Abstract
Developing tissue engineering approaches to generate functional vascular networks is important for improving treatments of peripheral and cardiovascular disease. Endothelial colony forming cells (ECFCs) are an endothelial progenitor cell (EPC) population defined by high proliferative potential and an ability to vascularize collagen-based matrices in vivo. Little is known regarding how physical properties of the local cell microenvironment guide vessel formation following EPC transplantation. In vitro evidence suggests that collagen matrix stiffness may modulate EPC vessel formation. The present study determined the ability of 3D collagen matrix physical properties, varied by changing collagen concentration, to influence ECFC vasculogenesis in vivo. Human umbilical cord blood ECFCs were cultured within matrices for 18 h in vitro and then fixed for in vitro analysis or implanted subcutaneously into the flank of immunodeficient mice for 14 days. We report that increasing collagen concentration significantly decreased ECFC derived vessels per area (density), but significantly increased vessel sizes (total cross sectional area). These results demonstrate that the physical properties of collagen matrices influence ECFC vasculogenesis in vivo and that by modulating these properties, one can guide vascularization.
Keywords: Collagen, ECM (extracellular matrix), vascular graft, mechanical properties, endothelial progenitor cell (EPC), in vivo
Introduction
Development of functional vascular networks is not only important for treatment of diabetes, peripheral vascular disease, and cardiovascular disease, but also a major limiting factor associated with current tissue engineering strategies targeting repair and regeneration of damaged or diseased tissue (Jain et al. 2005; Kawamoto and Asahara 2007). In fact, there are no tissue-engineered constructs presently available that have an inherent vascular network ready to be connected to the host vascular system (Kannan et al. 2005). Endothelial progenitor cells (EPCs) have been recently identified as a cell population that is naturally released into the circulation and homes to areas undergoing vessel formation in vivo (Kawamoto and Asahara 2007). Furthermore, these cells have the ability to integrate into injured or disease sites including tumors, ischemic skeletal and cardiac muscle, and ulcers (Asahara et al. 1999; Mund et al. 2009).
Developing cell delivery systems is necessary for improving targeted, localized, vasculogenic therapies. Localized cell delivery via biomaterial carriers that mimic the extracellular matrix (ECM) has been shown to improve cell survival and retention leading to improved revascularization and decreased cell number requirements (Chavakis et al. 2005; Suuronen et al. 2006; Jiang et al. 2008). In addition, biomaterial-based cell delivery systems (including implantable tissue engineered grafts) could provide microenvironmental cues to guide vessel formation and to serve as a template for tissue regeneration (Suuronen et al. 2006; Silva et al. 2008). However, little is known regarding how relevant ECM physical (structural, mechanical) properties can influence vascular cell behavior and vessel formation, which limits their use in the design of such cell delivery strategies.
Endothelial progenitor cells (EPC) are a cell population released into circulation to participate in vessel formation in both physiological and pathological settings and offer great potential as a cell source for vasculogenic therapies (Asahara et al. 1997; Ingram et al. 2005). Recent evidence suggests that the long-term vessel forming EPC, called endothelial colony forming cells (ECFC), are derived from a rare circulating subset and from resident endothelium of established blood vessels in man (reviewed in (Hirschi et al. 2008)). A variety of hematopoietic cell subsets also participate in neoangiogenesis and often are included with the EPC terminology (reviewed in (Hirschi et al. 2008)). In vitro investigations into the role of endothelial cells (EC) in vasculogenesis have found that the process is regulated by dynamic interactions with the collagen fibril component of the ECM (Ingber and Folkman 1989; Bell et al. 2001). Integrin binding of EC to tissue substrate, EC-matrix adhesion, collagen fibril contraction and reorganization, and ECM degradation (proteolysis) and deposition, have all been shown to serve a role in vessel formation (Hynes et al. 1999; Bell et al. 2001; Davis et al. 2002; Stupack and Cheresh 2002; Davis and Saunders 2006). This suggests that altering cell-ECM interactions through altered ECM physical properties may serve as a potential mechanism for cell delivery systems to modulate vessel formation. However, only a few in vitro studies have directly investigated how local 3D matrix (representing simplified 3D ECM) physical properties affect vessel formation.
In vitro investigations in simplified 3D matrices prepared from natural (e.g. collagen or fibrin) or synthetic biopolymers (e.g. poly-L-lactide acid oligopeptides) have demonstrated that matrix physical properties, such as apparent matrix stiffness and fibril or ligand density, can significantly alter EPC and differentiated EC vessel formation (Korff and Augustin 1999; Vailhe et al. 2001; Sieminski et al. 2004; Sieminski et al. 2007; Ghajar et al. 2008; Krishnan et al. 2008). In particular, RGD mediated, integrin-dependent binding to collagen fibrils supports the development of sufficient matrix-transduced tensional forces necessary for EC sprouting and tube formation (Korff and Augustin 1999). This also exemplifies the difficulty of discerning the mechanisms by which matrix physical properties induce EPC/EC response, for example in differentiating between biochemical and biophysical signaling arising from collagen-integrin binding (Ruegg and Mariotti 2003; Larsen et al. 2006). The stiffness of self-assembled matrices affects cell-matrix force balance and cell spreading, migration, and matrix remodeling, all of which are critical components of vessel formation (Salazar et al. 1999; Sieminski et al. 2007). Varying collagen concentration of polymerized 3D collagen matrices, which alters matrix stiffness and fibril density, has been shown to alter EC lumen size and tube length (Sieminski et al. 2004; Yamamura et al. 2007; Krishnan et al. 2008). These results provide initial evidence that specific collagen matrix physical properties are important parameters determining the ability of matrices to guide vessel formation. However, these in vitro studies have not been followed up with investigations addressing whether these matrix physical properties impact vessel formation in vivo, which are necessary for translation to clinical applications.
The present study tested whether collagen matrix physical properties known to effect EC vessel formation in vitro, namely collagen fibril density and stiffness, had significant effects on the formation of functional blood vessels by EPCs in vivo. We used an established in vivo transplantation model (Schechner et al. 2000; Enis et al. 2005; Melero-Martin et al. 2007; Yoder et al. 2007; Au et al. 2008), where ECFC, a specific high proliferative EPC population (Ingram et al. 2004), were cultured within 3D collagen matrices prior to transplantation into the flank of NOD/SCID mice. This model was also used previously to define the vasculogenic potential of ECFC (Yoder et al. 2007). Changing the physical properties of the 3D collagen matrices was found to significantly affect the density and morphology (cross-sectional area) of ECFC derived blood vessels within the implants. Importantly, this work demonstrates the capacity of ECM-based cell delivery systems to incorporate physical cues (in addition to biochemical cues) for guiding vessel formation in vivo.
Materials and methods
Culture of ECFCs
Human umbilical cord blood ECFC, representing pooled populations from 3 patients, were obtained from Endgenitor Technologies, Inc (Indianapolis, IN) and cultured as previously described (Ingram et al. 2004). ECFC were used between passage 6 and 8 for all experiments.
Polymerization of 3D collagen matrices
Cellularized collagen matrix implants were cast as previously described with minor modifications (Schechner et al. 2000). ECFC (2 × 106 cells/ml) were suspended in a solution of 0.5 to 3.5 mg/ml (final collagen concentration) rat tail type I collagen (BD Biosciences, Bedford, MA), 100 ng/ml human fibronectin (Millipore, Temecula, CA), 1.5 mg/ml sodium bicarbonate (Sigma-Aldrich, St. Louis, MO), 10% FBS, 25 mM HEPES, 30% complete EGM-2, and EBM-2 (Lanza, Walkersville, MD). Collagen-cell suspensions were kept at 4°C during mixing and pH adjusted to 7.4 using 1 M NaOH. Collagen-cell suspensions were pipetted into wells of 12-well plates (1 ml/well), allowed to polymerize at 37°C for 30 min, and covered with complete EGM-2 for overnight incubation at 37°C, 5% CO2.
Microstructural analysis of 3D collagen matrices
Confocal reflection microscopy (CRM) was used to visualize the 3D fibril microstructure of collagen matrices (Voytik-Harbin 2001). Matrices were polymerized in Lab-Tek chambered coverglass slides (Nunc, Thermo Fisher Scientific, Rochester, NY), overlaid with phosphate buffered saline (PBS), and imaged on an Olympus Fluoview FV1000 confocal system adapted to an Olympus 1X81 inverted microscope with a 60X UPlanSApo water immersion objective (Olympus, Tokyo, Japan). Images were collected from 5 random locations (20 μm off coverglass) of 2 independent matrices (n = 10 images). Fibril volume fraction, a measure of fibril density, was calculated as the percentage of fibril voxels (size 0.1 × 0.1 × 0.1 μm3) to total image voxels for each image using Matlab (Mathworks, Natick, MA) as described previously (Kreger and Voytik-Harbin 2009). Fibril voxels were determined by applying a threshold value chosen mathematically as the center of the concave bend in the sigmoidal decay curve of fibril volume fraction versus threshold value for that image. From original CRM images, fibril diameters of 16 fibrils (random, but spaced equally apart) were measured in 3 images for each matrix formulation (n = 48 fibrils) using Imaris software (Bitplane Inc, St. Paul, MN).
Mechanical analysis of 3D collagen matrices
Viscoelastic properties were determined for each collagen matrix with and without ECFCs (2X106 cells/ml) using an AR-2000 rheometer (TA Instruments, New Castle, DE) adapted with a 40 mm diameter parallel plate geometry, humidity trap, and peltier heater in the base plate. Samples were polymerized on the rheometer for 30 min at 37°C and matrix shear modulus was measured in oscillatory shear at 1% strain, 1 Hz (predetermined from linear viscoelastic regions). Shear modulus was decomposed into phase components: shear storage modulus (G′) and shear loss modulus (G″), which are related by the phase shift (δ, tan δ = G″/G′). Matrix compressive behavior was then measured by compressing (unconfined) matrices with the plate geometry at a rate of 10 μm/s. Stress-strain plots were generated, where compressive strain was calculated as 1 − L/L0 (L = height and L0 = initial height) and stress was calculated as normal force divided by plate area. Compressive modulus (Ec) was calculated using linear regression as the slope of the stress-strain curve from 0 to 5% strain. Mechanical analysis was performed on 3–5 independent matrices per matrix formulation.
Confocal Imaging of cellularized collagen matrices following in vitro culture
Cellularized collagen matrices were cultured in vitro for 18 h and then fixed with 4% paraformaldehyde. Matrices were stained overnight with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen, Carlsbad, CA), to label nuclei, and fluorescein isothiocyanate (FITC) conjugated Ulex europeus type I agglutinin (UEA-1) lectin (Sigma-Aldrich), to label cell membranes. Matrices were then washed with PBS and bisected. 3D images were collected using confocal microscopy.
Transplantation of cellularized collagen matrices
Cellularized collagen matrices were implanted into the flanks of 6–8 week old NOD/SCID mice as previously described (Yoder et al. 2007). Briefly, cellularized collagen matrices were cultured in vitro for 18 h, bisected, and each half (0.5 ml) was implanted into a different mouse and opposite flank (left or right, 2 matrices per mouse). Six to ten matrices were implanted for each treatment group (n = 6–10) with mouse gender and site (e.g. right/left flank) randomized. After 14 days, mice were euthanized and matrices were harvested and fixed in formalin free zinc fixative (BD Biosciences). Explants were then paraffin embedded, bisected along major axis, and sectioned (6 μm thick) for immunohistochemical analysis. All animal protocols were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Immunohistochemistry of explanted matrices
Sections were stained to differentiate human ECFC from host (mouse) cells as previously described (Yoder et al. 2007). Briefly paraffin-embedded tissue sections were deparaffinized and immersed in retrieval solution (Dako, Carpenteria, CA) for 20 min at 95–99°C. Slides were incubated at room temperature with anti-human CD31 (hCD31, clone JC70A, Dako, Carpinteria, CA) followed incubation with Labeled Streptavidin-Biotin2 System, Horseradish Peroxidase (LSAB2 System, HRP, Dako), then developed with 3,3′-diaminobenzidine (DAB, Dako) solution. Slides were counter-stained with hematoxylin.
Analysis of ECFC vessel formation
The number of ECFC (hCD31+) and host (hCD31−) blood vessels, defined as endothelial lined spaces filled with red blood cells (RBC), were counted from explant sections. Blood vessel profiles were obtained for a total of sixteen sections and accounted for approximately 20–25% of the explant volume. All sections were randomly selected from the middle portions of explants to bias for ECFC formed vessels. For vessel area measurements, at least 40 hCD31+ blood vessels were imaged from each explant using a Leica DM 4000B microscope (Leica Microsystems, Bannockburn, IL) with attached Spot-KE digital camera (Diagnostic Instruments, Sterling Heights, MI). Explant matrix size and vessel areas were measured using Metamorph (Molecular Devices, Sunnyvale, CA). Total vascular area, an indication of the vessel area fraction, was calculated as (number of hCD31+ vessels)*(average hCD31+ vessel area) for each explant.
Statistical analysis
All values are presented as mean ± standard error (SE). Statistical significance between groups was determined by Student’s t-test (pooled, two-tail comparisons) using SAS software (SAS Institute Inc, Cary, NC). A value of p<0.05 was considered significant.
Results
Characterization of matrix physical properties
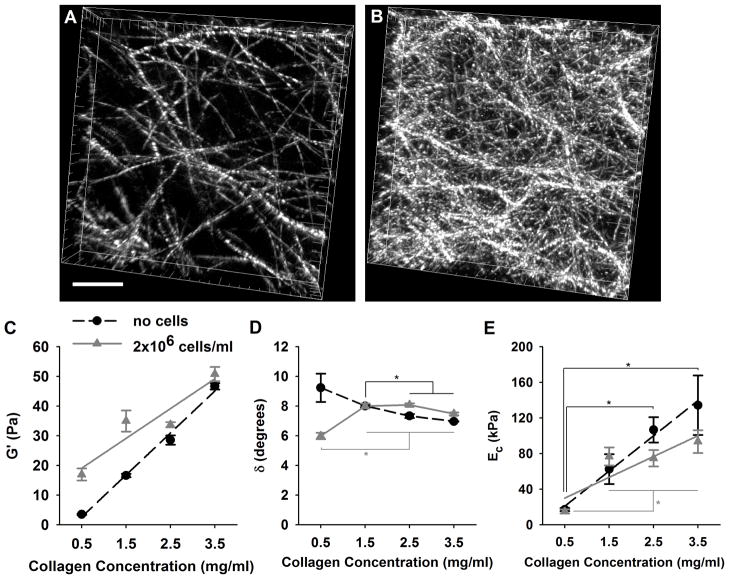
Collagen concentration of the polymerized reactions was varied to systematically modulate matrix physical properties (e.g. fibril density and stiffness) known to affect in vitro EPC vessel formation. CRM analysis showed that varied collagen concentration significantly altered local 3D fibril microstructure (Fig. 1A,B). Fibril volume fraction (fibril density, FD) calculated from CRM images increased linearly with increasing collagen concentration (C) and could be described by the following equation FD = 2.59C+7.37 (R2=1). Average fibril diameter did not change significantly with collagen concentration (396 ± 53 nm, mean ± standard deviation across all concentrations). Mechanical testing of the matrices in oscillatory shear showed that matrix G′ was positively correlated with the collagen concentration and therefore fibril density (Fig. 1C). G′ varied as a function of fibril density as described by the equation G′ = 14.14FD – 4.45 (R2 = 0.991). Small G″ and δ indicated that matrix shear response was dominated by the solid collagen fibril phase of the matrices (Fig. 1D). Interestingly, δ showed a modest but linear decrease with collagen concentration as described by δ = −0.75C + 9.39 (R2 = 0.936).
Fig. 1.
3D collagen matrix physical properties varied with collagen concentration. Fibril density increased linearly with collagen concentration as shown in 3D CRM images of 0.5 (A) and 2.5 (B) mg/ml matrices (scale bar = 10 μm). Shear storage modulus (G′ lines = linear regression trend lines), increased with increasing collagen concentration (p<0.05 between concentrations, n=3–5 for mechanical analyses) and with the addition of ECFCs (2 × 106cells/ml, gray lines, p<0.05 at each concentration except 3.5 mg/ml). Matrix fluid-like behavior, indicated by δ (D), was significantly affected by collagen concentration (* denotes p<0.05 within cell groups) and ECFC addition (p<0.05 at each concentration except 1.5 mg/ml). Matrix Ec (E) similarly increased with collagen concentration (* denotes p<0.05 within cell groups), but did not change significantly with ECFC addition (p>0.05 at all concentrations).
Similar to G′, Ec (indicative of compressive resistance) increased with increasing collagen concentration (Fig. 1E). This suggests that the increased fibril density restricted interstitial fluid flow (increased hydraulic resistance). The relationship between Ec and fibril density could be described as Ec = 15.27FD – 111.32 (R2 = 0.9887).
To evaluate whether the addition of the ECFC altered matrix mechanical properties, tests were repeated with matrices containing (2 × 106 ECFC/ml, gray lines Fig. 1C–D). Cell addition significantly affected G′ but not EC (p < 0.05). With cells, G′ significantly increased at each collagen concentration except 3.5 mg/ml (p=0.2), suggesting that the effect diminished with increasing concentration and mechanical integrity. δ was also significantly affected for low fibril density, 0.5 mg/ml matrices. These results may be explained by the cells acting to alter fibril-fibril interactions (e.g. fibril-fibril connectivity formed by adherence of cells to multiple fibrils) in the cell-collagen composite material. Importantly, ECFC addition did not disrupt the ability of varied collagen concentration to vary matrix biophysical properties, thus demonstrating embedded ECFC were still exposed to similar changes in matrix stiffness and fibril density mediated by the varying collagen concentrations.
In summary, a systematic increase in the collagen concentration of the polymerization reaction yielded matrices with increased fibril density as well as increased matrix stiffness, as related by G′ or Ec. Matrices prepared at different collagen concentrations therefore present cells with significantly different physical microenvironments (Table 1).
Table 1.
Summary of collagen matrix physical properties.
| Collagen Concentration (mg/ml) | Fibril Volume Fraction (%) | G′ (Pa) | δ (degrees) | Ec (kPa) |
|---|---|---|---|---|
| 0.5 | 8.65 ± 0.29 | 3.50 ± 0.17 | 9.24 ± 0.95 | 17.17 ± 1.71 |
| 1.5 | 11.25 ± 0.47 | 16.64 ± 0.57 | 8.00 ± 0.17 | 62.51 ± 16.80 |
| 2.5 | 13.83 ± 0.60 | 28.54 ± 1.57 | 7.33 ± 0.22 | 106.63 ± 14.28 |
| 3.5 | 16.42 ± 0.61 | 46.67 ± 1.06 | 6.96 ± 0.03 | 134.25 ± 33.35 |
ECFC vessel formation in vitro
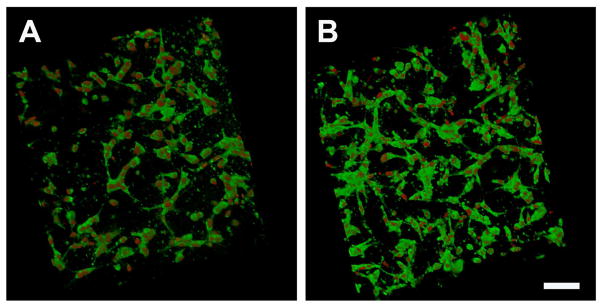
To characterize how ECFC responded to the difference collagen-fibril microenvironments, cellularized matrices were cultured for 18 hours and analyzed using confocal microscopy. In general, the ECFC response at the time of implantation was similar for all matrix microenvironments (Fig. 2). It should be noted that the ECFC cell densities measured within the low and high concentration matrices after 18 hours of culture were not statistically different (data not shown).
Fig. 2.
Confocal analysis of ECFC morphology within 3D collagen matrices with varied physical properties after 18 hours of in vitro culture and immediately prior to subcutaneous implantation. Cellularized matrices were labeled with DAPI (red), and UEA-1 Lectin (green) for visualization of nuclei and ECFCs, respectively. ECFC showed cord like networks but no apparent lumen formation in any collagen matrix formulation at this time point. While low concentration matrices (A, 0.5 mg/ml) appear to have a lower cell density compared to higher concentration matrices (B, 3.5 mg/ml, Scale bar A,B = 100 μm) the cell number is not different between the two conditions.
ECFC vessel formation in vivo
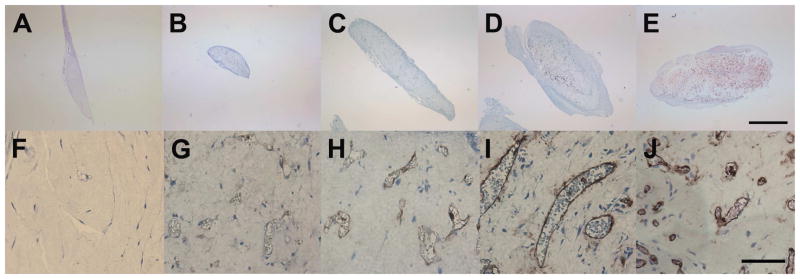
ECFC seeded matrices were implanted into the flank of NOD/SCID mice for 14 days. Matrices were then harvested and investigated for remodeling and blood vessel formation. In most cases, matrices were adherent to the underlying muscular tissue and not to the sub-dermal fascia. Gross observation indicated that, implanted matrices remodeled to a different extent depending on the fibril microstructure mechanical properties of the implant, with lower concentration matrices compacting to smaller sizes (Fig. 3). Explanted matrices were surrounded by a fibrous layer (capsule) of circumferentially oriented soft tissue (host connective and muscle tissues). This capsule was used to visually define the outer edge of the matrix for quantitative analysis. In some cases, ECFC (hCD31+) and chimeric (hCD31+ and hCD31−) vessels were observed outside of the matrix. While these vessels were not included in analysis, they indicate ECFC migration and/or proliferation occurs during the active anastomosis and vessel formation processes.
Fig. 3.
Histochemical analysis of explanted ECFC matrices. Matrices, removed after 14 days, remodeled to a different extent dependent on collagen concentration. Representative sections (of n≥6 implants in different mice) are shown for 0.5 (B,C), 1.5 (C,H), 2.5 (D,I), and 3.5 (E,J) mg/ml matrices (scale bars A–E = 1 mm, F–J = 250 μm). Lower concentration matrices contracted and degraded to a greater degree than higher concentration matrices (A–D). All matrices, except no cell controls (0.5 mg/ml A,F), were able to direct ECFCs to form functional hCD31+ blood vessels which contained RBCs.
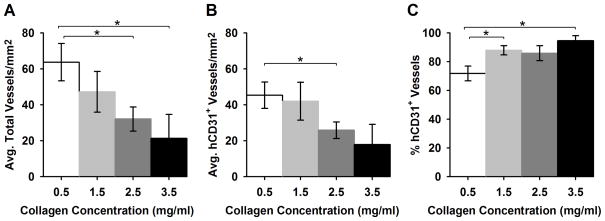
While ECFC functional blood vessels were observed in all ECFC containing matrices, the densities and morphologies of the vessels appeared to vary depending on the collagen concentration or matrix physical properties of the implant (Fig. 3 bottom row). Average total (ECFC and host) vessels per area and ECFC (hCD31+) vessels per area decreased significantly with increasing matrix collagen concentration from 63.7 ± 10.4 and 45.3 ± 7.3 vessels/mm2 at 0.5 mg/ml to 21.3 ± 13.4 and 17.9 ± 11.3 vessels/mm2 at 3.5 mg/ml, respectively (Fig. 4). Interestingly, the percentage of hCD31+ vessels increased with increasing matrix concentration from a mean of 71% to 84% of the total vessels measured. This may relate to the differences in the extent of implant remodeling and thus, total size of the implant. Additionally, control matrices (no ECFC) did not contain host blood vessels, indicating ECFC were required for vessel formation under the conditions employed in the present studies.
Fig. 4.
Quantification of RBC-containing vessel density within explanted ECFC matrices. Average total vessels per area (A) and hCD31+ vessels per area (B) decreased with increasing collagen concentration (* denotes p<0.05 between groups). Interestingly, the percentage of hCD31+ vessels (out or the total ECFC and chimeric vessels) increased with increasing concentration (C).
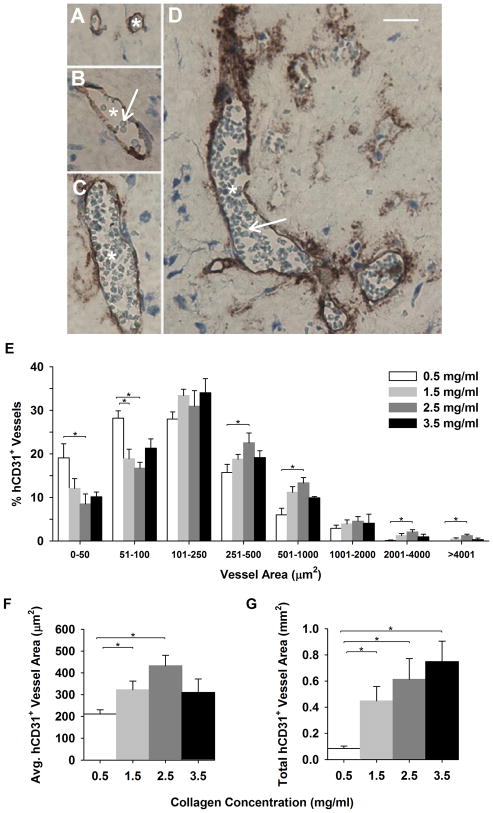
To determine the effect of matrix concentration and physical properties on vessel morphology, vessel areas were measured (Fig 5). At low collagen concentration (0.5 mg/ml), matrices exhibited hCD31+ vessels with smaller average areas compared to 1.5 and 2.5 mg/ml matrices (Fig. 5A). This was reflected in a shifted population distribution of hCD31+ vessel areas (e.g. 0.5 mg/ml collagen implants displayed a significant increase in vessels with a smaller area and less vessels with larger area than implants comprised of 2.5 mg/ml collagen, Fig. 5C) and resulted in significantly smaller mean vessel area (p < 0.05, Fig. 5A). To account for the differences in matrix compaction and vessel density and morphology, total hCD31+ vascular area was calculated (= avg. vessel density * avg. vessel area) and was found to increase with collagen concentration. Importantly, this indicates that vessel density measurement alone is not a sufficient measure of vessel formation, as it may not reflect changes in the size of the implant and the vessels following matrix and vessel remodeling in the host.
Fig. 5.
Analysis of RBC-containing vessels areas within explanted ECFC matrices. Light micrographs of explant histological sections show representative hCD31+ vessels from multiple matrices with different areas between 51 and 100 μm2 (A), between 501 and 1000 μm2 (B), between 1001 and 2000 μm2 (C), and greater than 4000 μm2 (D, scale bar A–D = 100 μm, A–D arrows –RBCs, asterisks -hCD31+ vessels). The distribution of hCD31+ vessel areas (E), average hCD31+ vessel area (F), and total hCD31+ vascular area (G) were measured for each collagen concentration (*denotes p<0.05 between groups). Vessel morphology was significantly altered by matrix collagen concentration, with increasing concentration shifting towards increased average areas and total vessel areas.
Discussion
Current strategies involving direct injections of EPCs and other stem/progenitor cells into tissues or the circulation have lacked efficacy largely owing to the limited survival of cells, inefficient and inconsistent retention of cells following injection into tissues, and lack of a suitable cellular microenvironment to modulate differentiation and vessel formation (March and Johnstone 2004). One mechanism to improve cell based therapies is to design biomaterial carriers with relevant biochemical and biophysical features that direct vessel formation in a predictable and reproducible fashion. While little is known regarding how specific physical properties of biomaterial correlate with EPC vessel formation in vivo, in vitro studies have shown certain physical properties of collagen matrices, namely fibril density and stiffness, can influence vessel formation (Korff and Augustin 1999; Sieminski et al. 2004; Yamamura et al. 2007). In this study, we have shown that altering the physical properties of polymerized collagen-fibril matrices influence ECFC blood vessel formation in vivo using an established animal transplant model. More specifically, collagen fibril density and matrix stiffness, varied by changing collagen concentration, were found to affect implant remodeling (contraction and biodegradation) as well as ECFC (hCD31+) vessel density, proportion of ECFC to host vessels (% hCD31+), and blood vascular areas. A unique finding was that while cellularized matrices produced at increasing collagen concentration showed a decrease in overall vessel density, an increase in average vessel area and total vascular area was observed, suggesting the potential for increased blood perfusion. These findings demonstrate that matrix physical properties may directly impact ECFC-mediated vascularization and therefore represent an important design criterion for cell delivery strategies.
The design of microenvironments (niches) for guiding cell behavior through the right combination of biochemical and biophysical signals is a universal theme to tissue engineering (Ghosh and Ingber 2007). However, identification of specific relationships between physical properties and cell responses are difficult to control and assess due to the complexity of cell-ECM interactions. In vitro studies have shown that integrin mediated adhesion of vascular ECs to collagen fibrils of the ECM is required for cell survival (Meredith and Schwartz 1997), proliferation (Assoian and Schwartz 2001), and migration (Senger et al. 2002). Furthermore, the magnitude of cell traction force relative to the apparent stiffness of a collagen matrix modulates in vitro capillary morphogenesis, including average network length, vessel area, and wall area (Korff and Augustin 1999). In other words, the tensional force balance that occurs as a result of the dynamic responsiveness of the cytoskeleton and the deformation resistance (stiffness) offered by the surrounding collagen fibril network provides critical contextual cues that control cell decision making and fate (Mammoto and Ingber 2009). While these mechanisms and relationships need further elucidation, our present objective was to first demonstrate that quantifiable differences in physical features of the local collagen-fibril microenvironment would have measurable impact on ECFC vessel formation in vivo.
Increasing the concentration of the polymerizable collagen yields 3D matrices with increased fibril density, no change in fibril diameter, and increased matrix strength and stiffness (Roeder et al. 2002; Kreger et al. In Press). These changes in biomechanical properties created grossly different physical microenvironments within which ECFC could be entrapped and cultured. The fibril microstructure and mechanical properties of the collagen matrices were quantified in the presence and absence of ECFCs. In the absence of cells, an increase in collagen concentration from 0.5 mg/ml to 3.5 mg/ml yielded a 2-fold increase in fibril density which translated to a 13-fold increase in G′ and an 8–fold increase in Ec. As expected, linear relationships were observed between fibril density and stiffness measures (G′ and Ec). While the range of fibril densities and matrix stiffness tested is significantly lower than that found in the ECM of mature tissues (≥ 40 mg/ml collagen), these properties more closely approximate provisional or immature ECM as occurs during development or wound healing (Miller and Rhodes 1982). Inclusion of ECFCs (2X106 cells/ml) within the construct had moderate and somewhat mixed effects on the mechanical properties of the matrices. It is plausible that within low concentration matrices, cells are serving as reinforcements of low density fibril-fibril interactions through formation of cell-matrix adhesions. In contrast, cells seeded within high fibril density matrices cause discontinuities within the dense fibril network thereby causing a decrease in overall mechanical integrity. This interplay between collagen matrix concentration and cell density as critical determinants of tissue construct mechanical behavior has been described previously (Marquez et al. 2006).
We have shown for the first time that varied matrix physical properties modulates ECFC (hCD31+) blood vessel densities and total vascular areas. Specifically, increased collagen concentration resulted in larger vessel areas, but less of them, whereas decreased collagen concentration resulted in higher densities of smaller area vessels. Vessel areas ranged broadly from several micrometers to greater than 4000 μm2, which was larger than expected. Capillary areas have been reported previously in the range of 50–78μm2 and 30–300 μm2 for mouse and human, respectively (Wiedeman 1963). Interestingly average vessel areas for all experimental groups measured between 200μm2 and 425 μm2 and decreased with collagen concentration, an effect which has been recently reported within in vitro models (Sieminski et al. 2004). It should be noted that vessel densities are routinely reported for in vivo implant models (Schechner et al. 2000; Melero-Martin et al. 2007; Yoder et al. 2007), and if we had applied such an analytical approach we would have missed the effect of altering the matrix properties. Thus, we propose that total vessel areas should be added as a new metric for evaluating vessel formation in ECM implants. Our results suggest that more attention to vessel details, such as areas, will help elucidate how matrix physical properties influence vessel formation and have obvious implications for reperfusion/revascularization strategies.
Fibril density and matrix stiffness of the cellularized constructs also influenced the size of the remodeled implant at two weeks, which is likely also having an effect on vascularization (Ghajar et al. 2008). The ability of host cells, soluble factors and nutrients to infiltrate and diffuse into the implant affects the rate and extent of vessel formation and anastomosis with host vessels. Indeed previous reports have demonstrated a role for perivascular (Au et al. 2008; Melero-Martin et al. 2008) and circulating angiogenic cells (Hur et al. 2004; Silva et al. 2008) in supporting nascent vessel formation. Further, perivascular cells have been shown to induce basement membrane deposition by endothelial cells resulting in alterations in integrin expression in vitro (Stratman et al. 2009). Our findings show that decreased matrix collagen concentration supports greater host cell infiltration resulting in a higher percentage of host (hCD31-) vessels. Thus it appears that varying collagen concentration and associated matrix physical properties affects a number of parameters that drive functional vessel formation in vivo.
The eventual translation of such collagen-based vehicles for cellular therapies requires the ability to precision-tune relevant matrix physical and biochemical properties in a highly reproducible manner. In the present study, matrices were prepared with commercial rat tail collagen based upon its precedence in the literature and to assist in comparison of results obtained previously with this animal implant model (Yoder et al. 2007). Unfortunately, routinely used commercial and laboratory collagens are often poorly specified and demonstrate significant variation in purity and polymerization potential (e.g., polymerization kinetics and fibril microstructure-mechanical properties) Kreger et al., 2010(Abraham et al. 2008; Kreger and Voytik-Harbin 2009). These collagens represent predominantly monomeric (single collagen molecule) compositions and therefore recapitulate a narrow range of fibril architectures and mechanical properties since they do not incorporate the natural cross-links that serve to direct, stabilize, and strengthen intermolecular and interfibrillar interactions inherent to the ECM (Even-Ram and Yamada 2005; Sabeh et al. 2009). As such, use of such collagens for creating 3D tissue constructs yields a limited range of fibril architectures, slow assembly rates, and low and inconsistent mechanical integrity, which ultimately translates into a lack of reproducibility in biological response (Johnson et al. 2007).
Future work will focus on application of collagen polymer formulations that are well characterized in terms polymerization (fibril-forming) properties as well as their molecular composition. In fact, an acid-solubilized type I collagen formulation derived from pig skin recently has been developed which comprises both collagen monomers and oligomers (at least two collagen molecules covalently attached by a natural cross-link). This formulation yields matrices with highly reproducible physical properties even when derived from different source hides (Kreger et al. In Press), Furthermore, this collagen formulation supports ECFC-derived vessel formation over an expanded range of fibril microstructure-mechanical properties (data not shown).
In conclusion, we demonstrated that material and physical properties of collagen-fibril matrices, namely the architecture of fibrils and their related stiffness, modulate functional blood vessel formation by in-vivo delivered ECFCs. The principles and concepts address some of the current problems associated with cell-based therapies and may contribute to the design and optimization of clinically-useful delivery strategies for ECFCs as well as other stem and progenitor cell populations. Now, having established that changing collagen concentration can induce significant differences in vessel formation in vivo, it is important to investigate the mechanisms that mediate these biophysical effects. A thorough understanding of the molecular mechanisms underlying matrix-induced changes in cellular behavior will contribute to the development of effective delivery matrices for ECFC-based cellular therapies as well as ECM-based therapeutic strategies aimed at reprogramming the host cell vascular repair response in vivo.
Acknowledgments
We would like to acknowledge Beverly Waisner and Joanne Kuske for their technical assistance. This work was supported in part by funds from the Riley Children’s Foundation (M.C.Y.), from award number F30HL096350 from the National Heart, Lung, and Blood Institute (P.J.C) and from the Collaborative Biomedical Research Pilot Grant sponsored jointly by Indiana University School of Medicine and Purdue University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham LC, Zuena E, et al. Guide to collagen characterization for biomaterial studies. J Biomed Mater Res B Appl Biomater. 2008;87(1):264–85. doi: 10.1002/jbm.b.31078. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11(1):48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Au P, Tam J, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SE, Mavila A, et al. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114(15):2755–2773. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- Chavakis E, Aicher A, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201(1):63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Bayless KJ, et al. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268(3):252–75. doi: 10.1002/ar.10159. [DOI] [PubMed] [Google Scholar]
- Davis GE, Saunders WB. Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc. 2006;11(1):44–56. doi: 10.1038/sj.jidsymp.5650008. [DOI] [PubMed] [Google Scholar]
- Enis DR, Shepherd BR, et al. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(2):425–430. doi: 10.1073/pnas.0408357102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17(5):524–32. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Ghajar CM, Chen X, et al. The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J. 2008;94(5):1930–1941. doi: 10.1529/biophysj.107.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv Drug Deliv Rev. 2007;59(13):1306–18. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Ingram DA, et al. Assessing Identity, Phenotype, and Fate of Endothelial Progenitor Cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Yoon CH, et al. Characterization of Two Types of Endothelial Progenitor Cells and Their Different Contributions to Neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24(2):288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Bader BL, et al. Integrins in vascular development. Braz J Med Biol Res. 1999;32(5):501–10. doi: 10.1590/s0100-879x1999000500002. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989;109(1):317–30. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, et al. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105(7):2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Jain RK, Au P, et al. Engineering vascularized tissue. Nat Biotechnol. 2005;23(7):821–3. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wang B, et al. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem. 2008;103(1):321–34. doi: 10.1002/jcb.21416. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Leight JL, et al. Demystifying the effects of a three-dimensional microenvironment in tissue morphogenesis. Methods Cell Biol. 2007;83:547–83. doi: 10.1016/S0091-679X(07)83023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan RY, Salacinski HJ, et al. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26(14):1857–75. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Asahara T. Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheter Cardiovasc Interv. 2007;70(4):477–84. doi: 10.1002/ccd.21292. [DOI] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112(Pt 19):3249–58. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- Kreger ST, Bell BJ, et al. Polymerization and Matrix Physical Properties as Important Design Considerations for Soluble Collagen Formulations. Biopolymers. doi: 10.1002/bip.21431. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger ST, Voytik-Harbin SL. Hyaluronan concentration within a 3D collagen matrix modulates matrix viscoelasticity, but not fibroblast response. Matrix Biol. 2009;28(6):336–46. doi: 10.1016/j.matbio.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan L, Underwood CJ, et al. Effect of mechanical boundary conditions on orientation of angiogenic microvessels. Cardiovasc Res. 2008;78(2):324–32. doi: 10.1093/cvr/cvn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, V, Artym V, et al. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18(5):463–71. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009 doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- March KL, Johnstone BH. Cellular approaches to tissue repair in cardiovascular disease: the more we know, the more there is to learn. Am J Physiol Heart Circ Physiol. 2004;287(2):H458–63. doi: 10.1152/ajpheart.00343.2004. [DOI] [PubMed] [Google Scholar]
- Marquez JP, Genin GM, et al. Cellular and matrix contributions to tissue construct stiffness increase with cellular concentration. Ann Biomed Eng. 2006;34(9):1475–82. doi: 10.1007/s10439-006-9160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero-Martin JM, De Obaldia ME, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103(2):194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero-Martin JM, Khan ZA, et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109(11):4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Jr, Schwartz MA. Integrins, adhesion and apoptosis. Trends Cell Biol. 1997;7(4):146–50. doi: 10.1016/S0962-8924(97)01002-7. [DOI] [PubMed] [Google Scholar]
- Miller EJ, Rhodes RK. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Mund JA, Ingram DA, et al. Endothelial progenitor cells and cardiovascular cell-based therapies. Cytotherapy. 2009;11(2):103–13. doi: 10.1080/14653240802714827. [DOI] [PubMed] [Google Scholar]
- Roeder BA, Kokini K, et al. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng. 2002;124(2):214–22. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- Ruegg C, Mariotti A. Vascular integrins: pleiotropic adhesion and signaling molecules in vascular homeostasis and angiogenesis. Cell Mol Life Sci. 2003;60(6):1135–57. doi: 10.1007/s00018-003-2297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, et al. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185(1):11–9. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar R, Bell SE, et al. Coordinate Induction of the Actin Cytoskeletal Regulatory Proteins Gelsolin, Vasodilator-Stimulated Phosphoprotein, and Profilin during Capillary Morphogenesisin Vitro. Experimental Cell Research. 1999;249(1):22–32. doi: 10.1006/excr.1999.4460. [DOI] [PubMed] [Google Scholar]
- Schechner JS, Nath AK, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A. 2000;97(16):9191–6. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, et al. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol. 2002;160(1):195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieminski AL, Hebbel RP, et al. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297(2):574–84. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Sieminski AL, Was AS, et al. The stiffness of three-dimensional ionic self-assembling peptide gels affects the extent of capillary-like network formation. Cell Biochem Biophys. 2007;49(2):73–83. doi: 10.1007/s12013-007-0046-1. [DOI] [PubMed] [Google Scholar]
- Silva EA, Kim ES, et al. Material-based deployment enhances efficacy of endothelial progenitor cells. Proceedings of the National Academy of Sciences. 2008;105(38):14347–14352. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, et al. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114(24):5091–101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack DG, Cheresh DA. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci STKE. 2002;119:pe7. doi: 10.1126/stke.2002.119.pe7. [DOI] [PubMed] [Google Scholar]
- Suuronen EJ, Veinot JP, et al. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(1):I138–44. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- Vailhe B, Vittet D, et al. In vitro models of vasculogenesis and angiogenesis. Lab Invest. 2001;81(4):439–52. doi: 10.1038/labinvest.3780252. [DOI] [PubMed] [Google Scholar]
- Voytik-Harbin SL. Three-dimensional extracellular matrix substrates for cell culture. Methods Cell Biol. 2001;63:561–81. doi: 10.1016/s0091-679x(01)63030-9. [DOI] [PubMed] [Google Scholar]
- Wiedeman MP. Dimensions of blood vessels from distributing artery to collecting vein. Circ Res. 1963;12:375–8. doi: 10.1161/01.res.12.4.375. [DOI] [PubMed] [Google Scholar]
- Yamamura N, Sudo R, et al. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng. 2007;13(7):1443–53. doi: 10.1089/ten.2006.0333. [DOI] [PubMed] [Google Scholar]
- Yoder MC, Mead LE, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]