Nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease (NAFLD) have been recognized entities in adult and pediatric medicine for more than 2 decades (1,2). Long-term follow-up studies of adults with NASH have confirmed that fibrosis can progress to cirrhosis (3). Individuals with NAFLD who have hepatic fibrosis and/or inflammation at presentation can progress to more severe fibrosis or cirrhosis and have a higher mortality when compared with individuals with steatosis alone (4–6).
Nonalcoholic fatty liver disease is recognized as the most common cause of elevated liver enzymes in children. A recent autopsy series demonstrated that the estimated prevalence of NASH was 2.96% (22/742) in children. Furthermore, 23% of children with NAFLD in this study had NASH, whereas bridging fibrosis or cirrhosis was seen in 9% of children with NASH in this study (7).
Long-term natural history data of pediatric NAFLD or NASH are not available. Certain components of routine laboratory tests may be predictive of NAFLD pattern and advanced fibrosis, but these serologic markers do not at present have adequate sensitivity or specificity to replace liver biopsy data to distinguish NASH from NAFLD (8). Serum alanine aminotransferase (ALT) and aspartate animotransferase (AST), for example, are not reliable measures of histological improvement in NASH (9).
Thus, liver tissue histology becomes a critical requirement for accurate diagnosis and evaluation of fibrosis progression in patients with NAFLD or NASH. The invasive nature and inherent risk of percutaneous liver biopsy, coupled with the debatable benefit of liver biopsies in guiding therapy or changing clinical outcomes, have prevented gaining a full understanding of the natural history and progression rates of NASH in both children and adults. This report presents 2 children with NASH whose serial liver biopsies demonstrate progression of hepatic fibrosis in a short period of time. This report attempts to highlight that worsening of hepatic fibrosis can occur within childhood in patients with NASH.
CASE REPORTS
Patient A
A white male initially presented at 11 years of age for evaluation of elevated AST (59 IU/L) and ALT (134 IU/L). His weight was 67.7 kg and body mass index (BMI) was 28.2 kg/m2, both >97th percentile for age and sex. The child had a medical history of attention-deficit/hyperactivity disorder, vesicoureteral reflux, a tonsillectomy, and having had bilateral myringotomy tubes. His family history was significant for obesity, hypothyroidism, asthma, and type 1 diabetes mellitus. On physical examination, he was obese but without any acanthosis nigricans, hepatomegaly, or splenomegaly.
His AST and ALT continued to remain above the upper limit of normal for more than 3 months and he subsequently underwent exclusionary workup for causes of chronic liver injury other than NAFLD (detailed below). A liver biopsy was obtained as part of his initial workup, which showed steatohepatitis with mild inflammatory activity. A few of the portal tracts showed a mild chronic inflammatory infiltrate composed predominantly of lymphocytes and rare plasma cells. There was panlobular steatosis, predominantly macrovesicular steatosis with intermixed microvesicular steatosis. There was no appreciable ballooning identified. A few scattered foci of glycogenated nuclei were present predominantly in zone 1. There were a few scattered foci of acute inflammatory cells both singly and in rare collections scattered throughout the parenchyma. This was consistent with a NAFLD activity score (NAS-scoring system detailed below) of 4 of 8. Focally by trichrome staining there was delicate periportal fibrosis appreciated: stage 1c/4 (Fig. 1 and Table 1).
FIGURE 1.
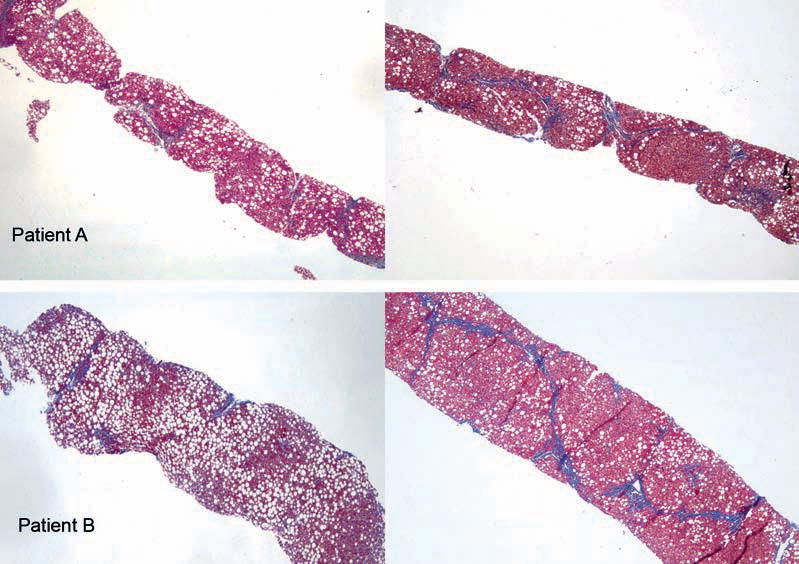
Trichrome stain of needle liver biopsy tissue (original magnification × 4) from patients A and B at time of initial (left panels) and subsequent follow-up (right panels) presentation. Trichrome stain highlights the areas of advanced fibrosis. Macro- and microvesicular steatoses are easily seen.
TABLE 1.
Subject characteristics at initial presentation and follow-up
| Patient A |
Patient B |
|||
|---|---|---|---|---|
| Biopsy no. 1 | Biopsy no. 2 | Biopsy no. 1 | Biopsy no. 2 | |
| Age, y | 11 | 16 | 10 | 13 |
| Weight, kg | 67.7 | 122.2 | 75.1 | 103.6 |
| BMI, kg/m2 | 28.2 | 38.6 | 31.8 | 36.05 |
| AST, IU/L | 59 | 114 | 112 | 256 |
| ALT, IU/L | 134 | 172 | 240 | 390 |
| GGT, U/L | 73 | 105 | 97 | 89 |
| Platelet count, 103/mcL | 355 | 252 | 262 | 239 |
| NAFLD Activity Score | 4/8 | 4/8 | 4/8 | 6/8 |
| Fibrosis Stage | 1c/4 | 3/4 | 1c/4 | 3/4 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; BMI = body mass index; GGT = γ-glutamyl transpeptidase; NAFLD = nonalcoholic fatty liver disease; NASH = nonalcoholic steatohepatitis.
He was advised to start 400 IU/day of vitamin E taken orally once per day. In addition, he was provided nutritional and exercise counseling. He was subsequently lost to follow-up and returned to the clinic 5 years later at age 16 with a complaint of abdominal pain and a 10-U rise in his BMI (now 38 kg/m2 or >99th percentile). Physical examination revealed persistent obesity, multiple abdominal striae, and right upper quadrant abdominal tenderness. His liver edge was percussed 3 cm below the right costal margin. His ALT (154 IU/L) and AST (39 IU/L) were elevated (Table 1), and an abdominal computed tomography scan at this time showed diffuse fatty infiltration of his liver and possible splenomegaly.
A second liver biopsy was performed, which was again consistent with steatohepatitis. The second biopsy also had an NAS score of 4 of 8 with the following histological description: The majority of the portal tracts had varying degrees of inflammatory infiltrate composed predominantly of lymphocytes. The steatosis was predominantly macrovesicular with some microvesicular steatosis, although the amount of steatosis was less compared with the initial biopsy and now with a predominantly zone 1 pattern. A few glycogenated nuclei were present in zone 1. The number of lobular inflammatory foci increased compared with the initial biopsy. There were a few ballooned hepatocytes seen. The degree of fibrosis, as evidenced by trichrome staining, was markedly increased from the previous biopsy with the fibrosis advancing to a stage of 3 of 4 with portal, periportal, and perisinusoidal fibrosis with bridging seen on trichrome staining (Fig. 1 and Table 1).
Patient B
A white male initially presented at 10 years of age for evaluation of elevated AST (112 IU/L) and ALT (240 IU/L). His weight was 75.1 kg and BMI was 31.8 kg/m2, both >99th percentile for age and sex. The child had a medical history of gastroesophageal reflux, tonsillectomy, and adenoidectomy. His family history was significant for obesity, hypothyroidism, sleep apnea, hepatitis C, and steatohepatitis. On physical examination, he was obese but without any acanthosis nigricans, hepatomegaly, or splenomegaly.
His liver enzymes (AST and ALT) did not improve during the next 3 months and he subsequently underwent exclusionary workup for causes of liver injury (detailed below). A liver biopsy done as part of his initial workup showed that the portal tracts were mildly infiltrated by a lymphocytic infiltrate. The bile ducts and portal vessels were histologically unremarkable. The majority of the portal tracts had no significant fibrosis by trichrome staining; however, a few portal tracts had delicate “finger-like” projections of periportal fibrosis. There was marked, predominantly panlobular macrovesicular steatosis with minimal microvesicular steatosis. There were also a few foci of necrotic hepatocytes and of predominantly lymphocytic inflammation. There was no ballooning or glycogenated nuclei appreciated (Table 1 and Fig. 1). Overall, the steatohepatitis was consistent with minimal inflammatory activity (NAS 4/8) and mild fibrosis (stage 1c).
He was subsequently advised to lose weight and was provided nutritional and exercise counseling. He did not return for follow-up as advised, but 3 years later presented at age 13 with a complaint of abdominal pain and a 5-U rise in his BMI to 36.05 kg/m2 (>99th percentile). Physical examination revealed obesity, acanthosis nigricans, and hepatomegaly, with a liver edge percussed 2 cm below the right costal margin. His AST and ALT were still elevated (Table 1) and an ultrasound scan at this time showed diffuse fatty infiltration of his liver. A second liver biopsy was performed, which had the following histological description: There was mild expansion of the majority of portal tracts with an inflammatory infiltrate composed predominantly of lymphocytes with a rare plasma cell and few scattered neutrophils. The bile ducts were normal. The parenchyma had moderate patchy mixed microvesicular and macrovesicular steatoses estimated at 45% to 50%. The steatosis had no particular zonal pattern. Foci of lobular inflammation were present and rare glycogenated nuclei were identified. No acidophil bodies were identified. Mild ballooning was appreciated focally. The trichrome stain confirmed portal, periportal, and bridging fibrosis. The second liver biopsy was scored with an NAS of 6 of 8 and fibrosis stage 3 of 4 (Table 1 and Fig. 1).
Liver Histology Scoring
All of the liver biopsies described in this report were reviewed by a single pathologist (T.B.) as part of this study and were scored according to the NAS system, consisting of steatosis, lobular inflammation, and hepatocyte ballooning (10). Each biopsy was a single core with at least 8 portal tracts and considered adequate for interpretation. The fibrosis was staged as follows: stage 0: no fibrosis; stage 1A: mild perisinusoidal fibrosis only; stage 1B: moderate perisinusoidal fibrosis only; stage 1C: periportal fibrosis only; stage 2: periportal and perisinusoidal fibrosis; stage 3: as above with bridging fibrosis; and stage 4: cirrhosis.
Exclusionary Information
Both children had the following causes of hepatitis and liver enzyme elevation excluded: hepatitis B and C, Wilson disease, α-1 antitrypsin deficiency, muscular dystrophy, autoimmune hepatitis, hypothyroidism, and celiac disease.
DISCUSSION
We present 2 children with biopsy-proven NASH that was progressive in severity of fibrosis within the time span of 3 to 5 years. These children had mild fibrosis on initial liver biopsy but were subsequently lost to follow-up. Upon repeat presentation these children had gained more weight and had a persistent increase in their AST and ALT. Repeat liver biopsies demonstrated significant progression of liver fibrosis, highlighting that NASH can advance rapidly within childhood.
There have been reports that NAFLD or NASH may be a slowly progressing disease for adults, yet end-stage liver disease secondary to NASH is well recognized (3,4). There are multiple case reports of adults who have undergone liver transplantation for cirrhosis secondary to NASH (11). It has also been proposed that a large proportion of cryptogenic cirrhosis may actually have been caused by NASH (6,12). Furthermore, long-term follow-up studies have also shown that patients who have NASH have an increased risk of mortality when compared with those with only steatosis, even when controlled for BMI, age, and sex (5). Both of these children had significant increases in their obesity. Patient A went from the 97th percentile to >99th percentile BMI, whereas the patient B remained above the 99th percentile and had an increase of 5-U BMI
A potential limitation to the conclusions drawn from our case report is the possibility of sampling error inherent in the technique of liver biopsy (13). Although NASH activity scoring may vary significantly between 2 independent liver samples, agreement for fibrosis staging has been shown to be more robust in a recent study of 43 adults (agreement rate of 98% and kappa reliability test of 0.96) (14). Another limitation of our retrospective report is that we cannot comment on the progression of insulin resistance because no measures of insulin resistance (fasting glucose, insulin, or oral glucose tolerance tests) were consistently available to compare across all presentation time points.
Our cases had rapid progression of NASH within childhood, which raises concern that the natural history of NASH in some children may be aggressive. Both children in our report were morbidly obese for their age and sex (defined as BMI >99th percentile). Given the rising prevalence of extreme obesity in children, which affects an estimated 4% of children in the United States, there may be a subset of children with NASH who are at greater risk for developing end-stage liver disease in late adolescence or early adulthood (15). Our cases, therefore, clearly highlight the need for prospective natural history studies in children with NASH that include serial liver biopsies or at minimum a validated noninvasive measure of significant fibrosis (16).
The progression of liver fibrosis staging within a 3- to 5-year interval in these children lends support to the argument that NASH is not a benign entity, with progression to advanced fibrosis possible in childhood. Pediatric patients who already have NASH, in particular with fibrosis, should be studied closely for disease progression, and intensive weight management strategies should be implemented. NAFLD and NASH have also been shown to improve dramatically following weight loss surgery in adults, thus bariatric surgery could be a therapeutic option for selected extremely obese adolescents with advanced NASH, when medical and behavioral strategies for weight loss have failed (17).
Acknowledgments
This work was supported by NIH K23 DK080888 (S.A.X.), NIH K12 HD028827 (R.K.), and the Children’s Digestive Health and Nutrition Foundation Young Investigator Award (R.K.).
Footnotes
The authors report no conflicts of interest.
References
- 1.Moran JR, Ghishan FK, Halter SA, et al. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983;78:374–7. [PubMed] [Google Scholar]
- 2.Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710–23. doi: 10.1053/gast.2001.27126. [DOI] [PubMed] [Google Scholar]
- 3.Tseng PH, Liu CJ, Kao JH, et al. Disease progression in a patient with nonalcoholic steatohepatitis. J Formos Med Assoc. 2008;107:816–21. doi: 10.1016/S0929-6646(08)60196-5. [DOI] [PubMed] [Google Scholar]
- 4.Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 (suppl 1):S11–6. doi: 10.1097/01.mcg.0000168644.23697.31. [DOI] [PubMed] [Google Scholar]
- 5.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 6.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43 (suppl 1):S99–112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 8.Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–20. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomba R, Wesley R, Pucino F, et al. Placebo in nonalcoholic steatohepatitis: insight into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–8. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 11.Bonatti H, Falkensammer J, Sawyer R, et al. Fat luck: three siblings requiring liver transplantation for nonalcoholic steatohepatitis. Transpl Int. 2008;21:189–91. doi: 10.1111/j.1432-2277.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 12.Tellez-Avila FI, Sanchez-Avila F, Garcia-Saenz-de-Sicilia M, et al. Prevalence of metabolic syndrome, obesity and diabetes type 2 in cryptogenic cirrhosis. World J Gastroenterol. 2008;14:4771–5. doi: 10.3748/wjg.14.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 14.Larson SP, Bowers SP, Palekar NA, et al. Histopathologic variability between the right and left lobes of the liver in morbidly obese patients undergoing Roux-en-Y bypass. Clin Gastroenterol Hepatol. 2007;5:1329–32. doi: 10.1016/j.cgh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DS, Mei Z, Srinivasan SR, et al. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17. e2. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Nobili V, Parkes J, Bottazzo G, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136:160–7. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Mummadi RR, Kasturi KS, Chennareddygari S, et al. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]


