × 6 (saliva sample occasion: −1 (pre-manipulation), +1 (post-manipulation), +15, +30, +45, +60 min) ANOVA with repeated measures on the saliva sample occasion factor found, as expected, a significant interaction, F5,205 = 10.424, p < 0.001, η2 = 0.24) (figure 2). This significant interaction, together with the pattern evident in figure 2, shows that after the stress manipulation, cortisol levels were significantly increased by the cold water stress task but not by the warm water non-stress task.
Figure 2.
Salivary cortisol in nanomoles per litre at several time points across the experiment. The bar represents the time of the stress and the non-stress manipulation, respectively. Error bars indicate 1 s.e. Filled circles on a line, cold water; unfilled circles on a line, warm water non-stress; and shaded box, time of warm water/cold water procedure.
(ii). Stress induction: subjective stress ratings
The subjective and cardiovascular stress indices are shown in table 1, separately for the cold water stress and warm water non-stress conditions. The upper section of table 1 presents the mean subjective unpleasantness, stress and pain ratings. Participants in the cold water stress condition experienced the hand immersion as significantly more unpleasant, F1,42 = 79.47, p < 0.001, η2 = 0.65, stressful, F1,42 = 38.65, p < 0.001, η2 = 0.48, and painful, F1,42 = 168.79, p < 0.001, η2 = 0.81, than did participants in the warm water non-stress condition.
Table 1.
Subjective stress ratings as well as systolic and diastolic blood pressure (mmHg) and heart rate (beats per minute) before (pre), during and after (post) hand immersion in warm or cold water in the two treatment groups. (Data represent M ± s.e.m.)
| warm water test | cold-pressor test | |
|---|---|---|
| subjective stress ratings (0–100) | ||
| unpleasant | 1.90 ± 5.12 | 58.57 ± 30.04** |
| stressful | 5.24 ± 11.67 | 41.42 ± 25.74** |
| painful | 0.476 ± 2.18 | 64.29 ± 22.71** |
| systolic blood pressure (mm Hg) | ||
| pre | 130.15 ± 9.34 | 127.15 ± 10.79 |
| during | 139.46 ± 15.91 | 172.52 ± 20.41** |
| post | 132.53 ± 15.28 | 135.05 ± 13.91 |
| diastolic blood pressure (mm Hg) | ||
| pre | 71.65 ± 9.27 | 71.19 ± 15.14 |
| during | 78.04 ± 12.54 | 99.15 ± 17.72* |
| post | 74.98 ± 13.77 | 78.41 ± 17.52 |
| heart rate (beats per minute) | ||
| pre | 73.02 ± 11.90 | 73.14 ± 11.14 |
| during | 73.27 ± 10.55 | 79.98 ± 9.89* |
| post | 73.01 ± 9.87 | 70.86 ± 11.51 |
*p < 0.05 compared with warm water test.
**p < 0.001 compared with warm water test.
(iii). Stress induction: systolic blood pressure
Participants’ mean systolic blood pressure, by stress condition and assessment occasion, are shown in the second section of table 1. The stress induction procedure elevated systolic blood pressure in the cold water stress condition compared with the warm water non-stress condition, as shown by a 2 (stress condition: cold water stress, warm water non-stress) × 3 (assessment occasion: before, during, and after hand immersion) mixed ANOVA that found the interaction between factors significant, F2,78 = 35.56, p < 0.001, η2 = 0.47. Post hoc analyses of intergroup differences at each of the three assessment occasions revealed a significant difference in systolic blood pressure only during hand immersion in the water, F1,39 = 33.63, p < 0.001, η2 = 0.46.
(iv). Stress induction: diastolic blood pressure
Comparable results were obtained for diastolic blood pressure, the means of which are displayed by stress condition and assessment occasion in the third section of table 1. A mixed 2 × 3 ANOVA of these means found that the interaction was significant, F2,78 = 21.20, p < 0.001, η2 = 0.35, and post hoc analyses of these means further revealed a significant difference between the stress and non-stress groups only during hand immersion in the water, F1,39 = 19.53, p < 0.001, η2 = 0.334.
(v). Stress induction: heart rate
The mean heart rates of participants in each of the two stress conditions, by assessment occasion, are shown in the last section of table 1. The stress condition × assessment occasion interaction was also significant, F2,78 = 13.82, p = 0.42, and post hoc analyses of these means again found a significant difference between groups only during hand immersion, F1,39 = 4.40, p < 0.04, η2 = 0.10.
(b). Startle magnitude
To analyse the effects of stress on approach motivation to similar and dissimilar mates, we employed a mixed design ANOVA with the between subject factor stress condition (cold water stress, warm water non-stress) and the within-subject factor picture content (four levels: self-resembling, other-resembling, not-manipulated and neutral). To be able to analyse the effects of stress on approach motivation to similar and dissimilar mates, we had to first show that affective startle modulation actually took place. As expected, startle response magnitude was affected by picture content (F3,126 = 17.55, p < 0.001, η2 = 0.30). Startle probes elicited larger blink responses during foreground presentation of neutral pictures than during presentation of self-resembling erotic female nudes (p < 0.001), other-resembling erotic female nudes (p < 0.001) and non-manipulated erotic female nudes (p < 0.001), showing the expected startle inhibition for appetitive pictures.
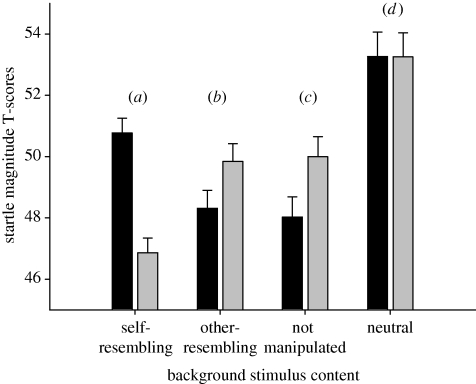
The experimental findings of central importance tested the hypothesis that the startle activity in the different erotic picture categories differed between the cold water stress and the warm water control group. The interaction between stress condition (cold water stress, warm water non-stress) and picture content (self-resembling, other-resembling, non-manipulated and neutral) for startle magnitude was significant (F3,126 = 6.796, p < 0.001, η2 = 0.132). Replicating earlier findings (Lass-Hennemann et al. submitted), in the warm water non-stress group foreground presentation of pictures of self-resembling erotic female nudes led to a larger inhibition of the startle reflex compared with other-resembling erotic female nudes (p < 0.05) and non-manipulated erotic female nudes (p < 0.01), indicating a higher approach motivation to self-resembling erotic pictures. In the cold water stress group, however, self-resembling erotic female nudes led to less inhibition of the startle reflex compared with other-resembling (p < 0.05) and non-manipulated erotic female nudes (p < 0.05), indicating a higher approach motivation to other-resembling and non-manipulated erotic pictures. Startle reactivity was larger in the presence of self-resembling than other-resembling female nudes in 16 of 22 participants in the stress condition and smaller in the presence of self-resembling female nudes in 16 of 21 participants in the control condition (figure 3).
Figure 3.
Blink magnitude to startle stimuli during foreground presentation of four different picture contents: (a) photographs of erotic female nudes with manipulated facial similarity to the participant (self-resembling), (b) photographs of erotic female nudes with manipulated facial similarity to another participant (other-resembling), (c) photographs of erotic female nudes whose facial characteristics were non-manipulated (not-manipulated), and (d) neutral pictures in the stress and the control group. Data are reported as T-scores. Error bars indicate 1 s.e. Black shaded boxes, cold water; grey shaded boxes, warm water.
(c). Self-report pleasure and arousal ratings
Analysis of picture valence ratings revealed a main effect of content (F3,126 = 54.68, p < 0.001, η2 = 0.56) (table 2). All three erotic picture categories were rated to be more pleasant than the neutral pictures (all ps < 0.001). A significant stress condition × picture content interaction showed that the stress and control groups differed in their valence ratings for the four picture categories. Post hoc tests showed that participants in the cold water stress group rated self-resembling female nudes to be less pleasant than other-resembling (p < 0.05) or non-manipulated erotic female nudes (p < 0.05) (F3.63 = 29.09, p < 0.001, η2 = 0.58), while participants in the warm water non-stress group rated self-resembling erotic female nudes to be more pleasant than other-resembling female nudes (p < 0.001) and non-manipulated female nudes (p < 0.001) (F3.63 = 36.55, p < 0.001, η2 = 0.63). Both groups rated all erotic picture categories to be more pleasant than neutral pictures (all ps < 0.001). Group t-tests showed that the difference in valence ratings for self-resembling female nudes between the two groups was significant (T42 = −3.897, p < 0.001).
Table 2.
Mean reports of rated pleasure and arousal of the cold water stress and the warm water non-stress group when viewing self-resembling erotic pictures, other-resembling erotic pictures, not manipulated erotic pictures and neutral pictures. (Note: data represents mean ± s.d.)
| warm water test | cold-pressor test | |
|---|---|---|
| self-resembling erotica | ||
| pleasure ratings (1–9) | 7.60 ± 1.10 | 6.26 ± 1.19 |
| arousal ratings (1–9) | 5.84 ± 1.52 | 5.62 ± 1.17 |
| other-resembling erotica | ||
| pleasure ratings (1–9) | 6.57 ± 1.21 | 6.82 ± 0.92 |
| arousal ratings (1–9) | 5.77 ± 1.59 | 6.14 ± 1.21 |
| not manipulated erotica | ||
| pleasure ratings (1–9) | 6.25 ± 1.19 | 7.02 ± 1.09 |
| arousal ratings (1–9) | 5.82 ± 1.71 | 5.60 ± 1.11 |
| neutral pictures | ||
| pleasure ratings (1–9) | 5.00 ± 0.75 | 5.00 ± 0.63 |
| arousal ratings (1–9) | 2.54 ± 1.42 | 1.99 ± 0.85 |
Analysis of arousal ratings also revealed a main effect of picture content (F3,126 = 165.02, p < 0.001, η2 = 0.79). All three erotic subsets were rated to be more arousing than neutral pictures (p < 0.001). However, there was no interaction between stress condition and picture content (F3,126 = 1.93, p = 0.13). Both groups’ arousal ratings for the four picture categories were comparable with each other. Overall, these findings indicate that participants in the stress group experienced self-resembling erotic pictures as less pleasant than participants in the control group, although both groups experienced all three erotic categories to be equally arousing.
4. Discussion
Humans usually prefer similar mates. However, mating preferences in animals have been shown to be influenced by stress. In this study, we asked the question whether stress influences men's preference for similar mates. To test this, we employed two independent methods to measure sexual attraction: subjective valence and arousal ratings, and affective modulation of startle. We predicted that stress would modulate affective startle inhibition in the presence of pictures of self-resembling erotic female nudes. For non-stressed participants, we replicated earlier findings showing that the startle response magnitude was smaller during foreground presentation of photographs of self-resembling female nudes compared with other-resembling female nudes and non-manipulated female nudes (Lass-Hennemann et al. submitted). In the stress group, we observed the opposite pattern: startle response magnitude was larger during foreground presentation of self-resembling female nudes compared with other-resembling female nudes and non-manipulated female nudes. The results for subjective valence ratings were in the same direction: participants in the cold water stress group rated self-resembling erotic female nudes to be less pleasant than other-resembling female nudes and non-manipulated female nudes, while participants in the warm water non-stress group rated self-resembling pictures to be more pleasant than other-resembling and non-manipulated female nudes. However, this effect was not found for arousal ratings: arousal ratings for the three erotic picture categories did not differ between the stress and the control group. Importantly, none of our participants detected the digital alteration of the erotic female nudes. Therefore, we succeeded in creating pictures of erotic female nudes with a subtle and undetected resemblance to the participant.
Our findings indicate that under non-stressful circumstances, facial self-resemblance increases attractiveness ratings and the activity of neurobiological structures involved in approach motivation, suggesting that similar potential mates lead to greater approach motivation and, thus, to higher sexual attractiveness than dissimilar mates. Under stressful conditions, however, these mating preferences seem to be reversed. Our findings indicate greater approach motivation to other-resembling female nudes and non-manipulated female nudes under stressful circumstances, and thus to higher sexual attractiveness of dissimilar mates compared with similar mates. Importantly, this result was not only found for subjective ratings, but also for modulation of startle magnitude. Subjective ratings are a voluntarily controllable measure, one that may be confounded by person variables such as social desirability and demand characteristics. Startle magnitude, on the other hand, is well-validated measure of the activity of neurobiological circuits involved in approach motivation that is not directly under the voluntarily control of the participant.
Interestingly, we did not find an effect for subjective arousal ratings. All erotic picture categories were rated to be equally arousing. This might be explained in two ways. First, participants might not have been as familiar with the concept of arousal. While valence is a concept that is widely used in everyday life to indicate the emotional valence of something, arousal is less common in everyday life and more related to negative emotional experiences. Therefore, participants might have not been able to make distinctions as subtle as those for subjective valence ratings. Second, the difference between the neutral and the erotic picture categories is much higher for arousal ratings compared with valence ratings. Therefore, a significant difference within the erotic picture categories might have been harder to detect.
Our results are in line with animal studies showing that stress reduces preference for those mates that are preferred under non-stressful circumstances (Hingle et al. 2001; Kavaliers & Ossenkopp 2001). Recent studies have shown that humans tend to prefer similar mates (Griffths & Kunz 1973; Zajonc et al. 1987; Bereczkei et al. 2002, 2004). This is remarkable, because optimal outbreeding theory (Bateson 1983) proposes that individuals would show a preference for mates that are not similar to oneself, because of the costs of inbreeding. This prediction is in line with human and animal genetic mate choice studies that show a preference for major histocompatibility complex dissimilarity in potential partners (for a review, see Havlicek & Roberts 2009). However, heritable benefits may be more important in short-term mates (Gangestad & Simpson 2000) and preferences for potential cues of genetic quality have been found to be stronger in short-term than in long-term contexts (Little et al. 2002; Penton-Voak et al. 2003). When men pursue long-term mating strategies, they usually invest in their children: because paternity is often uncertain for men, in a long-term relationship men place greater value on characteristics such as trustworthiness and sexual loyalty (Buss & Schmitt 1993). Research has shown that people tend to find self-resembling faces to be more trustworthy than dissimilar faces (DeBruine 2002, 2005). Therefore, it seems likely that men who pursue a long-term relationship should prefer self-resembling mates.
As described above, life-history theory predicts that the optimal reproductive strategy for individuals in stressful environments is to maximize current reproduction to minimize the chances of lineage extinction (Stearns 1992). A way to maximize current reproduction is to have short-term relationships instead of long-term relationships, and it has been shown that individuals who experienced psychosocial stress have more short-term relationships than individuals without a history of psychosocial stress (Koehler & Chisholm 2009). Therefore, it seems likely that, in our study, stress altered men's mating preference by making positive features of possible short-term mates (dissimilarity) more attractive than positive features of possible long-term mates (trustworthiness).
Of course, it is unlikely that a short physical stressor may totally switch men's mating preferences, as stressful situations are quiet common in everyday life. However, affective startle modulation is not a direct measure of mate choice, it is simply a measure of the approach motivation towards certain mates. This may represent the first step in a complex chain that results in mate selection. As such, startle may be a very basic indicator of approach motivation which increases the probability of, but does not completely determine, specific mating decisions.
Chronically stressful environments should increase the outbreeding tendencies, because inbreeding may lead to offspring that are not genetically diverse enough to deal with the varying circumstances that a risky and stressful environment poses on them. Thus, the same mechanism may account for chronic and acute stress, but might only become relevant to behaviour in chronically stressful environments, in which the approach motivation towards dissimilar mates may be constantly higher.
Of course, we can only speculate about the underlying mechanisms at this point. Future studies should investigate the effects of chronic stress on mating preferences and may also incorporate more measures of approach motivation and sexual attraction such as a penile/vaginal photoplethysmograph to measure changes in blood flow in the penis/vagina in order to gather a more direct measure of the sexual arousal to similar versus dissimilar mates.
One limitation of our study may be that the startle reflex is not only modulated by affective valence, but also by other factors such as attention. One might argue that non-stressed participants unconsciously recognized themselves in the pictures and therefore paid more attention to the self-resembling erotic female nudes as indicated by a stronger startle inhibition to self-resembling erotic female nudes. Of course, we cannot rule out that attention to self may play a role in these results. However, some important points speak against the attention hypothesis. First, the subjective valence ratings of the participants show the same effect as the startle modulation does. Second, an attention effect might explain the startle inhibition to self-resembling pictures in the control group. However, it may not explain the startle inhibition to other-resembling and non-manipulated erotic female nudes in the stress group. Furthermore, stress usually has a facilitating effect on perception and attention. Owing to these arguments, we believe that it is unlikely that the control group recognized themselves, while the stress group did not. Thus, we believe that our results remain valid and that startle inhibition reflects the activity of neurobiological structures involved in approach motivation. However, future studies should incorporate paradigms designed to measure attention to the different stimuli as well as measures of approach motivation and sexual arousal.
Our results provide further support for facial self-resemblance as a potential modulator of sexual attractiveness. Furthermore, our study is, to our knowledge, the first to show that stress influences human mating preferences, and provides further support for the influence of stress on mate choice and sexual behaviour. This study also illustrates the value of using the startle response as a measure of approach motivation in these studies.
Acknowledgements
This research was approved by the responsible local ethics committee and all participants gave their written informed consent.
We wish to thank D. Perrett and B. Tiddeman for the use of their image manipulation software. J.L.-H., C.E.D., L.K.K., A.S. and H.S. are members of the International Research Training Group ‘Psychoneuroendocrinology of Stress: from molecules and genes to affect and cognition’ funded by the German Research Foundation (Deutsche Forschungsgemeinschaft: DFG), grant GRK 1389/1.
Endnote
IAPS picture numbers used in this study were 7000, 7002, 7004, 7006, 7009, 7010, 7020, 7025, 7035, 7040.
References
- Bateson P.1983Optimal outbreeding. In Mate choice (ed. Bateson P.), pp. 257–278 Cambridge, MA: Cambridge University Press [Google Scholar]
- Bereczkei T., Gyuris P., Koves P., Bernath L.2002Homogamy, genetic similarity, and imprinting; parental influence on mate choice preferences. Pers. Individ. Diff. 33, 677–690 (doi:10.1016/S0191-8869(01)00182-9) [Google Scholar]
- Bereczkei T., Gyuris P., Weisfeld G. E.2004Sexual imprinting in human mate choice. Proc. R. Soc. Lond. B 271, 1129–1134 (doi:10.1098/rspb.2003.2672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T. D., Cuthbert B. N., Filion D. L., Hackley S., Lipp O. V., Van Boxtel A.2005Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42, 1–15 (doi:10.1111/j.1469-8986.2005.00271.x) [DOI] [PubMed] [Google Scholar]
- Bradley M. M., Lang P. J.1994Measuring emotion: the self-assessment Manikin and the semantic differential. J. Behav. Ther. Exp. Psych. 25, 49–59 (doi:10.1016/0005-7916(94)90063-9) [DOI] [PubMed] [Google Scholar]
- Bradley M. M., Cuthbert B. N., Lang P. J.1999Affect and the startle reflex. In Startle modification: implications for neuroscience, cognitive science and clinical science (eds Dawson E., Schell A. M., Böhmelt A. H.), pp. 157–186 Cambridge, MA: Cambridge University Press [Google Scholar]
- Bradley M. M., Codispoti M., Cuthbert B. N., Lang P. J.2001Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion (Wash. DC) 1, 276–298 [PubMed] [Google Scholar]
- Buss D. M., Schmitt D. P.1993Sexual strategies theory: an evolutionary perspective on human mating. Psychol. Rev. 100, 204–232 (doi:10.1037/0033-295X.100.2.204) [DOI] [PubMed] [Google Scholar]
- Debruine L. M.2002Facial resemblance enhances trust. Proc. R. Soc. Lond. B 269, 1307–1312 (doi:10.1098/rspb.2002.2034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruine L. M.2005Trustworthy but not lust-worthy: context-specific effects of facial resemblance. Proc. R. Soc. B 272, 919–922 (doi:10.1098/rspb.2004.3003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendorfer R. A., Kirschbaum C.1992Synthesis of a cortisolbiotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J. Steroid Biochem. 43, 683–692 (doi:10.1016/0960-0760(92)90294-S) [DOI] [PubMed] [Google Scholar]
- Gangestad S. W., Simpson J. A.2000The evolution of human mating: trade-offs and strategic pluralism. Behav. Brain Sci. 23, 573–587 (discussion 587–644). (doi:10.1017/S0140525X0000337X) [DOI] [PubMed] [Google Scholar]
- Griffths R., Kunz P.1973Assortative mating: a study of physiognomic homgamy. Soc. Biol. 20, 448–453 [DOI] [PubMed] [Google Scholar]
- Havlicek J., Roberts S. C.2009MHC-correlated mate choice in humans: a review. Psychoneuroendocrinology 34, 497–512 (doi:10.1016/j.psyneuen.2008.10.007) [DOI] [PubMed] [Google Scholar]
- Hingle A., Fowler K., Pomiankowski A.2001The effect of transient food stress on female mate preference in the stalk-eyed fly Cyrtodiopsis dalmanni. Proc. R. Soc. Lond. B 268, 1239–1244 (doi:10.1098/rspb.2001.1647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M., Ossenkopp K. P.2001Corticosterone rapidly reduces male odor preferences in female mice. NeuroReport 12, 2999–3002 (doi:10.1097/00001756-200109170-00049) [DOI] [PubMed] [Google Scholar]
- Koehler N., Chisholm J. S.2009Early psychosocial stress affects men's relationship length. J. Sex Res. 46, 366–374 (doi:10.1080/00224490902773996) [DOI] [PubMed] [Google Scholar]
- Koukounas E., Mccabe M. P.2001Sexual and emotional variables influencing sexual response to erotica: a psychophysiological investigation. Arch. Sex Behav. 30, 393–408 (doi:10.1023/A:1010261315767) [DOI] [PubMed] [Google Scholar]
- Koukounas E., Over R.2000Changes in the magnitude of the eyeblink startle response during habituation of sexual arousal. Behav. Res. Ther. 38, 573–584 (doi:10.1016/S0005-7967(99)00075-3) [DOI] [PubMed] [Google Scholar]
- Lang P. J., Bradley M. M., Cuthbert B. N.1999International affective picture system (IAPS) Gainsville, FL: NIMH Centre for the Study of Emotion and Attention. [Google Scholar]
- Lass-Hennemann J., Deuter C. E., Kuehl L. K., Schulz A., Blumenthal T. D., Schachinger H.Submitted Do humans prefer similar or dissimilar mates? Facial self-resemblance influences physiological reactions but not subjective ratings to erotic stimuli. [Google Scholar]
- Lass-Hennemann J., Schulz A., Nees F., Blumenthal T. D., Schachinger H.2009Direct gaze of photographs of female nudes influences startle in men. Int. J. Psychophysiol. 72, 111–114 (doi:10.1016/j.ijpsycho.2008.11.001) [DOI] [PubMed] [Google Scholar]
- Levenston G. K., Patrick C. J., Bradley M. M., Lang P. J.2000The psychopath as observer: emotion and attention in picture processing. J. Abnor. Psychol. 109, 373–385 (doi:10.1037/0021-843X.109.3.373) [PubMed] [Google Scholar]
- Little A. C., Jones B. C., Penton-Voak I. S., Burt D. M., Perrett D. I.2002Partnership status and the temporal context of relationships influence human female preferences for sexual dimorphism in male face shape. Proc. R. Soc. Lond. B 269, 1095–1100 (doi:10.1098/rspb.2002.1984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S.1999Parasitized female guppies do not prefer showy males. Anim. Behav. 57, 1129–1134 (doi:10.1006/anbe.1998.1064) [DOI] [PubMed] [Google Scholar]
- Lovallo W.1975The cold pressor test and autonomic function: a review and integration. Psychophysiology 12, 268–282 (doi:10.1111/j.1469-8986.1975.tb01289.x) [DOI] [PubMed] [Google Scholar]
- Penton-Voak I. S., Little A. C., Jones B. C., Burt D. M., Tiddeman B. P., Perrett D. I.2003Female condition influences preferences for sexual dimorphism in faces of male humans (Homo sapiens). J. Comp. Psychol. 117, 264–271 (doi:10.1037/0735-7036.117.3.264) [DOI] [PubMed] [Google Scholar]
- Prause N., Janssen E., Hetrick W. P.2008Attention and emotional responses to sexual stimuli and their relationship to sexual desire. Arch. Sex Behav. 37, 934–949 (doi:10.1007/s10508-007-9236-6) [DOI] [PubMed] [Google Scholar]
- Schwabe L., Haddad L., Schachinger H.2008HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 33, 890–895 (doi:10.1016/j.psyneuen.2008.03.001) [DOI] [PubMed] [Google Scholar]
- Stearns S. C.1992The evolution of life histories New York, NY: Oxford University Press [Google Scholar]
- Tiddeman B. P., Perrett D., Burt D. M.2001Prototyping and transforming facial textures for perception research. IEEE Comput. Graph. Appl. Res. 21, 42–50 (doi:10.1109/38.946630) [Google Scholar]
- Zajonc R. B., Adelmann P. K., Murphy S. T., Niendenthal P. M.1987Convergence in the physical appearances of spouses. Motiv. Emotion 11, 335–346 (doi:10.1007/BF00992848) [Google Scholar]