Abstract
This study investigated the effects of mild calorie restriction (5%) on body weight, body composition, energy expenditure, feeding behavior, and locomotor activity in female C57BL/6J mice. Mice were subjected to a 5% reduction of food intake relative to baseline intake of ad libitum mice for 3 or 4 weeks. In experiment 1, body weight was monitored weekly and body composition (fat and lean mass) was determined at weeks 0, 2, and 4 by dual-energy X-ray absorptiometry. In experiment 2, body weight was measured every 3 days and body composition was determined by quantitative magnetic resonance weekly, and energy expenditure, feeding behavior, and locomotor activity were determined over 3 weeks in a metabolic chamber. At the end of both experiments, CR mice had greater fat mass (P < 0.01) and less lean mass (P < 0.01) compared with AL mice. Total energy expenditure (P < 0.05) and resting energy expenditure (P < 0.05) were significantly decreased in CR mice compared with AL mice over 3 weeks. CR mice ate significantly more food than AL mice immediately following daily food provisioning at 1600 hrs (P < 0.01). These findings showed that mild CR caused increased fat mass, decreased lean mass and energy expenditure, and altered feeding behavior in female C57BL/6J mice. Locomotor activity or BAT thermogenic capacity did not appear to contribute to the decrease in energy expenditure. The increase in fat mass and decrease in lean mass may be a stress response to the uncertainty of food availability.
Keywords: mild calorie restriction, energy expenditure, UCP1, body composition, meal pattern
INTRODUCTION
Calorie restriction (CR) is the most reproducible experimental paradigm known to slow aging and increase lifespan in a wide range of animal species, including mice and rats (1). Aside from slowing the intrinsic rate of aging, CR provides many health benefits. It delays or prevents many age-related diseases like cardiovascular disease and cancers (2).
CR is widely used in animal studies to investigate systems involved in energy regulation. Traditionally, experimental mammalian models of CR have restricted calorie intake from 30–60% (3). In response to moderate or severe energy restriction, CR animals experience a complex physiological and behavioral response via alteration of body weight, body composition, energy expenditure, and locomotor activity. CR causes an alteration of body composition by significantly decreasing body weight, body fat mass, and/or fat-free mass in humans and rodents (4–6). It has been suggested that the anti-aging phenotype may be linked to changes in body composition after CR, particularly the reduction of fat mass (7). CR leads to changes in energy expenditure by altering resting metabolic rate (RMR), physical activity, and the thermogenic effect of food. Further, the reduction in energy expenditure is likely to occur only during the period of negative energy balance, and there will be no relative difference after energy balance is reestablished after weight loss (8). In contrast, the metabolic rate in CR rats is higher than predicted from their altered body composition (9). A debate concerning the effects of CR on energy expenditure partly results from the normalization of body composition, which adds a new question of how to explain the contribution of energy expenditure to the aging phenomena.
Thyroid hormones modulate metabolism and thermogenesis, and are particularly important during CR (10, 11). Serum T3 and T4 are reduced in calorie restricted-animals for the conservation of energy (10, 11). However, decreased serum T3 was only observed during the weight loss period in humans (8). Uncoupling protein 1 (UCP1), a unique protein located in the inner membrane of mitochondria, allows protons to leak across the inner mitochondrial membrane, reducing ATP production and producing heat (12). It has previously been shown that UCP1 plays an important function in BAT adaptive thermogenesis, and is regulated by serum T3 (12). Moreover, the sympathetic nervous system plays a pivotal role in the regulation of expression and activity of UCP1 by the release of norepinephrine (12).
Besides the alteration of body composition and energy expenditure, CR can induce behavioral adaptations in locomotor activity and feeding behavior in humans, mice, and rats (13–15). Such behavioral responses include a reduction in spontaneous activity and a lowered body temperature, or alternatively an increase in foraging type hyperactivity (6, 15). However, the effects of CR on feeding behavior and its contribution to energy metabolism are poorly understood. The changes in meal pattern, including the size and frequency of meals, under CR had profound effects on the health and longevity of laboratory animals and humans, and a better understanding of the mechanisms may lead to novel approaches for disease prevention and treatment (16).
In general, the greater the level of CR (without causing malnutrition), the more beneficial it is for health (2). The classic CR regimen (~30–40% CR) is an unlikely reality for most humans to achieve in order to gain the proposed health and life-extending benefits. It remains to investigate the health benefits of lesser degrees of CR that may be more attainable for human studies (2). Although 5% CR is commonly used to promote consumption of total food allotments, even in control groups (13, 17), it is currently unclear whether mild CR (5%) is effective for increasing lifespan, attenuating age-related disease, and/or altering body composition normally associated with CR in mice.
The purpose of the present study was to investigate the influence of mild CR (5%) on body composition, energy expenditure, locomotor activity, and feeding behavior in female C57BL/6J mice.
METHODS AND PROCEDURES
Mice
Eight-week-old female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME, USA) were maintained under standard laboratory conditions on a 12/12 hour light/dark cycle in a temperature (22.0 ± 1.0 °C) and humidity (50%) controlled room. Animals were group housed for one week and then housed individually to decrease variability (18). In the first experiment, the mice were housed individually in normal mouse cages (31L×20W×15H cm) and provided food ad libitum (AL) for 6 weeks. The mice were subsequently subjected to a 5% calorie restriction (CR) or AL feeding (n=10/group) for 4 weeks. In the second experiment, 10-week-old mice were individually housed and acclimated to a TSE-Labmaster chamber (23.5L×13.5W×13H cm) for 3 weeks. During the third week, mice were weighed and randomly assigned to one of two groups; AL (n=7) or CR (n=8). CR was initiated at 13 weeks of age, and lasted for 3 weeks. For experiments 1 and 2, cages were cleaned on the final day of each week. Mice were provided with standard mouse chow (Teklad Global 16% Protein Rodent Diet, Harlan, Madision, WI, USA) throughout the experiment. By calorie, the mouse standard chow contained 11% from fat, 20% from protein, and 69% from carbohydrate. All procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Body weight, body composition and body fat distribution
Body weights were measured weekly (experiment 1) or every 3 days (experiment 2) at 1000 hrs. Body composition (fat and lean mass) was measured in vivo using either dual-energy X-ray absorptiometry (DXA; GE Lunar PIXImus software version 1.45, Lunar, Madison, WI, USA, experiment 1) or quantitative magnetic resonance (QMR, Echo 3-in-1, Echo Medical System, Houston, TX, USA, experiment 2). For DXA, all body composition scans were performed under anesthesia with isoflurane (3%) between 1000–1200h at weeks 0, 2 and 4. Body composition measurements with QMR were acquired the day before CR started, and weekly thereafter between 1300–1500 hours. Three hours prior to the scans, food was weighed, recorded, and removed from the cage. This period of fasting helped avoid any potential effect of gut fill on body composition results. At termination, fat pads, including interscapular brown adipose tissue (IBAT), inguinal white adipose tissue (IWAT), gonadal WAT (GWAT), subcutaneous WAT (SWAT), retroperitoneal WAT (RWAT), and mesenteric WAT (MWAT) were dissected carefully and weighed (accurate to 0.01g).
Feeding behavior
For experiment 1, the amount of food for the CR group was calculated according to 95% of the baseline intake of the AL group (AL=3.06±0.07g/day), and provided at 1600h every day throughout the experiment. For experiment 2, the initial 3-day’s food intake in CR mice was calculated according to 95% of the 3-day baseline measurement of AL mice. The food allotment for CR mice was then adjusted every 3 days based on the intake of AL mice, and provided at 1600hrs (light off at 1800h, and on at 0600h next day). Feeding behavior (including meal frequency and pattern of daily food consumption) was monitored by a feeding monitor system (Labmaster; TSE Systems, Bad Homburg, Germany) consisting of Macrolon type III cages equipped with food baskets connected to weight sensors. The basket of the feeding sensor was filled with normal food pellets (shaved into 1–2g pieces) and the drinking bottle was filled with autoclaved water. Food intake was measured continuously as a decrease in the weight of the food in the basket. Each time food was removed from the food container, the amount of food retrieved, and the timing of the event was recorded by the computer. The sawdust bedding was checked carefully, and spillage was recorded. Weight variations of the food basket were recorded every 9 minutes. Meal pattern analysis was conducted adopting a minimum inter-meal interval separating into two meals of 9 minutes (19).
Energy expenditure and locomotor activity
In experiment 2, total energy expenditure (TEE) and locomotor activity were analyzed using an indirect-calorimetry system (Labmaster; TSE Systems, Bad Homburg, Germany) over 3 weeks. Air-tight respiratory chambers with a flow rate of 0.45L/h were maintained at 22.0°C (± 1.0 °C). Energy expenditure was calculated using 1-minute samples for quantification of O2 consumption and CO2 production. Circadian energy expenditure (24 hours) was analyzed at baseline (day 0) and last day (day 20) of the experiment, and energy expenditure was averaged into 27 min periods per animal, and then was averaged for both groups. TEE was determined by calculating the average hourly energy expenditure over 22 hrs and then multiplying it by 24. Resting energy expenditure (REE) was calculated by averaging the three lowest 10 consecutive-minute periods of energy expenditure, with at least one hour between each period (20). This was then multiplied to generate a 24-hour REE (20). Locomotor activity was determined with infrared sensor pairs arranged in a grid pattern for horizontal (x, y level) activity. Movement was monitored continuously and reported as a total count every 9 minutes and expressed as counts/24 hrs.
Uncoupling protein 1 (UCP1) mRNA expression
Real time RT-PCR was conducted as previously described (21). On the day following 3 weeks treatment (21st day), mice were euthanized by decapitation and interscapular BAT was rapidly collected between 1000h to 1200h (food was provided at 1600h), frozen in liquid N2, and stored at −80°C. Total RNA was extracted using Trizol reagent (Life Technologies, Gathiersburg, MD) according to the manufacturer’s directions, after which cDNA was synthesized using a SuperScript III kit (Invitrogen). Multiplex Real Time RT-PCR was carried out using LUX-labeled oligonucleotide primers (Invitrogen) analyzed on a Chromo4 Instrument (MJ Research) for each gene of interest with Opticon MonitorTM software version 3.0. Primers for mouse UCP1 were as follows: UCP1 sense 5′-GGT CAG AAT GCA AGC CCA GAG-3′; UCP1 antisense 5′-CGG GTG TAA GCA TTG TAG GTC CCFAMG-3′. PCR conditions were 50°C for 2 min, 95°C for 2 min and then 45 cycles of 95°C for 15 sec, 60°C for 45 sec and 72°C for 30 sec. Certified housekeeping primer sets were purchased for the housekeeping genes (Invitrogen). The threshold cycle (CT value) corresponding to exponential amplification of PCR product during the log-linear phase for both the target gene (FAM labeled) and internal reference gene β-actin were analyzed for each sample in triplicate using the deltaCT comparative method to determine relative expression levels. Amplification efficiencies and assay validations were determined from control standard curves for both the gene of interest and the reference RNA generated for each 96-well assay. The intra-assay coefficient of variation was ≤3%.
Serum T3 assay
Trunk blood was collected and serum total T3 concentrations were determined in duplicate using a total T3 ELISA kit (Alpha Diagnostic, San Antonio, TX). The intra-assay coefficients of variation were ≤10% and the detection limit was 20ng/dL.
Statistical analyses
All statistical analyses were performed using SAS (Version 9.1; SAS Institute, Cary, NC). Repeated measures analysis of variance (ANOVA) was used to determine significant differences in body weight, food intake, body composition, total daily and resting energy expenditure, and activity between AL and CR mice over time. The post-hoc Bonferoni correction was used for the multiple comparisons in total and resting energy expenditure, and locomotor activity. Fat pad weights, serum T3 concentration, UCP1, and meal pattern were analyzed by student t-test. Data are reported as mean ± standard error of the mean (SEM). The criterion for statistical significance was P ≤ 0.05.
RESULTS
Experiment 1. Effects of 5% CR on body weight and composition over 4 weeks
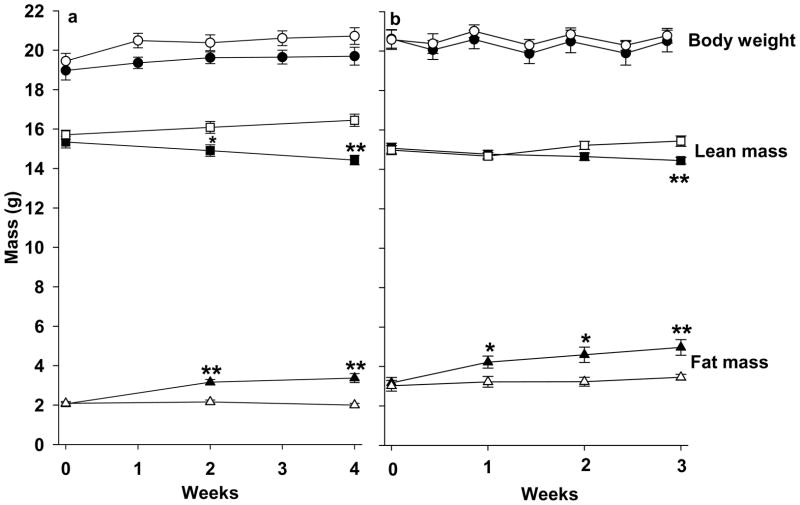
Five-percent CR induced a significant increase in body fat mass (P < 0.01) and a significant decrease in lean mass (P < 0.01), while AL mice remained relatively stable over 4 weeks. Relative to the AL mice, CR mice had a 68.5% greater fat mass (3.37 ± 0.23g vs. 2.00 ± 0.09g, P < 0.01), and a 12.3% lower lean mass (14.43 ± 0.24g vs. 16.45 ± 0.31g, P < 0.01) at the end of the treatment (Fig. 1a). Both CR and AL mice increased their body weight over 4 weeks, while no significant difference was found between the two groups at the end of the experiment (P = 0.11).
Figure 1.
Effects of 4-week (a) and 3-week (b) mild calorie restriction (5%, black symbols) and ad libitum (white symbols) on body weight, body fat, and lean mass. Values are means ± SE (Figure 1a, n=12/group; Figure 1b, n=7 for ad libitum and n=8 for calorie restriction group). *P<0.05; **P<0.01 compared to ad libitum mice at the same time.
Experiment 2. Effect of 5% CR on body weight and composition, energy expenditure, feeding behavior, and locomotor activity over 3 weeks
Body weight, body composition, and fat pad distribution
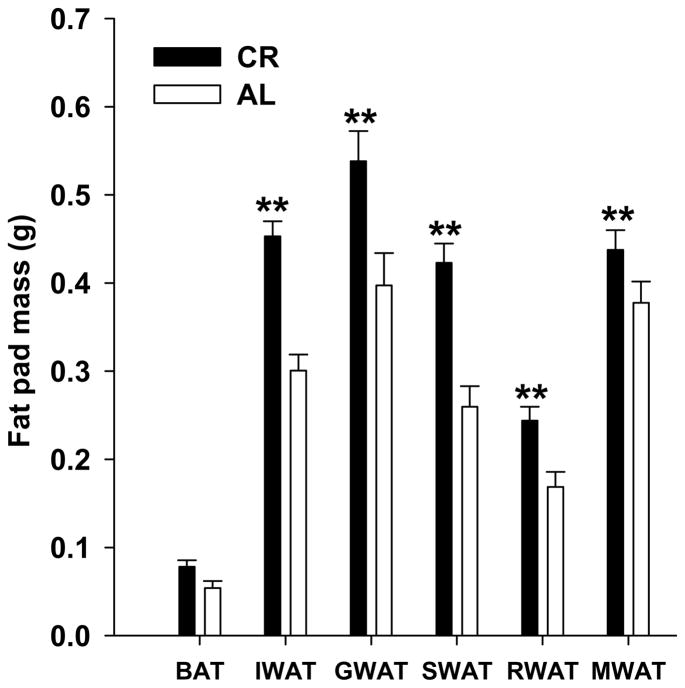
Five-percent CR induced significant changes in body composition without altering body weight. Body weight was relatively stable throughout the experiment in both AL and CR mice (P > 0.05). Relative to AL mice, CR mice showed an increased body fat mass (P < 0.01) and decreased lean mass over 3 weeks. CR mice had a 43.6% greater fat mass (4.97 ± 0.40g vs. 3.46 ± 0.15g, P < 0.01), and a 6.4% lesser lean mass (14.44 ± 0.17g vs. 15.43 ± 0.26g, P < 0.01) than AL mice at the end of the experiment (Fig. 1b). CR mice had significantly more IWAT, GWAT, SWAT, RWAT, and MWAT than AL mice after 3 weeks treatment (P<0.01). In contrast, there was no significant difference in the weight of IBAT between CR and AL mice (P = 0.17, Fig. 2).
Figure 2.
Effects of 3-week mild calorie restriction (5%) on the fat pad mass (BAT, brown adipose tissue; IWAT, inguinal white adipose tissue; GWAT, gonadal white adipose tissues; SWAT, subcutaneous white adipose tissue; RWAT retroperitoneal white adipose tissue, and MWAT, mesenteric white adipose tissue). Values are means ± SE (n=7 for ad libitum and n=8 for calorie restriction group). *P<0.05; **P<0.01 compared with ad libitum mice.
Energy expenditure, serum T3 and UCP1
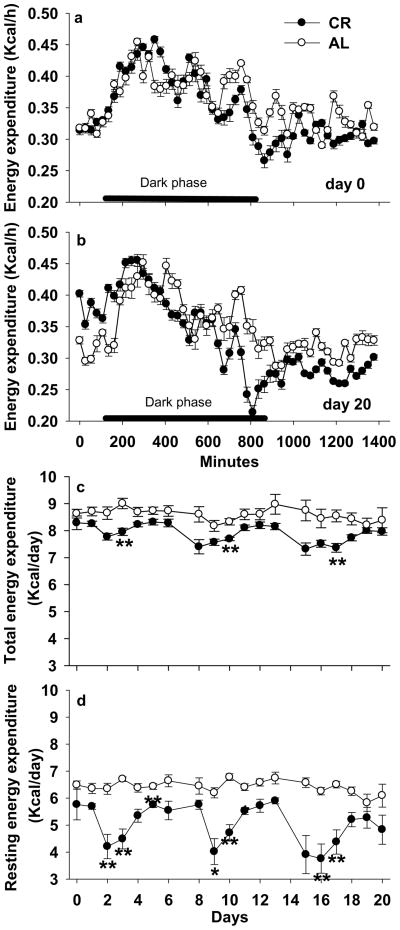
There was no significant difference in the circadian pattern of total energy expenditure (TEE, Kcal/h) at baseline (Day 0, P=0.276) and end of the experiment (Day 20, P=0.810). However, on day 20, TEE was lower in AL mice compared to CR mice in the first 2 hours after food provided (at 1600h, and light off at 1800h), but was lower in CR mice than AL mice in the later dark phase and thereafter.
TEE (Kcal/day, P < 0.05) and resting energy expenditure (REE, Kcal/day, P < 0.05) were significantly decreased in CR mice compared with AL mice over 3 weeks. TEE was 5.0% lower (7.97 ± 0.14 vs. 8.38 ± 0.46 kcal/day) and REE was 20.7% lower (4.83 ± 0.54 vs. 6.09 ± 0.43 kcal/day) in CR mice than AL mice after 3 weeks (Fig. 3). There was no significant difference in mRNA expression of UCP1 from BAT or total serum T3 between CR and AL mice (P > 0.05, Table 1).
Figure 3.
Effects of 3-week mild calorie restriction (5%) on (a) hourly energy expenditure at day 0; (b) hourly energy expenditure at day 20; (C) daily total energy expenditure; and (d) daily resting energy expenditure. Values are means ± SE (n=7 for ad libitum and n=8 for calorie restriction group). *P<0.05; **P<0.01 compared with ad libitum mice at the same time.
Table 1.
Effects of 3-week mild calorie restriction on total serum T3 concentration and mRNA expression.
| 5% calorie restriction | Ad libitum | P value | |
|---|---|---|---|
| Total serum T3 (ng/dL) | 81.95±8.10 | 79.55±8.74 | 0.844 |
| UCP1 mRNA (relative) | 1.135±0.074 | 1.067±0.051 | 0.474 |
Data presented as Mean ± SE.
Feeding behavior and locomotor activity
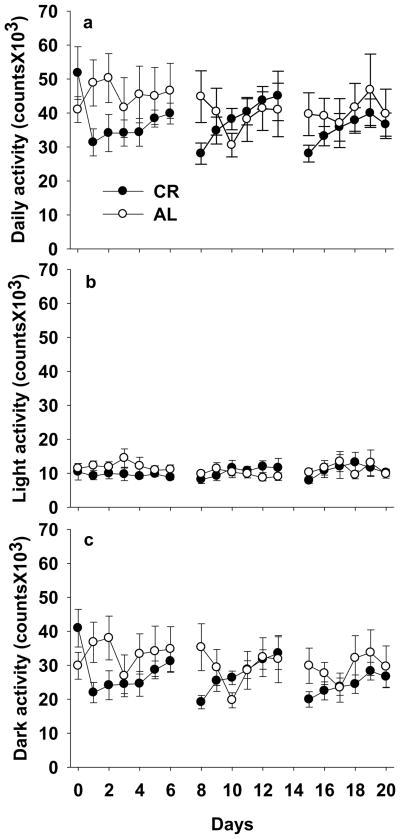
CR induced significant differences in feeding behavior. For two hours after providing of food at 1600 hrs, CR mice ate significantly more food over a significantly greater number of meals (Table 2). Total food consumption during the 12 hours of darkness did not differ until the 3rd week when CR mice consumed significantly less than AL mice (1.85 ± 0.26 vs 2.57 ± 0.16g, P<0.05); however, there was no significant difference in the number of meals consumed. From lights on at 0600 hrs until food provisioning at 1600 hrs, CR mice ate significantly less with dramatically fewer feeding bouts than AL mice (Table 2). Mild CR had no effect on locomotor activity (Fig. 4).
Table 2.
Effects of 3-week mild calorie restriction on daily food intake and number of meals.
| 5% calorie restriction | Ad libitum | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 1 | Week 2 | Week 3 | Baseline | Week 1 | Week 2 | Week 3 | |
| Food intake (g/day) | ||||||||
| Total | 3.59±0.18 | 3.61±0.07 | 3.41±0.08 | 3.21±0.10 | 3.60±0.26 | 3.87±0.13 | 3.64±0.20 | 3.46±0.18 |
| Before dark | 0.31±0.06 | 0.91±0.14** | 1.13±0.21** | 1.33±0.25** | 0.29±0.04 | 0.43±0.03 | 0.42±0.05 | 0.34±0.04 |
| During dark | 2.67±0.17 | 2.64±0.17 | 2.25±0.24 | 1.85±0.26* | 2.56±0.18 | 2.75±0.15 | 2.62±0.20 | 2.57±0.16 |
| After dark | 0.61±0.14 | 0.06±0.03** | 0.03±0.03** | 0.03±0.02** | 0.75±0.10 | 0.69±0.05 | 0.60±0.07 | 0.55±0.08 |
| Daily No. of meals | ||||||||
| Total | 42±2 | 32±2* | 30±3 | 29±3 | 44±3 | 42±3 | 36±2 | 37±4 |
| Before dark | 4±1 | 8.00±1** | 9±1** | 10±1** | 4±0 | 4±0 | 4±0 | 4±1 |
| During dark | 30±2 | 23±3 | 21±3 | 19±3 | 29±3 | 28±2 | 24±1 | 25±2 |
| After dark | 9±1 | 1±1** | 1±1** | 1±0** | 11±1 | 10±1 | 8±1 | 9±2 |
Data presented as Mean ± SE.
P<0.05,
P<0.01 compared between 5% calorie restriction and ad libitum mice at matched time point.
Before dark: from when food was provided at 1600h until 1800h (light off); during dark: from light off (1800h) until 600h next day (light on); after dark: from light on at 600h to 1600h when food was provided.
Figure 4.
Effects of 3-week mild calorie restriction (5%) on daily locomotor activity (a), light activity (b), and dark activity (c). Values are means ± SE (n=7 for ad libitum and n=8 for calorie restriction group). *P<0.05; **P<0.01 compared with ad libitum mice at the same time.
DISCUSSION
In the present study, mild CR (5%) resulted in a significant change in body composition without altering body weight. Relative to AL mice, CR mice had greater fat mass and lower lean mass. CR resulted in a reduction in TEE and REE relative to AL mice. CR induced an alteration of feeding behavior, where CR mice consumed dramatically more food upon food provisioning.
A growing body of literature has demonstrated that moderate or severe CR (30–60%) leads to significant changes in body composition including reduced body fat mass and/or lean mass, with or without weight loss (4–6, 22, 23). For example, 25% CR in humans resulted in a clear decline of body fat mass and fat-free mass after 6 months (5). In 6 month-old mice, 55% CR resulted in a 71% reduction in total fat mass after 6 months (22). However, these changes are age-specific, and the loss of overall weight in aged mice was due primarily to a loss of lean mass, unlike a loss in fat mass in adult mice (22). To our knowledge, there are no reports of the effect of mild CR on body composition in human or rodent models. In the current study, female C57BL/6J mice restricted by 5% showed an unexpected response by increasing fat mass and decreasing lean mass. These changes were confirmed in two independent studies using two methods of body composition analysis, DXA and QMR, and then confirmed at necropsy with dissected fat pad weights.
In the present study, CR mice consumed the vast majority of their food prior to lights on at 0600 hr and had little or no food from 0600 to 1600 hrs. When food was given at 1600 hrs, CR mice consumed nearly four times as much food over a two-hour period compared to AL mice (Table 2; week 3). It is unclear why the 5% CR mice in this study responded by increasing fat mass and decreasing lean mass. Dietz (24) suggested that food choice or physiological adaptations in response to episodic food shortages could cause increased body fat and decreased lean mass in both rodents and humans. Women with normal weight had significantly greater body fat and less lean mass after cyclic dieting (25). However, the association between feeding behavior and body composition is less reported. Possible explanations include the increased fat content of food consumed, binge eating after diet restriction, and disordered eating patterns (e.g. reduced meal frequency) under food restriction conditions (24, 26, 27). It is of interest that we found similar feeding behavior responses in female mice restricted by only 5% (i.e., changes in eating pattern, reduced meal frequency, and binge eating). The association between feeding behavior and fat accumulation in mild CR mice is unknown. However, the gorging behavior observed in CR mice implies that compressing caloric intake into a short time may alter energy partitioning including the changes of digestible or metabolically energy intake, which may result in more effective triglyceride production and storage in fat depots. Moreover, female and male rodents typically play different roles in the survival of the species, and would be expected to respond differently to food scarcity. The response to mild CR in male mice should be tested even though it seems there are no sex-dependent differences in lipid metabolism (28).
Food uncertainty or limitation may also explain by small mammals inhabiting temperate and arctic regions exhibit annual cycles in body weight. Siberian hamsters (Phodopus sungorus) lose body weight in the fall, while collared lemmings (Dicrostonyx groenlandicus) lose weight in spring, when going into periods of food limitation (29, 30). Body composition, including not only body fat, but also lean mass (metabolically active tissues like skeletal muscles), was decreased accompanied with weight loss. These body composition changes in field mammals can be mimicked by altering the photoperiod, even in the presence of ad libitum food (20, 29–31). Lindstedt (1985) hypothesized that a smaller body size reduced the absolute energy requirement by reducing the metabolic rate to compensate for the food limitation (32). In the present study, 5% CR induced a significant decrease in total and resting energy expenditure, which was maintained throughout the restriction period and associated with a significant decrease of lean mass. This was similar to the changes in energy expenditure experienced by seasonal rodents independent of fat mass consideration. Therefore, the accumulation of total body fat in female C57BL/6J mice might result from the adjustment of energy expenditure to meet the stress of uncertain food availability. Further studies are necessary to test this hypothesis either by changing the meal time or by spreading out the food allocation to CR mice to prevent the gorging behavior. A common feature of moderate and severe CR in rats and mice is a decrease in energy expenditure due to the loss of metabolically active tissues, like skeletal muscles and organ tissues (33). Mild CR, in this study, induced decreased lean mass in female C57BL/6J mice after 3 weeks, which might partially explain the reduction of energy expenditure. Unfortunately, we did not determine the makeup of the fat-free mass to see whether it was muscle or organ tissue that was lost. Also, it was not possible to adjust energy expenditure by lean mass as there was no statistically significant relationship between lean mass and TEE or REE.
To further understand the mechanism of the decrease of energy expenditure under mild CR, serum total T3 and UCP1 in BAT, two factors that influence energy expenditure, were investigated. After 3 weeks of mild CR, total serum T3 levels and UCP1 mRNA did not display any significant differences between groups. Weinsier et al. (8) had noted that a decrease of serum T3 occurred during negative energy balance, but did not persist during a period of sustained maintenance of a reduced body weight. The finding that mild CR had no effect on serum T3 differs from previous studies showing that under moderate and severe restriction, serum T3 was reduced in rats (10, 11). BAT plays an important role in the regulation of energy expenditure and body temperature in small mammals, which depends on its specific protein-UCP1. Several studies suggest that moderate or severe CR decreases UCP1 mRNA and protein expression in BAT of rodents, contributing to energy conservation (34, 35). The different response in T3 and UCP1 between mild and severe CR suggests that the decreased energy expenditure in mild CR may not be due to the thyroid hormone metabolism or UCP1 mediated BAT thermogenesis. CR is believed to suppress the sympathetic activity to reduce thermogenesis in BAT (36), and associated with the reduction in body temperature (13, 37). Considering that the reduction in energy expenditure preceded the decreased lean mass, it is possible that the sympathetic activities in mice under mild CR are suppressed, and induce a lower body temperature but was not measured. An important point which arises from the current work is that the UCP1 mRNA was measured at end of the experiment, and considering as index of BAT thermogenesis, which might not reflect the chronic condition and feeding cycle very well.
CR could lead to an alteration of body fat mass or lean mass by changes not only in metabolic rate, but also due to changes in energy expenditure induced by changes in locomotor activity. The present study did not show any differences in locomotor activity, which agrees with other studies in mice and rats (6, 17, 38). Evans et al (2005) found that male FBNF1 rats did not show any difference in locomotor activity between AL group and a weight maintenance group (5% CR) (17). Twenty-five percent (25%) CR did not induce significant changes in physical activity after 3 or 6 months in humans (39). Three weeks of mild CR did not induce any changes in locomotor activity or activity during light or dark, which means that these animals did not reduce their locomotor activity to decrease energy expenditure in response to mild food restriction. Besides the reduction of activity and adaptation in BAT thermogenesis, CR induced torpor may be another possibility to conserve energy in mice. The lean mice restricted by 30–40% for 12–14 weeks displayed daily torpor phenomena, and was associated with a remarkable decrease in rectal temperature (40). Similarly, Overton et al. (2004) verified torpor could be induced by a single night of fasting or a few days CR (13). In the present studies, we found a remarkable decrease of energy expenditure in dark phase before lights on (around 500–600h). It can not be excluded whether torpor existed at that time period because body temperature was not measured.
CONCLUSIONS AND PERSPECTIVE
Mild CR altered body composition, energy expenditure, and meal patterns in female C57BL/6J mice. The increase in fat and decrease in lean mass may be a stress response to uncertain food availability. Locomotor activity and BAT thermogenesis do not appear to contribute to the decreased energy expenditure in this study. Some aging studies use 5% CR as their control group to maintain body weight and food intake. Many of the life-extending benefits of severe CR are associated with a reduction in fat stores. Whether fat accumulation under mild CR is linked to accelerated aging and related pathologies such as cancer or diabetes remains to be investigated. Further research is needed to determine if the accumulation of body fat was caused by the food reduction or the timing of food provision.
Acknowledgments
This work was supported by the UAB Small Animal Phenotyping Core of the Clinical Nutrition Research Unit (P30DK56336), the Neuroscience Blueprint Center Grant (P30NS057098), the Diabetes Research and Training Grant (P60DK079626), and the Center for Metabolic Bone Disease (P30AR046031. MBC and DLS were supported by F32DK064532. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
Reference List
- 1.Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132(2):171–6. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127(1):1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337(14):986–94. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escriva F, Gavete ML, Fermin Y, et al. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol. 2007;194(1):131–41. doi: 10.1677/joe.1.07043. [DOI] [PubMed] [Google Scholar]
- 5.Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92(3):865–72. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J Gerontol A Biol Sci Med Sci. 2006;61(8):781–94. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- 7.Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5(1):13–9. doi: 10.1111/j.1467-789x.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 8.Weinsier RL, Nagy TR, Hunter GR, Darnell BE, Hensrud DD, Weiss HL. Do adaptive changes in metabolic rate favor weight regain in weight-reduced individuals? An examination of the set-point theory. Am J Clin Nutr. 2000;72(5):1088–94. doi: 10.1093/ajcn/72.5.1088. [DOI] [PubMed] [Google Scholar]
- 9.Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech Ageing Dev. 2005;126(6–7):783–93. doi: 10.1016/j.mad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91(8):3232–5. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 11.Passadore MD, Griggio MA, Nunes MT, Luz J. Effects of ageing on the energy balance of food-restricted rats. Acta Physiol Scand. 2004;181(2):193–8. doi: 10.1111/j.1365-201X.2004.01281.x. [DOI] [PubMed] [Google Scholar]
- 12.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 13.Overton JM, Williams TD. Behavioral and physiologic responses to caloric restriction in mice. Physiol Behav. 2004;81(5):749–54. doi: 10.1016/j.physbeh.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Physiol Behav. 2008 doi: 10.1016/j.physbeh.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rising R, Lifshitz F. Energy expenditures & physical activity in rats with chronic suboptimal nutrition. Nutr Metab (Lond) 2006;3:11. doi: 10.1186/1743-7075-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–60. doi: 10.1146/annurev.nutr.25.050304.092526. [DOI] [PubMed] [Google Scholar]
- 17.Evans SA, Parsons AD, Overton JM. Homeostatic responses to caloric restriction: influence of background metabolic rate. J Appl Physiol. 2005;99(4):1336–42. doi: 10.1152/japplphysiol.01380.2004. [DOI] [PubMed] [Google Scholar]
- 18.Nagy TR, Krzywanski D, Li J, Meleth S, Desmond R. Effect of group vs. single housing on phenotypic variance in C57BL/6J mice. Obes Res. 2002;10(5):412–5. doi: 10.1038/oby.2002.57. [DOI] [PubMed] [Google Scholar]
- 19.Jurgens HS, Schurmann A, Kluge R, et al. Hyperphagia, lower body temperature, and reduced running wheel activity precede development of morbid obesity in New Zealand obese mice. Physiol Genomics. 2006;25(2):234–41. doi: 10.1152/physiolgenomics.00252.2005. [DOI] [PubMed] [Google Scholar]
- 20.Powell CS, Blaylock ML, Wang R, Hunter HL, Johanning GL, Nagy TR. Effects of energy expenditure and Ucp1 on photoperiod-induced weight gain in collard lemmings. Obes Res. 2002;10(6):541–50. doi: 10.1038/oby.2002.73. [DOI] [PubMed] [Google Scholar]
- 21.He X, Schoeb TR, Panoskaltsis-Mortari A, et al. Deficiency of P-selectin or P-selectin glycoprotein ligand-1 leads to accelerated development of glomerulonephritis and increased expression of CC chemokine ligand 2 in lupus-prone mice. J Immunol. 2006;177(12):8748–56. doi: 10.4049/jimmunol.177.12.8748. [DOI] [PubMed] [Google Scholar]
- 22.Colman RJ, Nam G, Huchthausen L, Mulligan JD, Saupe KW. Energy restriction-induced changes in body composition are age specific in mice. J Nutr. 2007;137(10):2247–51. doi: 10.1093/jn/137.10.2247. [DOI] [PubMed] [Google Scholar]
- 23.Racette SB, Weiss EP, Villareal DT, et al. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61(9):943–50. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietz WH. Does hunger cause obesity? Pediatrics. 1995;95(5):766–7. [PubMed] [Google Scholar]
- 25.Manore MM, Berry TE, Skinner JS, Carroll SS. Energy expenditure at rest and during exercise in nonobese female cyclical dieters and in nondieting control subjects. Am J Clin Nutr. 1991;54(1):41–6. doi: 10.1093/ajcn/54.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Chaput JP, Gilbert JA, Tremblay A. Relationship between food insecurity and body composition in Ugandans living in urban Kampala. J Am Diet Assoc. 2007;107(11):1978–82. doi: 10.1016/j.jada.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Townsend MS, Peerson J, Love B, Achterberg C, Murphy SP. Food insecurity is positively related to overweight in women. J Nutr. 2001;131(6):1738–45. doi: 10.1093/jn/131.6.1738. [DOI] [PubMed] [Google Scholar]
- 28.Martin B, Pearson M, Kebejian L, et al. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148(9):4318–33. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heldmaier G, Steinlechner S. Seasonal control of energy requirements for thermoregulation in the Djungarian hamster (Phodopus sungorus), living in natural photoperiod. J Comp Physiol B. 1981;142:429–37. [Google Scholar]
- 30.Nagy TR, Gower BA, Stetson MH. Endocrine correlates of seasonal body mass dynamics in the collared lemmings (Dicrostonyx groenlandicus) Amer Zool. 1995;35:246–58. [Google Scholar]
- 31.Bartness TJ, Demas GE, Song CK. Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med (Maywood ) 2002;227(6):363–76. doi: 10.1177/153537020222700601. [DOI] [PubMed] [Google Scholar]
- 32.Lindstedt SL. Seasonality, fasting endurance, and body size in mammals. Am Nat. 1985;125:873–8. [Google Scholar]
- 33.Nelson KM, Weinsier RL, Long CL, Schutz Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56(5):848–56. doi: 10.1093/ajcn/56.5.848. [DOI] [PubMed] [Google Scholar]
- 34.Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab. 2007;293(1):E316–E326. doi: 10.1152/ajpendo.00710.2006. [DOI] [PubMed] [Google Scholar]
- 35.Valle A, Catala-Niell A, Colom B, Garcia-Palmer FJ, Oliver J, Roca P. Sex-related differences in energy balance in response to caloric restriction. Am J Physiol Endocrinol Metab. 2005;289(1):E15–E22. doi: 10.1152/ajpendo.00553.2004. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell NJ, Stock MJ. Effect of chronic food restriction on energy balance, thermogenic capacity, and brown-adipose-tissue activity in the rat. Biosci Rep. 1982;2(8):543–9. doi: 10.1007/BF01314214. [DOI] [PubMed] [Google Scholar]
- 37.Duffy PH, Feuers RJ, Leakey JA, Nakamura K, Turturro A, Hart RW. Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev. 1989;48(2):117–33. doi: 10.1016/0047-6374(89)90044-4. [DOI] [PubMed] [Google Scholar]
- 38.Santos-Pinto FN, Luz J, Griggio MA. Energy expenditure of rats subjected to long-term food restriction. Int J Food Sci Nutr. 2001;52(2):193–200. [PubMed] [Google Scholar]
- 39.Martin CK, Heilbronn LK, de JL, et al. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007;15(12):2964–73. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 40.Himms-Hagen J. Food restriction increases torpor and improves brown adipose tissue thermogenesis in ob/ob mice. Am J Physiol. 1985;248(5 Pt 1):E531–E539. doi: 10.1152/ajpendo.1985.248.5.E531. [DOI] [PubMed] [Google Scholar]