Abstract
Good decision making for fisheries and marine ecosystems requires a capacity to anticipate the consequences of management under different scenarios of climate change. The necessary ecological forecasting calls for ecosystem-based models capable of integrating multiple drivers across trophic levels and properly including uncertainty. The methodology presented here assesses the combined impacts of climate and fishing on marine food-web dynamics and provides estimates of the confidence envelope of the forecasts. It is applied to cod (Gadus morhua) in the Baltic Sea, which is vulnerable to climate-related decline in salinity owing to both direct and indirect effects (i.e. through species interactions) on early-life survival. A stochastic food web-model driven by regional climate scenarios is used to produce quantitative forecasts of cod dynamics in the twenty-first century. The forecasts show how exploitation would have to be adjusted in order to achieve sustainable management under different climate scenarios.
Keywords: ecological forecasting, risk assessment, climate change, sustainable management, Baltic Sea, cod
1. Introduction
Ecosystems worldwide have already shown clear evidence of change in response to global warming (Walther et al. 2002; IPCC 2007; Rosenzweig et al. 2008). Because ecosystems provide vital goods and services, our ability to anticipate future changes is of great concern. In order to be of value in decision making and management, ecological forecasts should explicitly account for uncertainties and provide quantitative assessments of the risks associated with management actions (Clark et al. 2001). Forecasting in agriculture and forestry is today fairly well developed (Easterling & Apps 2005), based on a long tradition of large-scale experimentation and modelling (Adams et al. 1990; Joyce et al. 1995), however many ecological models ignore key sources of uncertainty, thereby providing incomplete information for evaluating risk (Clark et al. 2001). Although large uncertainty should not impede efforts to anticipate change, difficulties in evaluating impacts of climate change on marine ecosystems have resulted in a lack of reliable forecasts for fisheries production (Brander 2007). By adopting holistic ecosystem-based approaches, which consider both internal and external drivers (deYoung et al. 2004; Pikitch et al. 2004) we can include many sources of uncertainty and provide quantitative risk assessments for future fisheries production under different climate and fisheries management scenarios.
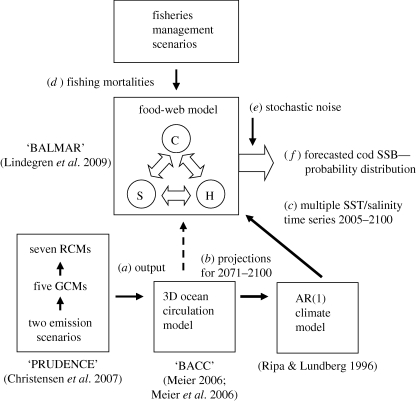
Atlantic cod is among the commercially most important fish species in the North Atlantic. Many of the stocks have collapsed owing to the joint effects of overfishing (Myers et al. 1997; Hutchings 2000; Frank et al. 2005) and climate-driven declines in productivity (Beaugrand et al. 2003; Lilly et al. 2008). Several studies have set out scenarios for cod stocks in the North Atlantic (Drinkwater 2005) and Baltic Sea (MacKenzie et al. 2007) based on global and regional climate projections. However these are qualitative scenarios which do not provide the information to assess risk from the joint effects of exploitation and environmental change (Clark et al. 2001). In order to provide quantitative risk assessments ecosystem-based models capable of integrating multiple drivers across trophic levels and properly including uncertainty are essentially needed (Clark et al. 2001; deYoung et al. 2004). Statistical models have proved useful in incorporating key ecological and environmental drivers and evaluating multiple sources of uncertainty (Walters et al. 1986; Harwood & Stokes 2003; Hjermann et al. 2004). We used the BALMAR food-web model (Lindegren et al. 2009) integrating species interactions, between cod and the forage fish species herring (Clupea harengus) and sprat (Sprattus sprattus) with external forcing through fishing, zooplankton and climate (see the electronic supplementary material, figure S1) to assess the risks of future stock collapse under different climate scenarios. Coupled with a climate model we were able to address the main external and internal sources of uncertainty (figure 1) and provide not only quantitative forecasts for Baltic cod but advice for achieving sustainable fisheries in the twenty-first century.
Figure 1.
A coupled food web–climate model approach to forecasting Baltic cod stock dynamics in the twenty-first century. The BACC assessment uses a three dimensional (3D) ocean circulation model to explore the range of projected climate changes in the Baltic Sea region based on climate scenarios from the PRUDENCE ensemble model. The BACC assessment conducted ‘time-slice’ simulations for 2071–2100 but no transient time series are available. In order to facilitate coupling with the food-web model, an AR(1) climate model was used to simulate transient time series based on the future projections for each variable. Additionally, multiple simulations were run in order to represent the variability and uncertainty in climate forcing in the twenty-first century. Finally, the response of the cod stock to climate and fisheries management was studied by forcing the food-web model with fishing mortalities for cod (C) as well as for sprat (S) and herring (H). Cod stock dynamics are forecasted as a 95% probability distribution of future spawning stock biomass (SSB) by running multiple stochastic simulations including random noise. SST, sea surface temperature; RCM, regional climate model; GCM, global climate model; AR(1), first order autoregressive.
2. Material and methods
(a). The climate model
We base our climate forecasts on simulation outputs from the BALTEX Assessment of Climate Change for the Baltic Sea Region (BACC 2007). It applies a multi-model ensemble approach (developed by the PRUDENCE project; Christensen et al. 2007) based upon seven regional circulation models, five global circulation models, and two emission scenarios to explore the range of outcomes and to enhance confidence in projected climate changes in the Baltic Sea (Meier 2006; Meier et al. 2006). This approach takes into account projections for precipitation and run-off, causing a freshening (i.e. decreasing salinities) of the Baltic Sea, as well as the effect of wind-driven inflows of high-saline water from the North Sea. Existing projections for 2071–2100 do not include transient time series for different seasons, areas and depths (Meier 2006). Therefore, we simulated future time series representing not only the projected change but the uncertainty and variability in climate forcing during the coming 100 years using an first order autoregressive (AR(1)) climate model (Ripa & Lundberg 1996). We generated ‘red-shifted’ transient time series (Steele & Henderson 1984; Φt+1) for spring sea surface temperature (SST) and summer salinity (80–100 m) of the Gotland Basin (i.e. the largest and northernmost spawning area of cod in the Baltic Sea):
| 2.1 |
where εt is random ‘white’ noise (zero mean and unit variance), β a parameter specifying the degree of environmental variation and α a measure of the autocorrelation in the time series. The degree of autocorrelation was estimated by fitting an autoregressive time series model to the observed SST and salinity data from 1974 to 2004, where the α parameter corresponds to the first-order autoregression coefficient. In the case of salinity, a marked ‘red-shifted’ dynamics was found, i.e. autocorrelation (α = 0.66) was significantly larger than zero (which corresponds to uncorrelated ‘white’ noise). The strong degree of autocorrelation is owing to the relatively low inter-annual variability in bottom salinity conditions in the Baltic Sea. By contrast, the strong and rapid fluctuations in SST give rise to a lower degree of autocorrelation (α = 0.21). The future series (from 2005 to 2104) were simulated based on the actual mean, variance (β) and autocorrelation (α) of the observed time series from 1974 to 2004. To simulate the predicted increase in Baltic Sea SST by 3.5°C and decrease in salinity by 0.8 or 4.8 practical salinity unit (psu), i.e. dependent on the choice of global circulation model (Meier et al. 2006; BACC 2007; see the electronic supplementary material, figure S2b,c), a gradual trend in the mean over 100 consecutive years was added. We also simulated future climate time series over a large spectrum of environmental autocorrelation (i.e. α = 0 to 1) because of concerns that global climate change may influence population persistence through changes also in the autocorrelation structure of the environment (Wigley et al. 1998; Pounds et al. 1999). Although species responses may change from a positive to a negative over wide ranges in temperature (MacKenzie & Köster 2004), we assume that the responses of Baltic cod to salinity and sprat to temperature remain positive, at least within the relatively narrow ranges of salinity and temperature predicted here (MacKenzie & Köster 2004; Köster et al. 2005). Using these simulations as inputs, we forced our food web model bottom-up (figure 1), exploring the impact of climate change on the future dynamics of Baltic cod, sprat and herring. Because herring was not directly forced by climate, but rather indirectly through its main prey, Pseudocalanus acuspes (Möllmann et al. 2003; Voss et al. 2003), we simulated the climate effect as an indirect linear response to salinity mediated by Pseudocalanus acuspes (salinity-Pseudocalanus acuspes: r = 0.65, n = 26).
(b). The food-web model
We studied the dynamics of Baltic cod, sprat and herring using the BALMAR food-web model (Lindegren et al. 2009), a linear state-space model based on a theoretical approach for predicting long-term responses of populations to environmental change (Ives 1995; Ives et al. 2003). The approach, a first-order multivariate autoregressive model (MAR(1)) applies a statistical framework for modelling food-web interactions at multiple trophic levels (Ives et al. 2003) and essentially functions as a set of lagged multiple linear regression equations (one for each species of the food web) solved simultaneously to arrive at the most parsimonious model overall (Hampton & Schindler 2006). Written in state-space form, the MAR(1) model we used is given by:
| 2.2 |
and
| 2.3 |
where X is a vector of true, but unobserved spawning stock biomasses (SSB) of cod, sprat and herring, derived from multi-species stock assessment (MSVPA) in the Baltic Sea (ICES 1996). The matrix B is a 3 × 3 matrix of species interaction parameters, an analogue of the ‘community matrix’ used in food-web theory (May 1972; Pimm 1982). The vector U contains lagged values of mean annual fishing mortalities (F) and a number of selected climate and zooplankton variables known to affect recruitment of cod, sprat and herring (see the electronic supplementary material, table S1 in Lindegren et al. 2009). Hence, the parameters of the covariate matrix C represent the effects of commercial fishing, climate and zooplankton on the dynamics of cod, sprat and herring, respectively. The process error E(t) was normally distributed and temporally uncorrelated (see the electronic supplementary material, figure S1 in Lindegren et al. 2009). Parameters were estimated without constraints by maximum likelihood estimation using a Kalman filter (Harvey 1989). The Kalman filter is a recursive estimator that sequentially calculates the unobserved SSB values X(t) from the previous time step (t −1) using the model formula specified in equation (2.2). Predictions from the ‘hidden’ state are then updated using the actual observed SSB values, Y(t) (equation (2.3)). The Z matrix explains how the observations (Y) relate to the unobserved states (X). Except for observation noise, these are identical, and hence Z is an identity matrix and V a matrix of observation errors. Model fitting was performed on time series covering the period 1974–2004. Finally, the most parsimonious model in terms of the number of parameters and the explained variance was selected and validated (Lindegren et al. 2009).
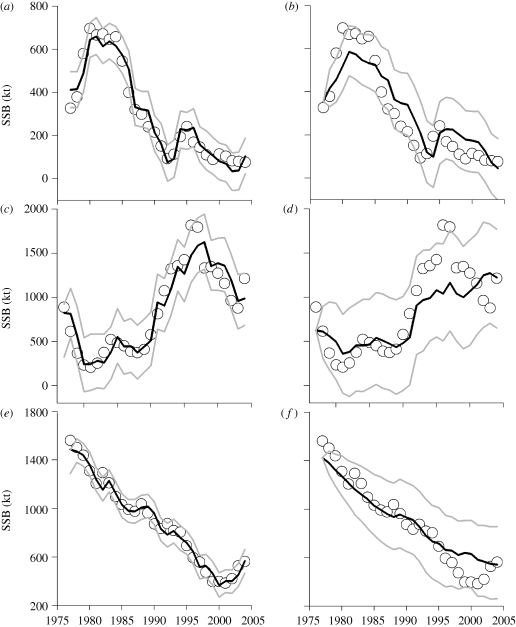
The final BALMAR model accurately captured the known mechanisms of species interactions within the Baltic Sea food-web (table 1; see the electronic supplementary material, figure S1), e.g. density-dependence/cannibalism (Sparholt 1994; Köster & Möllmann 2000; Neuenfeldt & Köster 2000), competition (Möllmann et al. 2005) and predation (Sparholt 1994). The model parameters also illustrate the negative effect of commercial fishing and the positive effect of zooplankton and climate (as represented by SST and salinity conditions) on growth, survival and recruitment of cod (Köster et al. 2005), sprat (MacKenzie & Köster 2004) and herring (Möllmann et al. 2005), respectively. The fitted model reproduced well the historical fish stock dynamics in the Baltic Sea (figure 2a,c,e). These are characterized by the collapse of the cod stock from high levels during the early 1980s, the large increase of the sprat stock since the late 1980s, and a constant decline of herring biomass. Furthermore, the food-web dynamics was simulated using only the first year SSB values as initial conditions (figure 2b,d,f). This procedure is fundamentally different from a simple fit to the data, as the observed values from the second year onwards are not used (Hjermann et al. 2004). The ability of the model to accurately recreate the past response of the fish community to climate and fisheries is a necessary basis for studying the effects of future changes in climate and fisheries management. All statistical analyses were conducted using the R software (www.r-project.org).
Table 1.
Parameter estimates and ecological mechanisms underlying the effects of species interactions, fishing and climate on Baltic cod, sprat and herring in the BALMAR food-web model (Lindegren et al. 2009).
| species interaction parameters; the community matrix (B) | |||
| cod | sprat | herring | |
| cod | 0.67a | 0 | 0.23e |
| sprat | −0.06d | 0.90b | 0 |
| herring | −0.09d | −0.10f | 0.87c |
| fishing, climate and zooplankton parameters; the covariate matrix (C) | |||
| fishing | climate | zooplankton | |
| cod | −0.91g | 0.08h | 0 |
| sprat | −0.34g | 0.08i | 0 |
| herring | −0.82g | 0 | 0.15j |
aDensity dependence/cannibalism (Sparholt 1994; Neuenfeldt & Köster 2000).
bFood competition/egg cannibalism (Köster & Möllmann 2000; Mackenzie & Köster 2004).
cFood competition (Möllmann et al. 2005).
dPredation mortality (Sparholt 1994; ICES 1996).
eForaging effect (Sparholt 1994; ICES 1996).
fInterspecific (food) competition (Sparholt 1994; Möllmann et al. 2005).
gFishing mortalities (ICES 2007).
hEgg/larvae survival (salinity dependent; Nissling 1994; Köster et al. 2005).
iEgg/larvae survival (SST dependent; Mackenzie & Köster 2004).
Figure 2.
Model validation by means of fitting and hindcasting the historical stock dynamics of (a,b) Baltic cod, (c,d) sprat and (e,f) herring. The left column shows the fit of the BALMAR food-web model (Lindegren et al. 2009), where SSB levels (black) accurately represent the observed dynamics (circles) of cod, sprat and herring from 1977 to 2004. (The degree of explained variance is: (a) 0.95; (c) 0.89 and (e) 0.98). The right column demonstrates hindcast SSB levels (black), where the historical stock dynamics were simulated based only on the starting biomasses (i.e. in 1977) as initial conditions. Grey lines are upper and lower 95% prediction intervals.
(c). Future climate and management scenarios
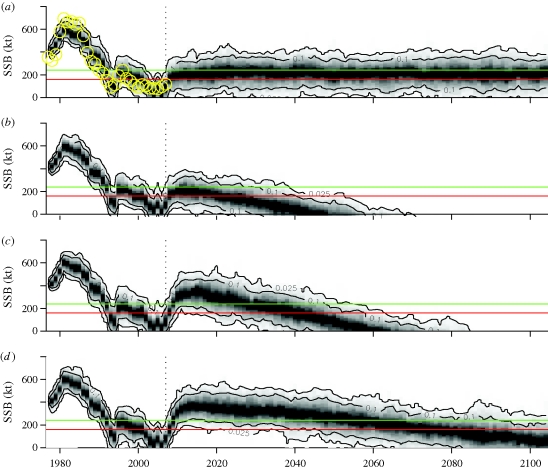
In order to study the effects of climate change on Baltic cod, we used a coupled climate–food web model approach (figure 1) in simulating four future scenarios for the Baltic Sea (a–d). Owing to large uncertainties in regional climate forecasting (Meier et al. 2006; BACC 2007; see the electronic supplementary material, figure S2) these scenarios aim to illustrate a number of probable ‘what if’—scenarios. Furthermore, the simulations apply a number of fishing strategies to study the role of fisheries management. In the first scenario (a), we provide a ‘control scenario’ where climate (SST and salinity) and fishing mortalities are kept at mean historical levels (F = 0.91; 1974–2004). In the second scenario (b), climate change is introduced by simulating an increasing trend in SST by 3.5°C and decreasing trend in salinity by 4.8 psu over 100 consecutive years. Fishing mortalities however remain fixed at mean historical levels. In scenario (c), the same climate change is combined with a reduction in cod fishing mortalities to the previously recommended precautionary fishing mortality (Fpa=0.6). The final scenario (d) simulates climate change with the same increase in SST by 3.5°C but with a smaller decrease in salinity by only 0.8 psu. Fishing mortalities remain at Fpa. For each scenario (a–d), we performed 1000 replicated stochastic simulations (see the electronic supplementary material, figure S3). At each time-step process noise was added to account for the main sources of uncertainty not covered by variability in climate, fishing or species interactions. By choosing a structurally simple model we acknowledge that we may not specifically address future variability stemming from (i) changes in species composition, e.g. owing to invasive species, (ii) changes in species distribution, (iii) drastic changes in ocean productivity or (iv) other anthropogenic disturbances (e.g. oil spills, etc.). Bearing in mind that these possible additional sources of variability are not included, the response of cod SSB to future scenarios (a–d) is presented as a 95 per cent probability distribution for each scenario.
(d). Adaptive management under climate change
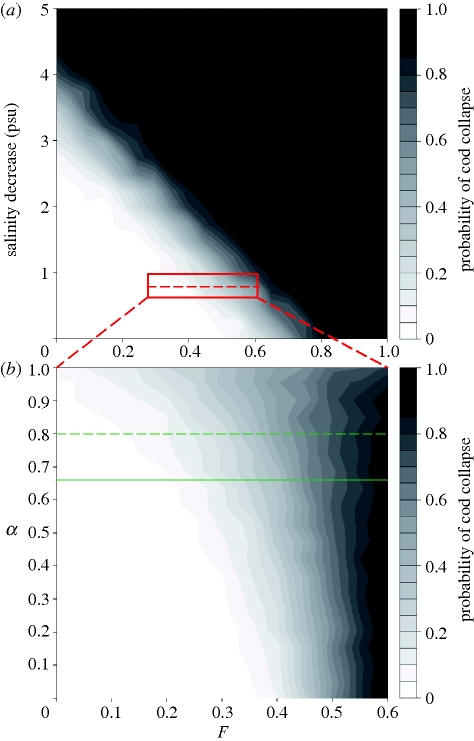
In order to investigate the synergistic effects of climate and fishing (Ottersen et al. 2006; Anderson et al. 2008) and derive sustainable exploitation levels for Baltic cod under different scenarios of climate change, we ran multiple simulations for each combination of salinity (i.e. projected decrease from 0 to 5 psu) and fishing (F from 0 to 1), keeping the projected increase in SST fixed at 3.5°C. The cod stock response was computed as the percentage of simulations where SSB drops below the limiting stock level (Blim=160 kt), hence a measure of the probability of stock collapse below ecologically safe levels. Given the concern that global climate change may influence population persistence through changes in the autocorrelation structure of the environment (Wigley et al. 1998), we additionally estimated the probability of stock collapse over a large spectrum of environmental autocorrelation (i.e. α = 0 to 1) with the projected increase in SST and decrease in salinity fixed at 3.5°C and 0.8 psu, respectively.
3. Results and discussion
(a). Ecological forecasting
Because the credibility of our forecasts depends in part on the ability to accurately recreate the past response of the fish community to climate and fisheries, we initiated our simulations in 1977 and forced the model by the observed climate and F levels until 2007. Despite slight underestimation at high stock levels and overestimation at low levels, the observed values were all within the confidence limits of the predictions (figure 2b). Based on this demonstrated ability to hindcast the past dynamics, we projected the response of cod to four future scenarios which include changes in both climate and fisheries management.
The first ‘control scenario’ without changes in climate and at mean historical F levels (1974 to 2004), predicts an initial increase and future mean biomass fluctuating slightly below the so-called precautionary stock level (Bpa) but above the limiting stock level (Blim; figure 3a). The initial increase in biomass, which is predicted in all four scenarios, is in accordance with recent observed dynamics as well as short-term stock assessment forecasts (ICES 2007) and is owing to improved salinity and oxygen conditions following inflow events from the North Sea (e.g. in 2003), combined with recent reductions in fishing mortalities. Owing to stochasticity in climate and process noise, the lower 95 per cent prediction interval however is close to zero, indicating a small probability (less than 2.5%) of extinction even without climate change. This probability increases if we add the slight tendency to overestimate biomass at low stock levels. Thus even if the mean climate does not change, current fishing mortality must be kept low in order to reduce the risk of extinction. In the second scenario (figure 3b), a combination of long-term change in SST (+3.5°C) and salinity (−4.8 psu) with mean F levels results in a forecast of initial stock recovery followed by a gradual decrease and probable extinction (more than 95%) of cod by the mid-2060s. The extinction can be explained by climate-driven recruitment failure (Köster et al. 2005) combined with high fishing pressure.
Figure 3.
Future climate and management scenarios and a 95% probability distribution of Baltic cod SSB. (a) A ‘control scenario’ where climate (SST and salinity) and fishing mortalities (F) fluctuate at mean 1974–2004 levels. Hindcasted simulations from 1977 to 2007 (i.e. based on the observed climate and F levels for these years) are compared with observed SSB (yellow circles) to validate the predictive accuracy of the model. (b) A predicted increase in mean SST by 3.5°C and decrease in mean salinity by 4.8 psu combined with mean F levels. (c) As in (b) but with F reduced to the previously recommended precautionary reference levels (Fpa). (d) Exploitation at Fpa but with a predicted decrease in salinity by only 0.8 psu. Solid horizontal lines mark the recommended ecological levels of Baltic cod, the precautionary stock level, Bpa (green) and limiting stock level, Blim (red). (Note that the use of these biomass reference points is currently being re-evaluated). Black contour lines show the 90 and 95% prediction intervals within which the cod stock dynamics of each replicated run fluctuates.
The third scenario shows that if fishing is reduced according to previously recommended precautionary reference levels (Fpa) then extinction may be postponed another 20 years (figure 3c), by which time the salinity drops below the absolute threshold level for cod recruitment in the Baltic Sea (Köster et al. 2005). Owing to differences in predicted salinities between global circulation models (see the electronic supplementary material, figure S2b,c), the fourth scenario includes only a minor decrease in salinity by 0.8 psu. However, the cod stock may not remain above ecologically safe levels during the coming 100 years (figure 3d), if exploitation is kept at Fpa. Hence, a strategy for adapting fishing mortalities to climate-driven stock production is necessary to ensure the persistence of Baltic cod into the twenty-second century.
(b). Adaptive management under climate change
The influence of both climate and fishing in determining cod stock persistence is apparent from the forecasting scenarios. Fishing reduces the age, size and geographical diversity of populations, making them more sensitive to climate-driven recruitment stress (Ottersen et al. 2006; Anderson et al. 2008). Furthermore, fish stock dynamics and persistence is influenced by ‘red-shifted’ (i.e. positively autocorrelated) marine climate, which by amplifying stock variance may ultimately increase the risk of extinction (Steele & Henderson 1984). Because Baltic cod is close to its physiological tolerance level, such changes in amplitude and duration of extreme climate events (i.e. the frequency and duration of Baltic inflow events) are very likely to have greater consequences than changes in mean values (Brander 2007).
In the first analysis, the probability of collapse increases steeply and non-linearly with F and decreasing salinities (figure 4a). If salinity decreases by only 0.8 psu cod is likely to persist, however if the decrease is 4.8 psu a collapse seems inevitable. The previously recommended precautionary fishing mortality (Fpa=0.6) results in approximately 50 per cent risk of collapse even without changes in climate, which is clearly not an acceptable level of precaution. If implemented, the current European Commission management plan (European Commission 2007) which gradually reduces exploitation to Fy (i.e. the target exploitation level of 0.3) may allow for sustainable exploitation of the cod stock, however, only given a decline in mean salinity of less than 1 psu.
Figure 4.
Probability of cod stock falling below ecologically safe levels (Blim) given future changes in climate and fishing mortalities. (a) Probabilities are calculated from simulations of mean decrease in salinity from 0 to 5 psu and F levels from 0 to 1. In (b) the additional effect of increasing environmental autocorrelation in future climate time series (i.e. alpha from 0 to 1) is investigated by studying the change in the slope of the ‘probability landscape’ at a projected salinity decline of 0.8 psu specifically (see red rectangle in (a)). Simulations were conducted over a range of fishing mortalities (F from 0 to 0.6) while the projected increase in SST remained fixed at 3.5°C. The green solid line marks the actual alpha in the salinity time series, while the dashed line aims to represent a correction for possible underestimations of forecasted global climate model autocorrelations, i.e. here set to a maximum of 20 per cent. White areas indicate low probabilities of cod biomass being below ecologically safe limits (Blim), while black areas demonstrate high probabilities.
In the second analysis, an added increase in environmental autocorrelation amplifies the probability of collapse (figure 4b) owing to an elevated risk of prolonged periods of poor salinity conditions for spawning and recruitment of Baltic cod. Furthermore, the effect is more pronounced at high levels of alpha (α) demonstrating an increasing synergy between fishing and climate effects in positively autocorrelated environments. However, given the minor underestimation of observed autocorrelations in global climate projections (Wigley et al. 1998; i.e. here illustrated by a maximum deviation of 20% from the observed level of alpha) the relative increase in the risk of stock collapse is rather limited (less than 10%). (Note however, that because population variance increase over time in ‘red-shifted’ marine environments (Steele & Henderson 1984), the relatively short time scale of our simulations (i.e. 100 years) may actually underestimate the long-term effects of environmental autocorrelation on stock persistence (i.e. normally simulated over 1000 years; Ripa & Lundberg 1996).) Nevertheless, it is evident that a sustainable strategy for managing exploitation must address several aspects of climate change and continuously be re-evaluated and adaptable as new information on current trends of the environmental drivers becomes available. In §3c we provide recommendations on exploitation levels that maximize long-term sustainable yield (MSY) under climate change and for an acceptable level of risk.
(c). Towards sustainable development in the Baltic sea
In contrast to the traditional (and indeed controversial) use of MSY, which aims to maximize biological production of target stocks irrespective of climate and indirect effects of non-target species (Hilborn 2007), MSY is here defined as the theoretical equilibrium yield that can be safely harvested from a stock under different climate conditions defined by both direct effects on recruitment and indirect effects of species interactions on growth and survival of Baltic cod (i.e. through the prey species sprat and herring). We initially computed the theoretical carrying capacity (K) of Baltic cod at zero exploitation and at various salinity and SST levels resembling the projected changes in Baltic Sea climate by 2100. The reproductive volume (MacKenzie et al. 2000), i.e. the maximum geographical range and habitat available for successful reproduction of Baltic cod is largely determined by salinity (and oxygen) levels in the Gotland Basin, the largest and northernmost spawning area of cod in the Baltic Sea (see the electronic supplementary material, figure S2). Our simulated K, reaching mean levels of 630 kt under historical salinity and SST levels (figure 5a) corresponds closely to peak SSB levels during the early 1980s (670 kt), a period referred to as ‘the gadoid outburst’ when cod inhabited almost the entire Baltic Sea (Bagge et al. 1994). At K the cod population is mainly limited by intra-specific competition for the available resources, i.e. food-availability and reproductive habitat. Hence, the theoretical K reached is owing to the combined outcome of food-availability through the interactions with the prey species herring and sprat and the positive effect of salinity on the size of the reproductive habitat. Additionally, both SST and the availability of the key zooplankton prey, Pseudocalanus acuspes indirectly limit cod K by influencing the recruitment of the prey species. Thus, our simulations appear to capture the main external and internal mechanisms explaining climate effects on carrying capacity of Baltic cod.
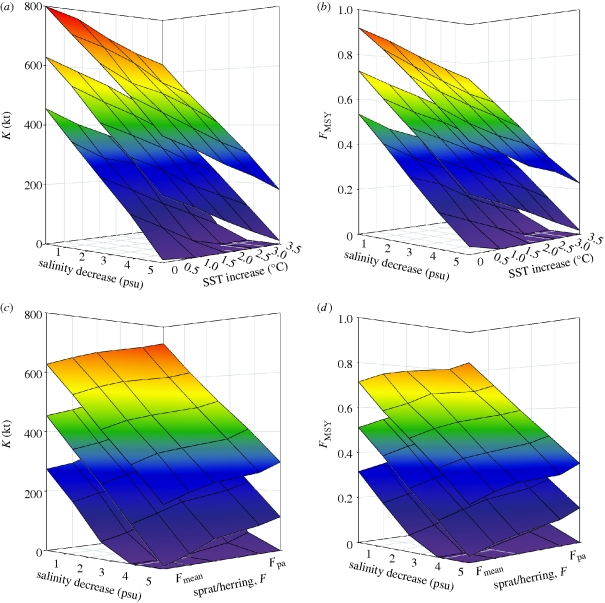
Figure 5.
A sustainable management strategy for Baltic cod under different scenarios of climate change. In (a, b) the theoretical carrying capacity (K) and corresponding maximum sustainable fishing mortality (FMSY) for Baltic cod is shown for each combination of projected changes in salinity and SST. In order to address the indirect effects of species interactions on cod stock response to climate change, the lower panels (c,d) include fishing pressures for sprat and herring, given a projected increase in SST by 3.5°C. Fishing mortalities range from mean historical levels (Fmean) to the recommended precautionary levels (Fpa) for sprat and herring, respectively (see the electronic supplementary material, figure S5). The middle planes represents mean K and FMSY while upper and lower planes, respectively, illustrate the upper and lower confidence levels of each scenario.
The biomass supporting MSY (BMSY) is derived as the point of maximum population growth and is located at K/2. By solving the model equation (i.e. equation (2.2)) for the level of F that under given changes in salinity and SST conditions yields BMSY, the maximum sustainable fishing mortality (FMSY) is shown decreasing with both salinity and increasing SST (figure 5b). This can be explained by a direct salinity effect on cod recruitment (i.e. through egg and larvae survival; Köster et al. 2005) and an indirect SST effect channelled through species interactions; owing to increased recruitment of sprat and a competition-driven decline in herring eventually affecting the dominance and availability of prey for cod. Hence, the degree to which species interactions may either buffer or accentuate the cod stock response to climate change depends on the nature of both positive and negative feedback loops within the food-web (Ives 1995).
In order to illustrate the need for multi-species management in mitigating climate effects on Baltic cod, we repeated the above analysis including different fishing pressures on sprat and herring as well. Thus, for each combination of salinity and fishing mortalities, i.e. ranging from mean historical levels (Fmean) to the recommended precautionary levels (Fpa) for sprat and herring, respectively (see the electronic supplementary material, figure S4), we estimated the theoretical K and FMSY for cod given a projected increase in SST by 3.5°C (figure 5c,d). As in the previous analysis both K and FMSY decrease with salinity conditions, however we may partially offset the indirect SST effect on cod by adapting fishing pressures to the recruitment potential of sprat and herring, respectively. Note however that accounting for these indirect SST effects through prey availability may not be sufficient to overcome any additional impacts of higher SST acting on oxygen consumption rates in the deep basins of the Baltic Sea, thus potentially reducing oxygen conditions and the size of the reproductive habitat for Baltic cod (MacKenzie et al. 2007).
4. Conclusions
The simulations presented above are intended to provide guidance in developing sustainable management for Baltic cod and to show the risks associated with different strategies. To that end, our ecosystem-based approach provides quantitative stock forecasts and suggests adaptive management actions to mitigate negative effects on future fisheries production under climate change. Furthermore, our ecosystem-based approach is intended to inform and thus strengthen the institutional and political will needed for successful implementation and governance of future ecosystem based fisheries management in the Baltic Sea (Hilborn 2007). Despite the difficulties inherent in forecasting, fisheries scientists should face the challenges of providing reliable forecasts to managers and stake-holders planning for the future. Although specifically fitted to the Baltic Sea, a low-diversity ecosystem highly sensitive to climate change, the methodology presented here is flexible enough to be used for other ecosystems, drivers (environmental or anthropogenic) and uncertainties, given adequate time series for the particular ecosystem (Ives et al. 2003). Adopting similar ecosystem-based approaches elsewhere will move us towards long-term sustainability in marine fisheries worldwide.
Acknowledgements
We wish to thank M. Meier for support concerning climate forecasting and F. Köster for general comments and input. M.L. was funded by the EU Marie Curie EST project METAOCEANS (MEST-CT-2005-019 678) and C.M. by the German Science Foundation through the Excellence Cluster ‘Integrated Climate System Analysis and Prediction (CliSAP) at the University of Hamburg. This study is a contribution to the European Union 6th Framework Projects ‘Resolving Climatic Impacts on Fish Stocks’ (RECLAIM, 044 133) and ‘Understanding the mechanisms of stock recovery’ (UNCOVER, 022 717).
References
- Adams R. M., et al. 1990Global climate change and United-States agriculture. Nature 345, 219–224 (doi:10.1038/345219a0) [Google Scholar]
- Anderson C. N. K., Hsieh C.-H., Sandin S. A., Hewitt R., Hollowed A., Beddington J., May R. M., Sugihara G.2008Why fishing magnifies fluctuations in fish abundance. Nature 452, 835–839 (doi:10.1038/nature06851) [DOI] [PubMed] [Google Scholar]
- BACC 2006Assessment of climate change for the Baltic Sea Basin: the BALTEX assessment of climate change for the Baltic Sea region (BACC) Project Geesthacht, Germany: GKSS [Google Scholar]
- Bagge O. G., Thurow F., Steffensen E., Bay J.1994The Baltic cod. Dana 10, 1–29 [Google Scholar]
- Beaugrand G., Brander K. M., Lindley J. A., Souissi S., Reid P. C.2003Plankton effect on cod recruitment in the North Sea. Nature 426, 661–664 (doi:10.1038/nature02164) [DOI] [PubMed] [Google Scholar]
- Brander K. M.2007Global fish production and climate change. Proc. Natl Acad. Sci. USA 104, 19 709–19 714 (doi:10.1073/pnas.0702059104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. H., Carter T. R., Rummukainen M., Amanatidis G.2007Evaluating the performance and utility of regional climate models: the PRUDENCE project. Clim. Change 81, 1–6 (doi:10.1007/s10584-006-9211-6) [Google Scholar]
- Clark J. S., et al. 2001Ecological forecasts: an emerging imperative. Science 293, 657–660 (doi:10.1126/science.293.5530.657) [DOI] [PubMed] [Google Scholar]
- deYoung B., Heath M., Werner F., Chai F., Megrey B., Monfray P.2004Challenges of modelling ocean basin ecosystems. Science 304, 1463–1466 (doi:10.1126/science.1094858) [DOI] [PubMed] [Google Scholar]
- Drinkwater K. F.2005The response of Atlantic cod (Gadus morhua) to future climate change. ICES J. Mar. Sci. 62, 1327–1337 (doi:10.1016/j.icesjms.2005.05.015) [Google Scholar]
- Easterling W., Apps M.2005Assessing the consequences of climate change for food and forest resources: a view from the IPCC. Clim. Change 70, 165–189 (doi:10.1007/s10584-005-5941-0) [Google Scholar]
- European Commission 2007Official Journal of the European Union 1098, L248/1 [Google Scholar]
- Frank K. T., Petrie B., Choi J. S., Leggett W. C.2005Trophic cascades in a formerly cod-dominated ecosystem. Science 308, 1621–1623 (doi:10.1126/science.1113075) [DOI] [PubMed] [Google Scholar]
- Hampton S. E., Schindler D. E.2006Empirical evaluation of observation scale effects in community time series. Oikos 113, 424–439 (doi:10.1111/j.2006.0030-1299.14643.x) [Google Scholar]
- Harvey A. C.1989Forecasting, structural time series models and the Kalman filter Cambridge, UK: Cambridge University Press [Google Scholar]
- Harwood J., Stokes K.2003Coping with uncertainty in ecological advice: lessons from fisheries. Trends Ecol. Evol. 18, 617–622 (doi:10.1016/j.tree.2003.08.001) [Google Scholar]
- Hilborn R.2007Defining success in fisheries and conflicts in objectives. Mar. Policy 31, 153–158 (doi:10.1016/j.marpol.2006.05.014) [Google Scholar]
- Hjermann D. O., Stenseth N. C., Ottersen G.2004The population dynamics of Northeast Arctic cod (Gadus morhua) through two decades: an analysis based on survey data. Can. J. Fish. Aquat. Sci. 61, 1747–1755 (doi:10.1139/f04-115) [Google Scholar]
- Hutchings J. A.2000Collapse and recovery of marine fishes. Nature 406, 882–885 (doi:10.1038/35022565) [DOI] [PubMed] [Google Scholar]
- ICES. International council for the exploration of the sea report of the working group on multispecies assessments of Baltic fish. 1996 Report no. ICES CM 1996/Assess:2. [Google Scholar]
- ICES. International council for the exploration of the sea report of the Baltic fisheries assessment working group. 2007 Report no. ICES CM 2007/ACFM:15. [Google Scholar]
- IPCC 2007Intergovernmental panel on climate change 2007: synthesis report (Fourth assessment report). [Google Scholar]
- Ives A. R.1995Predicting the response of populations to environmental-change. Ecology 76, 926–941 (doi:10.2307/1939357) [Google Scholar]
- Ives A. R., Dennis B., Cottingham K. L., Carpenter S. R.2003Community interaction webs and zooplankton responses to planktivory manipulations. Ecol. Monogr. 73, 301–330 (doi:10.1890/0012-9615(2003)073[0301:ECSAEI]2.0.CO;2) [Google Scholar]
- Joyce L. A., Mills J. R., Heath L. S., McGuires A. D., Haynes R. W., Birdsey R. A.1995Forest sector impacts from changes in forest productivity under climate change. J. Biogeogr. 22, 703–713 (doi:10.2307/2845973) [Google Scholar]
- Köster F. W., Möllmann C.2000Egg cannibalism in Baltic sprat Sprattus sprattus. Mar. Ecol. Prog. Ser. 196, 269–277 (doi:10.3354/meps196269) [Google Scholar]
- Köster F. W., et al. 2005Baltic cod recruitment: the impact of climate variability on key processes. ICES J. Mar. Sci. 62, 1408–1425 (doi:10.1016/j.icesjms.2005.05.004) [Google Scholar]
- Lilly G. R., et al. 2008Decline and recovery of Atlantic cod (Gadus morhua) stocks throughout the North Atlantic. In Resiliency of gadid stocks to fishing and climate change (eds Kruse S. G. H., Drinkwater K., Ianelli J. N., Link J. S., Stram D. L., Wespestad V., Woodby D.), pp. 39–66 Fairbanks, AL: Alaska Sea Grant Program [Google Scholar]
- Lindegren M., Möllmann C., Nielsen A., Stenseth N. C.2009Preventing the collapse of the Baltic cod stock through an ecosystem-based management approach. Proc. Natl Acad. Sci. USA. 106, 14 722–14 727 (doi:10.1073/pnas.0906620106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie B. R., Köster F. W.2004Fish production and climate: sprat in the Baltic Sea. Ecology 85, 784–794 (doi:10.1890/02-0780) [Google Scholar]
- MacKenzie B. R., Hinrichsen H. H., Plikshs M., Wieland K., Zezera A. S.2000Quantifying environmental heterogeneity: habitat size necessary for successful development of cod Gadus morhua eggs in the Baltic Sea. Mar. Ecol. Prog. Ser. 193, 143–156 (doi:10.3354/meps193143) [Google Scholar]
- MacKenzie B. R., Gislason H., Möllmann C., Köster F. W.2007Impact of 21st century climate change on the Baltic Sea fish community and fisheries. Global Change Biol. 13, 1348–1367 (doi:10.1111/j.1365-2486.2007.01369.x) [Google Scholar]
- May R. M.1972Will a large complex system be stable? Nature 238, 413–414 (doi:10.1038/238413a0) [DOI] [PubMed] [Google Scholar]
- Meier H. E. M.2006Baltic Sea climate in the late twenty-first century: a dynamical downscaling approach using two global models and two emission scenarios. Climate Dyn. 27, 39–68 (doi:10.1007/s00382-006-0124-x) [Google Scholar]
- Meier H. E. M., Kjellström E., Graham L. P.2006Estimating uncertainties of projected Baltic Sea salinity in the late 21st century. Geophys. Res. Lett. 33, L15705 (doi:10.1029/2006GL026488) [Google Scholar]
- Möllmann C., Kornilovs G., Fetter M., Köster F. W., Hinrichsen H. H.2003The marine copepod, Pseudocalanus elongatus, as a mediator between climate variability and fisheries in the Central Baltic Sea. Fish. Oceanogr. 12, 360–368 (doi:10.1046/j.1365-2419.2003.00257.x) [Google Scholar]
- Möllmann C., Kornilovs G., Fetter M., Köster F. W.2005Climate, zooplankton, and pelagic fish growth in the central Baltic Sea. ICES J. Mar. Sci. 62, 1270–1280 (doi:10.1016/j.icesjms.2005.04.021) [Google Scholar]
- Myers R. A., Hutchings J. A., Barrowman N. J.1997Why do fish stocks collapse? The example of cod in Atlantic Canada. Ecol. Appl. 7, 91–106 (doi:10.1890/1051-0761(1997)007[0091:WDFSCT]2.0.CO;2) [Google Scholar]
- Neuenfeldt S., Köster F. W.2000Trophodynamic control on recruitment success in Baltic cod: the influence of cannibalism. ICES J. Mar. Sci 57, 300–309 (doi:10.1006/jmsc.2000.0647) [Google Scholar]
- Nissling A.1994Survival of eggs and yolk-sac larvae of Baltic cod (Gadus morhua L.) at low oxygen levels in different salinities. ICES Mar. Sci. Symp. 198, 626–631 [Google Scholar]
- Ottersen G., Hjermann D. O., Stenseth N. C.2006Changes in spawning stock structure strengthen the link between climate and recruitment in a heavily fished cod (Gadus morhua) stock. Fish Oceanogr. 15, 230–243 (doi:10.1111/j.1365-2419.2006.00404.x) [Google Scholar]
- Pikitch E. K., et al. 2004Ecosystem-based fishery management. Science 305, 346–347 (doi:10.1126/science.1098222) [DOI] [PubMed] [Google Scholar]
- Pimm S. L.1982Food webs London, UK: Chapman and Hall [Google Scholar]
- Pounds J. A., Fogden M. P. L., Campbell J. H.1999Biological response to climate change on a tropical mountain. Nature 398, 611–615 (doi:10.1038/19297) [Google Scholar]
- Ripa J., Lundberg P.1996Noise colour and the risk of population extinctions. Proc. R. Soc. Lond. B 263, 1751–1753 (doi:10.1098/rspb.1996.0256) [Google Scholar]
- Rosenzweig C., et al. 2008Attributing physical and biological impacts to anthropogenic climate change. Nature 453, 353–358 (doi:10.1038/nature06937) [DOI] [PubMed] [Google Scholar]
- Sparholt H.1994Fish species interactions in the Baltic Sea. Dana 10, 131–162 [Google Scholar]
- Steele J. H., Henderson E. W.1984Modelling long-term fluctuations in fish stocks. Science 224, 985–987 (doi:10.1126/science.224.4652.985) [DOI] [PubMed] [Google Scholar]
- Voss R., Köster F. W., Dickmann M.2003Comparing the feeding habits of co-occurring sprat (Sprattus sprattus) and cod (Gadus morhua) larvae in the Bornholm Basin, Baltic Sea. Fish. Res. 63, 97–111 [Google Scholar]
- Walters C. J., Stocker M., Tyler A. V., Westerheim S. J.1986Interaction between Pacific cod (Gadus macrocephalus) and herring (Clupea harengus pallasi) in the Hecate Strait, British Columbia. Can. J. Fish. Aquat. Sci. Aquat. Sci. 43, 830–837 [Google Scholar]
- Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J. M., Guldberg O. H., Bairlein F.2002Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- Wigley T. M. L., Smith R. L., Santer B. D.1998Anthropogenic influence on the autocorrelation structure of hemispheric-mean temperatures. Science 282, 1676–1679 (doi:10.1126/science.282.5394.1676) [DOI] [PubMed] [Google Scholar]