Abstract
“Atherosclerotic” abdominal aortic aneurysms (AAAs) occur with the greatest frequency in the distal aorta. The unique hemodynamic environment of this area predisposes it to site-specific degenerative changes. In this review, we summarize the differential hemodynamic influences present along the length of the abdominal aorta, and demonstrate how alterations in aortic flow and wall shear stress modify AAA progression in experimental models. Improved understanding of aortic hemodynamic risk profiles provides an opportunity to modify patient activity patterns to minimize risk of aneurysmal degeneration.
Keywords: Abdominal aortic aneurysm, hemodynamics, blood flow, inflammation, exercise
1. Introduction
AAA is a common and frequently lethal age-related disease process that affects 6% of men and 1% of women over the age of 60 (Ashton et al., 2002; Lawrence-Brown et al., 2001; Lederle et al., 2000; Lindholt et al., 2002). Although aneurysms may develop throughout the length of the aorta, abdominal aneurysms are at least 5 times more prevalent than thoracic or thoracoabdominal aneurysms. This review introduces the concept of “regional pathogenic risk” in aortic disease, describing how the unique hemodynamic conditions present in the infrarenal aorta may modulate biologic mechanisms predisposing to aortic degeneration. We examine the relationship between aortic flow conditions and aortic aneurysmal degeneration in both experimental models and in patients, and examine how exercise may favorably influence hemodynamic conditions to reduce risk for or progression of AAA disease. Current imaging modalities allow for evaluation of patient-specific hemodynamic patterns; derivation of AAA growth and rupture risks through computational modeling techniques are also discussed.
1.1. Systemic influences on AAA disease pathobiology
Risks of rupture and sudden death are most closely related to aneurysm diameter. Infrarenal aortae ≥ 3 cm in diameter are generally considered aneurysmal; AAAs > 6 cm have a 10 to 20% chance of rupture within 12 months, and one third of all AAAs eventually rupture if left untreated (Darling et al., 1977). Surgical intervention is currently the only treatment shown to be effective in preventing AAA rupture and aneurysm-related death. Elective repair is reserved for aneurysms considered at risk for rupture or clinical evolution (≥ 5.5 cm in diameter) based on the likelihood of aneurysm related-death exceeding the surgical risk. AAA rupture or complications following surgical treatment are responsible for an estimated 30,000 deaths per year (Kent et al., 2004); AAA is the 13th leading cause of adult mortality, and the 3rd leading cause of sudden death in men > 65 years of age (Cowan et al., 2006). Current management of early AAA disease (4.0 cm to 5.5 cm in diameter) calls for serial imaging and clinical surveillance, coupled with medical management of traditional atherosclerotic risk factors. Approximately 70% of small AAA will require surgical repair within 10 years of initial diagnosis (Brady et al., 2002). The modest efficacy of current medical interventions to prevent AAA progression has recently been reviewed (Baxter et al., 2008).
The increasing use of endovascular approaches to exclude, rather than resect, AAAs, has been accompanied by a nationwide decline in procedure-related morbidity and mortality (Dillavou et al., 2006). Despite improved perioperative outcomes, however, endovascular aneurysm repair (EVAR) has limitations of its own, including blood flow developing outside of the graft either early or late following placement, termed “endoleaks”, requiring lifelong imaging surveillance and occasional reintervention (Brewster et al., 2003). The overall value of EVAR vs. traditional open surgical repair has been difficult to assess in the last decade, due to almost continuous improvement in device and imaging technology. The results of a national prospective randomized trial comparing the two were recently published (Lederle et al., 2009). Two prospective trials have considered the potential benefits of EVAR management in AAA < 5.5 cm in diameter; the PIVOTAL trial (USA) recently ended enrollment (Ouriel K, 2009), unable to demonstrate improved outcomes or reduced costs with early procedural intervention. The CAESAR trial (Europe) is ongoing.
Prior screening studies have identified advanced age, male gender, cigarette smoking, family history, hypertension, obesity, hypercholesterolemia and concomitant coronary or cerebrovascular arterial occlusive disease as distinct AAA risk factors (Blanchard et al., 2000; Lederle et al., 1997; Lederle et al., 2000; Singh et al., 2001). Although epidemiologic associations are well recognized, the mechanisms promoting AAA disease development in each case are less well understood. Cigarette smoking is the most significant acquired risk; smokers have up to a 7-fold increased risk for AAA disease; AAA has the closest association to cigarette smoking than any other save lung cancer, and 90% of all AAA patients have been regular smokers at sometime during their lifetimes (Baxter et al., 2008; Lederle et al., 2003). Obesity represents another significant acquired risk; waist circumference and waist-to-hip ratio have been independently associated with AAA after adjustment for other known risk factors and serum levels of the pro-inflammatory adipokine, resistin, correlate strongly with aortic diameter (Golledge et al., 2007). Female gender, African American race, regular aerobic exercise and diabetes mellitus are protective against AAA disease. The disproportionate influence of environmental and behavioral risks in disease pathogenesis is highlighted by the fact that, excluding individuals with congenital aneurysm syndromes such as Marfan Syndrome or Ehlers Danlos Syndrome, positive family histories can be obtained from only 15% of patients with AAA disease (Kuivaniemi et al., 2008; Verloes et al., 1995).
Research efforts in AAA disease remain focused on understanding the patho-biological mechanisms underlying aneurysm development. Although circulating biomarkers are being investigated for their utility in assessment of AAA status (Dalman et al., 2006; Golledge et al., 2008), aneurysm diameter remains the principle clinical determinant of disease progression and rupture risk. Expansion rates average 2–3 mm/year, influenced by baseline diameter and the above mentioned systemic risk factors. On the basis of a systematic review from population-based, randomized, controlled screening trials, the United States Preventative Services Task Force concluded that AAA screening may reduce AAA-related mortality by 43% in men aged 65–75 years (Fleming et al., 2005). Supported by this evidence, the 2007 Screening Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) (Lee et al., 2009) amendment included ultrasound screening to the Initial Preventative Physical Examination (IPPE) as a new federally-funded benefit provided by Medicare in response to the high prevalence and lethality of AAA disease.
1.2. Regional influences on AAA disease pathobiology
In comparison to the well-recognized systemic AAA risk factors detailed in the preceding section, few studies have measured or defined regional pathogenic risks. The marked predilection for aneurysmal dilatation in the abdominal as compared to thoracic segments draws attention to physiologic and anatomic features unique to the distal aorta. The infrarenal aorta is the most common site of extracranial aortic aneurysm formation. Differential hemodynamic influences present along the length of the aorta may work in concert with other regional factors to explain this preferential distribution. Region-specific structural differences are well-recognized along the aorta; the elastin-collagen ratio declines along the length of the aorta, reducing elasticity and wall motion (Ailawadi et al., 2003a). Reduced distal aortic elasticity, in combination with augmented pressure due to pulse wave reflections from the aortic bifurcation and other downstream arteries, may increase wall strain and aneurysm suseptibility. Extracellular matrix integrity and proteolysis may also vary regionally; increased expression and activity of matrix metalloproteinase-9 (MMP-9) is present in the native infrarenal murine aorta compared to thoracic and aortic arch specimens. However, when the thoracic and abdominal segments are transposed, thoracic segments increased MMP-9 expression when transplanted to the abdomen, while abdominal segment demonstrated reduced MMP-9 expression when transplanted to the thoracic segment (Ailawadi et al., 2003b).
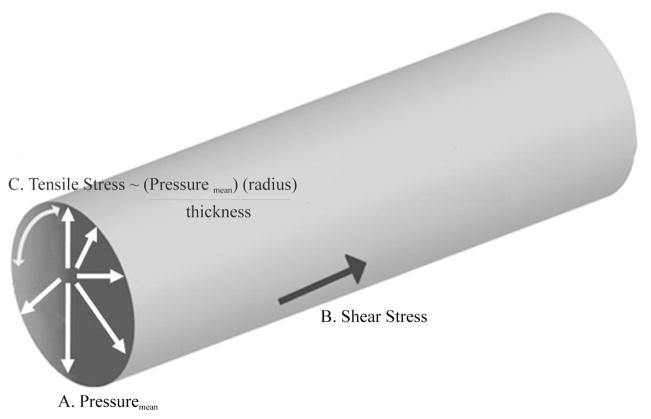
The term “hemodynamic forces” refer to the kinetic energy generated by the flow of blood through arteries and veins. Vascular endothelial and smooth muscle cells are constantly exposed to the dynamic influences of flowing blood. Cellular responses to these physical stimuli influence vessel wall homeostasis (Hsiai, 2008). Hemodynamic forces relevant to AAA pathogenesis can be resolved into three components: 1) wall shear stress (WSS), the tangential force exerted by moving blood along the axis of flow; 2) hydrostatic pressure, the perpendicular force acting on the vascular wall; and 3) relative wall strain (RWS), the circumferential stretch of the vessel wall exerted by cyclic luminal pressure changes and the resulting tensile stress (Figure 1).
Figure 1.
Hemodynamic forces relevant to AAA pathogenesis; hydrostatic pressure, the perpendicular force acting on the vascular wall (A), wall shear stress, the tangential force exerted by moving blood along the axis of flow (B), and tensile hoop stress, the stress in the aortic wall acting circumferentially and produced by the resulting pressure (C).
Hemodynamic conditions vary markedly along the aorta, from high Reynolds numbers at the aortic root to low and oscillatory shear conditions at the aortic bifurcation (Greve et al., 2006). Most relevant to AAA disease pathophysiology, and its predilection for the distal-most aortic segment, is the marked difference between resting aortic WSS in the thoracic and abdominal aorta. In suprarenal aortic segments, flow is antegrade throughout the cardiac cycle, providing continuous antegrade laminar WSS. In the infrarenal aorta, WSS values are lower, and reverse flow is present in late systole and diastole. In response to reduced distal arterial resistance and increased flow, such as is demonstrated in the response to even modest lower extremity exercise, WSS becomes antegrade and laminar throughout the cardiac cycle, mimicking those characteristic of more proximal aortic segments. These distinct regional differences in hemodynamic influences may account for some component of the differential aneurysm risk noted between the thoracic and abdominal aortic segments.
At the interface between blood and the vessel wall, vascular endothelial cells sense and respond to differential hemodynamic forces (Hsiai, 2008). Complementary in vitro data from human umbilical vein and bovine aortic endothelial cultures suggest several potential mechanisms by which exposure to steady physiologic shear stress may suppress pro-inflammatory gene expression (De Keulenaer et al., 1998). Following acute application of laminar SS to cultured endothelial cells, ion channel activation activates downstream signaling cascades such as the c-Jun N-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) pathways (Resnick et al., 2000), leading to shear-responsive gene transcription. In rodent models, increased antegrade shear stress stimulates anti-oxidant, anti-inflammatory, and anti-apoptotic aortic gene expression (Dalman, 2003). Known shear-responsive genes include ICAM-1, cycloxygenase-2, eNOS, Smad6, TGFβ1, copper zinc superoxide dismutase (SOD2), thrombomodulin and heme oxygenase-1 (HO-1) (Wasserman et al., 2002). These shear-responses may ultimately mitigate inflammation (Gimbrone et al., 1999) and proteolysis in the infrarenal aorta, suggesting mechanisms by which regional differences in aortic hemodynamic conditions may account for differential aneurysm risk.
2. Clinical relevance of aortic hemodynamic conditions
2.1. Resistive aortic hemodynamics
Several clinical associations highlight the pathogenic significance of resistive hemodynamic conditions on AAA progression. Patients with major limb amputation were found to be five times more likely to have AAAs > 40 years following injury than non-amputee patients matched for traditional AAA risk factors (Vollmar et al., 1989). Spinal cord injury (SCI) is also independently associated with increased prevalence of AAA disease. SCI greatly diminishes distal aortic blood flow and promotes chronically low antegrade and oscillatory shear stress conditions in the infrarenal abdominal aorta (Yeung et al., 2006). Multiple previous reports note increased AAA prevalence in symptomatic peripheral vascular disease (PVD) patients (Sandgren et al., 2001; van den Bosch et al., 2001). Although shared risk factors exist for both PVD and AAA, PVD is also associated with reduced lower extremity activity and resistive hemodynamic conditions in the infrarenal aorta. Resistive aortic hemodynamics may also promote expression of reactive oxygen species (ROS). The significance of oxidative stress in AAA disease has been demonstrated by increased ROS production and evidence of oxidative injury in human AAA compared with non-aneurysmal aortic segments (Miller et al., 2002); however, the exact mechanism by which sedentary lifestyle and associated resistive hemodynamics promote aneurysm formation remains unknown. Characterization of clinical hemodynamic conditions and their influences on aortic remodeling may identify novel therapeutic strategies, such as prescribed activity regimens, to minimize inflammation and aneurysmal degeneration in the infrarenal aorta.
2.2. Aortoiliac adaptation to spinal cord injury
To examine the influence of ambulation, and ambulation-related abdominal aortic hemodynamic conditions on aneurysm disease risk and progression, we performed abdominal aortic ultrasound imaging on a consecutive series of SCI patients aged ≥ 55 years old, who had been unable to walk for at least 5 years (Yeung et al., 2006). This patient cohort was chosen to represent the extreme examples of inactivity and resistive aortic hemodynamics. Aortic and iliac artery diameters in SCI patients (n = 123) were compared to those of age and risk-factor matched ambulatory control patients (n = 129) without prior diagnosis of abdominal or iliac aneurysm disease.
Each subject underwent fasting abdominal ultrasound imaging limited to the aorta and iliac arteries. Classification of aortic diameter was similar to previously reported studies, with normal defined as ≤ 2.0 cm, ≥ 2.5 cm classified as enlarged, and ≥ 3.0 cm aneurysmal (Lederle et al., 1997). Within the entire cohort, the average aortic diameter was larger in SCI than in control subjects (2.3cm SCI vs. 2.0cm control, p<0.01); however, iliac artery imaging demonstrated significantly reduced iliac diameter present in the SCI group. This latter observation was expected; chronic flow decreases result in the inward vascular remodeling and narrowing of lower extremity arteries in SCI patients (Yeung et al., 2006; Tang et al., 2008), but the co-existence of larger aortas and smaller iliac arteries had not been previously recognized as characteristics feature of SCI or adaptation to non-ambulatory existence.
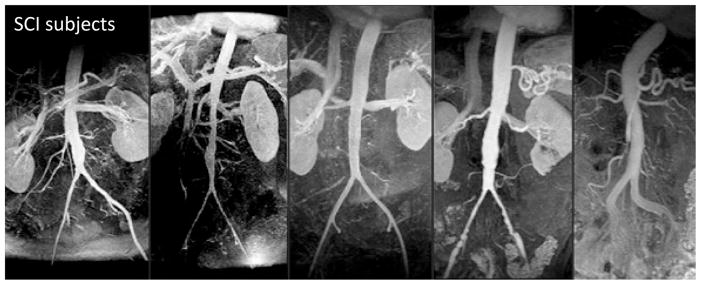
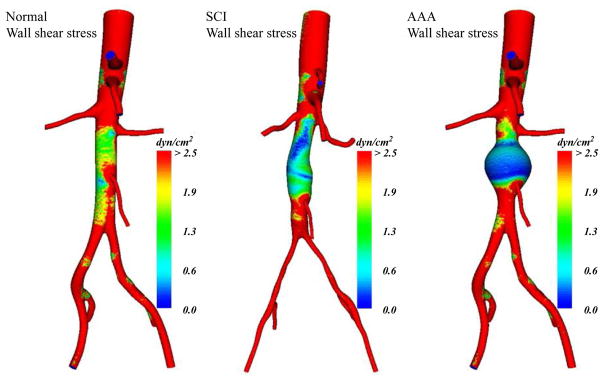
Aortic magnetic resonance angiography (MRA), in a subset of randomly selected SCI patients, demonstrated enlarged distal aortic segments adjacent to diminished iliac arteries (Figure 2, Yeung et al., 2006). Aortic hemodynamic conditions in SCI patients, as determined by cine phase-contrast magnetic resonance flow imaging (PC-MRI) and computational modeling, demonstrated marked reductions in antegrade flow and WSS throughout the cardiac cycle. When compared to “normal” activity patterns for the most sedentary category of ambulatory and healthy control subjects, time-averaged daily aortic WSS in non-ambulatory SCI patients was still less than 50% of controls (mean aortic WSS values 3.01 dyne/cm2 for SCI vs. 7.23 dyne/cm2 for control subjects, p=0.06). Increasing aortic diameter in the aneurysmal aorta produces even lower values of WSS (Figure 3, Dalman et al., 2006).
Figure 2.
Magnetic resonance angiography of the abdominal aorta in spinal cord injury (SCI) patients showing characteristic vascular phenotypes of ectatic distal aortic segments adjacent to diminished iliac arteries. Adapted from Yeung et al., 2006, J. Vasc. Surg. 44, 1254–1265 with permission.
Figure 3.
Geometric modeling of resting WSS estimates at peak systole of normal aorta, SCI aorta and 4cm AAA from left to right, respectively. Adapted from Dalman et al., 2006, Ann. N. Y. Acad. Sci. 1085, 92–109 with permission.
Although SCI patients also typically manifest disproportionately elevated traditional risks for AAA disease (Bauman and Spungen, 1994; Bauman et al., 1999; Zeilig et al., 2000), these risks (including cigarette smoking) were well controlled in our study, suggesting that sedentary existence or the hemodynamic consequences of SCI may represent a unique and independent AAA risk.
3. Modeling hemodynamic influences on AAA disease
3.1. Consequences of flow variability on experimental AAA diameter
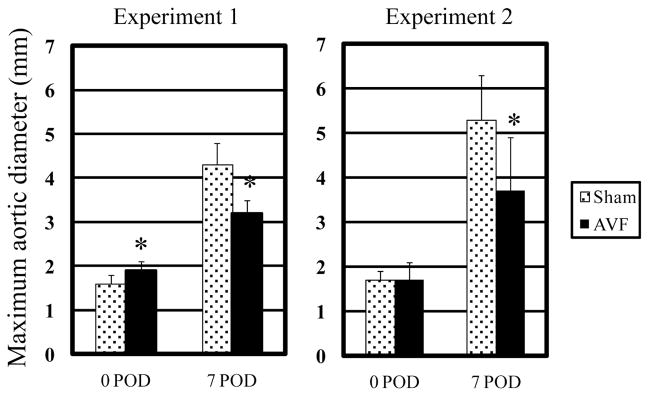
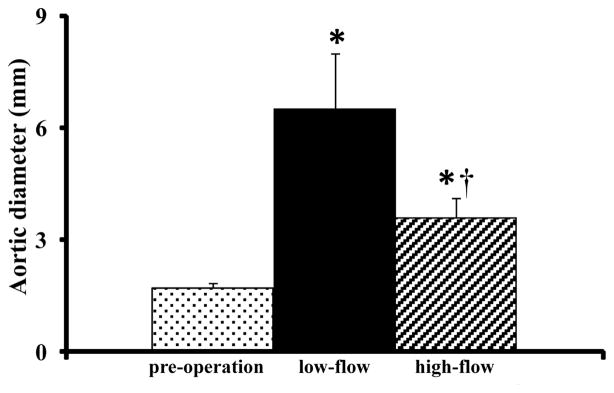
Since human AAA specimens obtained at the time of operative repair are typically acellular and atretic (representing end-stage disease), biologically relevant murine AAA models have been developed to better characterize the inciting events of aneurysm formation. To investigate the mechanisms linking hemodynamic forces and aneurysm pathogenesis, we incorporated variable flow conditions into the well-described rodent porcine pancreatic elastase (PPE) infusion AAA model (Anidjar et al., 1990). Intra-aortic elastase infusion reliably produces AAAs in rats and mice within 14 days. Sustained flow loading was accomplished by distal arteriovenous fistula creation (Abbruzzese TA, 1998), increasing aortic flow, WSS, and relative wall strain (RWS) > 300%, without influencing blood pressure or heart rate (Nakahashi et al., 2002). In normal aortae without PPE infusion, the increased flow, WSS and RWS induced by distal AVF creation stimulated modest aortic enlargement, an effect consistent with high flow-induced outward remodeling (Guzman et al., 1997; Karwowski et al., 1999; Tronc et al., 2000). Interestingly, this physiologic increase in aortic diameter in response to flow loading (Masuda et al., 1999) produced unexpected results in AAA models; AAA growth was reduced in the flow-loaded aorta, regardless whether high flow conditions were induced days before or after PPE infusion (Figure 4, Nakahashi et al, 2002). To simulate decreased blood flow conditions, unilateral common iliac artery ligation was performed, resulting in a 44% reduction in aortic flow compared to normal conditions. At 7 days post elastase infusion, AAA created in conjunction with unilateral common iliac artery ligation (LF-AAA) were almost twice as large as those created in high-flow aortae following AVF (HF-AAA group, Figure 5, Hoshina et al., 2003). Although AVF creation did increase heart rate, no significant changes in tail or central aortic blood pressure were noted under a wide range of experimental conditions (either femoral or aorto-caval AVF formation). From these experimental observations we concluded that variable flow conditions markedly influenced AAA progression in experimental models.
Figure 4.
Maximum aortic diameter at day 0, 7, or 14 days after PPE infusion (POD indicated postoperative day) as a function of normal flow (sham) or flow loading (AVF) applied either before (experiment 1) or after (experiment 2) PPE infusion (*p<0.05 against normal flow group). Reprinted from Nakahashi et al., 2002, Arterioscler. Thromb. Vasc. Biol. 22, 2017–2022 with permission.
Figure 5.
Changes in aortic diameter after PPE infusion with iliac artery ligation (low-flow) or femoral arteriovenous fistula creation (high-flow). (*p<0.05 against preoperative diameter, †p<0.01 compared with low-flow AAA). Reprinted from Hoshina et al., 2003, J. Vasc. Surg. 37, 1067–1074 with permission.
3.2. Consequences of flow variability on aneurysm inflammation and cellularity
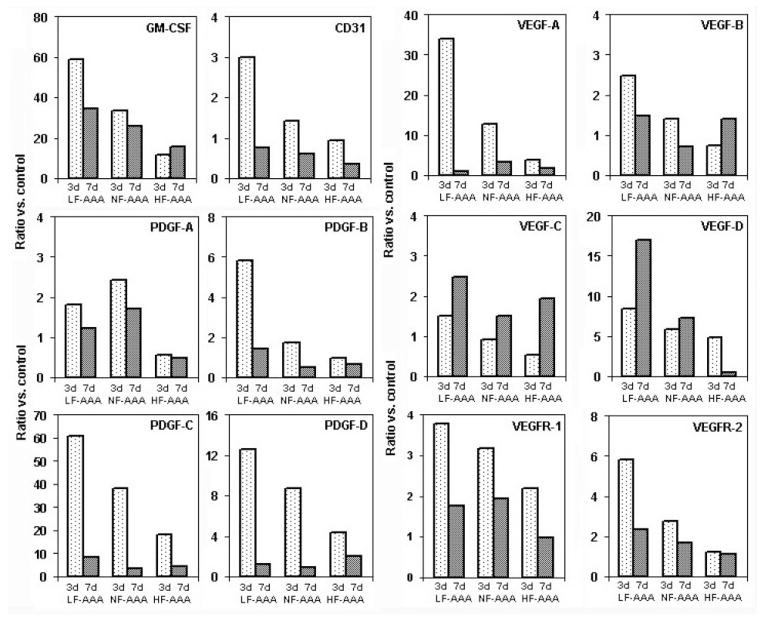
Variable flow conditions and resulting influences on aortic WSS regulate vascular remodeling through a complex interplay between cellular proliferation, monocyte adhesion and transmural migration, and extracellular matrix homeostasis. Hemodynamic influences on arterial remodeling include response to flow, pressure and wall strain (Langille et al., 1996). Because aortic cellularity represents such a critical element of ongoing mural integrity, we analyzed the morphologic and cellular consequences of variable flow conditions in experimental AAAs (Hoshina et al., 2003). HF-AAAs demonstrated decreased smooth muscle cell (SMC) apoptosis and cellular preservation within the aortic wall with qualitative evidence of elastin and collagen preservation compared to LF-AAAs. LF-AAAs demonstrated a greater density of mural neovessels, defined as single-cell layer capillaries proliferating in the medial and adventitia in association with increase mononuclear cell infiltration. Increased mural neovascularity, a significant feature of human AAA disease (Choke et al., 2006a; Choke et al., 2006b), may promote aortic aneurysm degeneration via mononuclear cell chemotaxsis and activation (Murakami et al., 2006). Using real-time reverse transcriptase polymerase chain reaction (RT-PCR) to amplify total RNA for vascular endothelial growth factor-D (VEGF-D), its specific receptor KDR, and platelet-derived growth factor-β (PDGF-β), we noted increased pro-angiogenic gene expression in LF-AAAs associated with increased mural neovascularity on CD-31 immunostaining.
Mural macrophage density in experimental AAA varied in an inverse dose-response relationship to luminal flow velocity and volume (Sho et al., 2004a). Within the aneurysm wall, macrophage density varied directly with distance from the lumen (media > adventitia), highlighting the relationship between the luminal interface and mural inflammation. In addition to direct influences on endothelial shear receptors, reduced antegrade or oscillatory WSS may promote monocyte binding and mural infiltration (Chatzizisis et al., 2007). Pro-inflammatory chemokine and cytokine expression (granulocyte macrophage-colony stimulating factor (GM-CSF) and monocyte chemoattractant protein (MCP-1) also varied inversely with luminal flow (Figure 6, Sho et al., 2004b).
Figure 6.
Flow-dependent expression of relevant cell markers, cytokines, growth factors and growth factor receptors. AAA mRNA expression analysis determined by real time PCR. Data reported as the ratio of target molecules versus normal control aortic tissue. Reprinted from Sho et al., 2004b, Arterioscler. Thromb. Vasc. Biol. 24, 1916–1921 with permission.
Variable hemodynamic conditions also influence localization and differentiation of circulating vascular progenitor cells. Vascular progenitor cells, identified as CD34+ (Sho et al., 2004b), have previously been recognized in medial and adventitial neovessel in human AAA specimens (Kobayashi et al., 2002). In the circulation, these adult peripheral blood cells may transdifferentiate into endothelial cells, macrophages or vascular SMCs at sites of arterial injury, potentially playing a critical role in vascular function (Xu et al., 2004; Zhang et al., 2004). In our variable flow models, HF-AAAs contained more mural CD34+/α-actin co-stained cells/cross-sectional area than NF- or LF-AAAs, suggesting that high shear environments directed differentiation towards maintenance of mural smooth muscle cellularity. In contrast, LF-AAA demonstrated proportionally more CD34+/CD31+ cells, in association with increased inflammation and neocapillary formation, suggesting that low shear conditions promoted differentiation of circulating vascular progenitor cells into adventitial neo-capillaries.
In subsequent experiments, we identified heme-oxygenase-1 (HO-1) as enzyme, previously recognized to be highly relevant to human aneurysm pathophysiology (Schillinger et al., 2002), that may be differentially regulated in experimental AAA by variable flow conditions (Nakahashi et al., 2002). In vascular cells, HO-1 catalyzes carbon monoxide, biliruibin, and biliverdin. These byproducts attenuate arterial injury and response through a variety of mechanisms, including decreased macrophage migration, reduction in pro-inflammatory cytokine production and gene expression via modifications of NFkB, AP-1, and STAT transcription factor activities (Tobiasch et al., 2001; Tulis et al., 2001). Mural HO-1 expression varied directly with flow velocity and volume in our variable flow models (HF- > NF- > LF-AAA), and expression was associated with variable reactive oxygen species (ROS) production (LF > NF > HF). Other shear-responsive, oxido-reductive genes expressed in high flow conditions include glutathione S-transferase and NAD(P)H:quinine oxidoreductase (Chen et al., 2002). These results suggest that flow-responsive anti-oxidant gene expression and ROS scavenging is one additional mechanism likely responsible for hemodynamic modulation of AAA disease.
The influence of variable flow conditions on aneurysm progression may not be limited solely to the abdominal aorta. The phenomenon of post-stenotic dilatation is well-recognized in many peripheral arterial beds, and recent observations suggest that areas of reduced shear predict subsequent enlargement and clinical progression of intra-cranial “berry” aneurysms (Hassan et al., 2005; Humphrey and Taylor, 2008). In the intracranial arterial circulation, mural adaptations to reduced WSS include significant increases in MMP activity, synthesis of extracellular matrix proteins, and apoptosis (Mimata et al., 1997; Humphrey and Taylor, 2008).
4. Translational Applications
4.1. Predicting AAA growth and rupture
While aortic diameter has generally proven to be an effective determinant of risk for aneurysm rupture and aneurysm-related mortality, some small AAAs continue to rupture prior to reaching surgical size thresholds, while other larger AAAs never require surgery. Increased precision in assessing risk for aneurysm rupture and clinical progression would improve current treatment outcomes while reducing the expense and risk of unnecessary reconstructive procedures performed for patients at low risk of clinical evolution. In a recent review by Vorp (2007), the aortic mural stress:strength ratio calculated for individual aneurysms is promoted as an effective determinant of rupture risk. The stress:strength analytic tool calculates the imbalance between wall stress and wall strength as the primary determinant of aneurysm progression. The time at which wall stress exceeds strength varies depending on patient-specific features and is not predicted by diameter determination alone.
Other parameters suggested to predict AAA growth and clinical evolution include intraluminal thrombus thickness (Stenbaek et al., 2000), wall tension (Hall et al., 2000), wall stress (Raghavan et al., 2000a), and vessel asymmetry (Doyle et al., 2009). Due to the accelerated loss of medial elastin, aortic aneurysm tissues are stiffer than non-aneurymal aorta, and this stiffness is accentuated in the circumferential direction (He and Roach, 1994; Raghavan and Vorp, 2000b; Vande Geest et al., 2006a). The utility of quantifying aortic stiffness as a predictor of aneurysm progression, while initially promising, has not been borne out in subsequent analyses (Sonesson et al., 1999; Humphrey and Taylor, 2008).
Peak wall stress has also been promoted as a sensitive and specific risk index; Fillinger et al. (2002) demonstrated that peak wall stresses calculated in vivo for AAAs near the time of rupture were significantly higher than peak stresses for electively repaired AAAs, even when matched for maximal diameter (ruptured, 47.7±6 N/cm2, emergent symptomatic, 47.5±4 N/cm2, elective repair 36.9±2 N/cm2, p=0.03) (Fillinger et al., 2002). Other investigations have identified the peak wall stress among AAA patients to be located on the posterior surface of the wall with statistically significant differences between aneurysmal and non-aneurysmal cases (Raghavan et al., 2000a). Subsequent studies used these methods to differentiate acuity and need for surgery in patients under observation for urgent intervention (Fillinger et al., 2003). This determinant, while reproducibly predictive of progression in investigational applications, has not achieved widespread acceptance or utilization in clinical practice.
Interest in the stress:strength balance equation led to the recent development of a novel predictive instrument, the rupture potential index (RPI), to predict clinical progression (Vande Geest et al., 2006b). Using well established three-dimensional reconstruction and finite element method techniques, wall stress simulations were created from CT scans of ruptured and non-ruptured repairs. The wall strength was estimated using a mathematical model which accounts for the spatially varying influences of ILT thickness, aneurysm wall dilation, and global influences of sex and family history. Between the two groups there were no significant differences in the maximum transverse diameters (6.8±0.3 cm vs 6.1±0.5 cm, p=0.26) or peak wall stress (46.0±4.3 N/cm2 vs 49.9±4.0 N/cm2, p=0.62). Ruptured AAAs demonstrated a significant decrease in minimum wall strength (81.2±3.9 N/cm2and 108.3±10.2 N/cm2, p=0.045) leading to a higher RPI value (ruptured = 0.48±0.05 vs nonruptured = 0.36±0.03, p=0.10). Although not reaching statistical significance, the lower p-value of the RPI compared to the results for diameter and peak wall stress comparisons suggested that RPI may be able to identify AAAs at increased risk for rupture. Subsequent work has demonstrated that inclusion of wall calcifications into the aortic finite element analyses alters calculated stress distributions and should therefore be included in the rupture risk assessment (Speelman et al., 2007). Although only a weak correlation was found between the calcification index (CI: percentage of total wall surface area occupied by calcifications) and peak stress, consistent with other studies (Li et al., 2008), their results suggested that the location and shape of the calcified regions, not only the relative amount, are considerations that influence AAA wall stress. The clinical utility of these and related indices remain to be determined – to date they have not gained widespread acceptance or application to clinical applications.
More recently, methods of determining vessel asymmetry in the anterior-posterior plane have been introduced into three-dimensional AAA models to determine the relationship between wall stress and geometrical parameters. In many aneurysms, diameter enlargement is asymmetric, with primarily anterior protrusion. The posterior region is often constrained from radial expansion by the adjacent spinal column. In a study of fifteen AAA patients, Doyle and colleagues demonstrated that posterior wall stress increases with anterior centerline asymmetry and therefore, assessment of the degree of bulging and asymmetry of an aneurysm may aid in surgical decision-making (Doyle et al., 2009). In comparison to a modified symmetric aneurysm, introduction of asymmetry increased peak wall stress by 48% and increased posterior wall stress by 38% between the two models.
With the advent of more accurate imaging techniques and reconstructive capabilities to determine wall stress and strength patterns in vivo, such biomechanical methods have produced promising early results in determining aneurysm risk profiles. Further investigation linking these hemodynamic modeling studies to potential interactions with biologic phenomena or inflammatory pathways, material properties of the aorta, and genetic or other known risk factors such as the effects of smoking or obesity will expand the relationship between biomechanical evaluation and clinical prognostication.
4.2. Influence of lower extremity exercise on local and systemic AAA risk factors
Previous studies of AAA prevalence did not consider the influence of reported activity, measured exercise capacity, or limb status in determining relative risk factors for aneurysm disease (Brady et al., 2004; Lederle et al., 1997). These indices of lower extremity activity and time-averaged aortic hemodynamic status may, as discussed above, be of significant relevance to the pathophysiology of AAA disease. Understanding the mechanisms by which exercise contributes to the prevention and regression of cardiovascular disease may potentiate the development of new and effective therapies either incorporating components of exercise therapies or pharmacologic mimicry of its salutary effects. Exercise, in combination with smoking cessation, is the most effective medical therapy for intermittent claudication (Weitz et al., 1996) presumably due, in part, to the hemodynamic alterations in the major vessels. In addition to these local effects, the systemic benefits of exercise training and physical fitness include a reduction in central adiposity and relevant inflammatory mediators such as C-reactive protein (CRP), TNF-α, IL-1 and IL-6 levels which may reduce AAA progression (Ford, 2002; Berg and Scherer, 2005). Mild levels of lower limb exercise in humans have been shown to increase flow rate and wall shear stress, as well as decrease oscillations in flow in the infrarenal abdominal aorta (Niezen et al., 1998; Pedersen et al., 1999; Taylor et al., 2002). Even short episodes of exercise can lead to biological changes in arteries exposed to increased blood flow and elevated shear stress to mediate cardiovascular fitness even after the cessation of physical activity (Green et al., 2004). These exercise induced changes in hemodynamic conditions have been hypothesized to inhibit atherosclerosis and enhance transport of cholesterol from the vessel wall (Tang et al., 2006). It has been demonstrated in vitro that the vascular endothelium can sense and respond to high shear environments by alignment in the direction of flow, upregulation of vasodilators and antioxidants such as endothelium derived relaxation factor/nitric oxide (Schalet et al., 1997), and downregulation of vasoconstrictors, inflammatory molecules (Vainas et al., 2003), and adhesion proteins (Tang et al., 2006). Exercise has long been recognized to reduce all-cause mortality and vascular-related complications in patients with CV disease (Myers et al., 2002). However, in addition to the widely known systemic benefits achieved with exercise, the protective influences of increased antegrade flow may provide a feasible and physiologic means for altering regional hemodynamics and attenuating AAA progression.
4.3. Biomechanical consequences of lower extremity activity
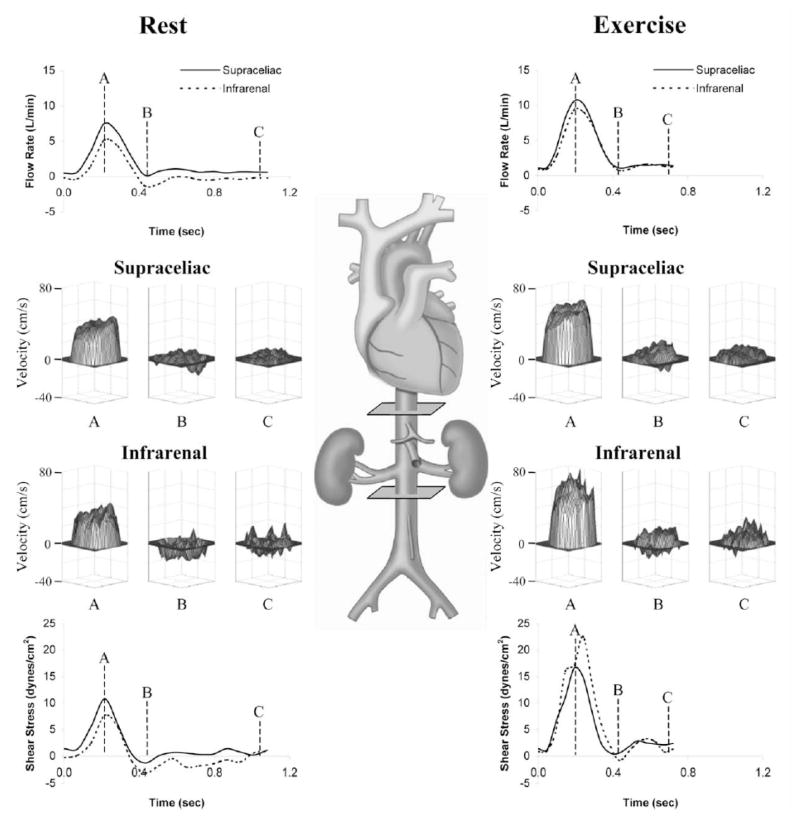
Novel computational flow simulations provide a means to quantify and visualize complex temporally-resolved hemodynamic changes along the entire abdominal aorta with high resolution. To test the ability of lower extremity exercise to modify aortic flow in the infrarenal aorta, hemodynamic conditions were quantified in healthy subjects aged 50–70 years (Cheng et al., 2003b) during rest and exercise. Previous MR analyses of volume flow rate have been limited to motionless states, however for these experiments, an MR-compatible cycle was built into the General Electric 0.5T open magnet to measure variable blood flow velocities in the abdominal aorta in vivo (Cheng et al., 2003c). The MR-compatible stationary cycle allows for dynamic evaluation of subjects that sit upright in the bore and pedal, achieving higher levels of exercise in a natural position (Taylor et al., 2002). Cine PC-MRI techniques were used to obtain anatomic and through-plane velocity maps perpendicular to the abdominal aorta during rest and exercise conditions. Application of a level set segmentation method was used quantify velocity data, blood flow rate, WSS and temporal oscillations of flow. Both supraceliac and infrarenal blood flow increased significantly from rest to exercise (Figure 7, Cheng et al, 2003b). Wall shear stress also increased with exercise from 2.0±0.7 dynes/cm2 to 7.3±2.4 dynes/cm2 in the supraceliac aorta and 1.4±0.8 dynes/cm2 to 16.5±5.1 dynes/cm2 at the infrarenal level (both p<0.001) (Cheng et al., 2003a). Compared to a younger group of subjects aged 20–30 years old (Tang et al., 2006), the older subjects were noted to have lower mean supraceliac WSS with greater oscillations at rest but the magnitude of change towards improved hemodynamic conditions with exercise was noted to be more dramatic than the younger group. These findings demonstrated that 1) moderate levels of exercise create aortic hemodynamic conditions that may reduce pro-inflammatory and pro-aneurysmal conditions within the abdominal aorta, and 2) the benefits of hemodynamic alteration may be more significant for older subjects given their increased adverse baseline conditions at rest.
Figure 7.
Schematic of the human aorta with imaging planes at the supraceliac and infrarenal levels and flow data from a representative healthy subject, aged 59, at rest and during cycling exercise. Blood flow rate waveforms (top) show significant increases in flow at both the supraceliac and infrarenal levels from rest (left) to exercise (right) throughout the cardiac cycle. Also note that reversal of flow at the infrarenal level at rest is eliminated during exercise. Velocity surface plots are shown for the supraceliac and infrarenal levels of the aorta at rest (left) and during exercise (right) at peak systole (A), end systole (B), and end diastole (C). Blood velocities increase from rest to exercise for all cardiac phases, and most of the negative blood velocities near the walls of the supraceliac and infrarenal levels at end systole (B) at rest (left) become positive during exercise (right). Wall shear stress plots (bottom) reveal that nearly all of the negative wall shear stress present in the infrarenal aorta during diastole at rest is eliminated with exercise. Reprinted from Cheng et al., 2003b, Atherosclerosis. 168, 323–331 with permission.
4.4. Therapeutic considerations
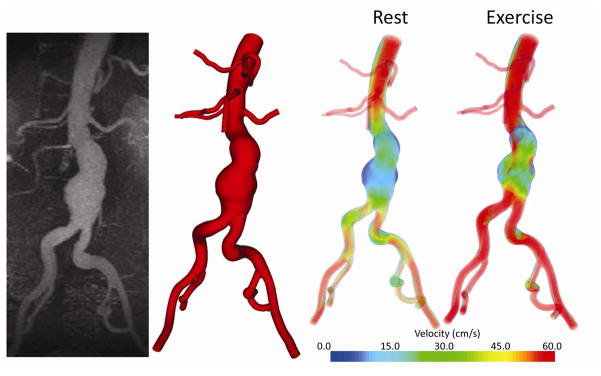
Given the potential benefits that lower extremity exercise may confer to aneurysm risk and disease progression, recently initiated a randomized, controlled clinical trial of supervised exercise training to limit progression of early AAA disease. As a component of this study, subject-specific geometrical models from MRA and PC-MRI datasets indicate that exercise effectively eliminates areas of low WSS and increases turbulence within the aneurysm as compared to rest (Figure 8, White and Dalman, 2008). The low, oscillatory and stagnant flow seen at rest in the AAA is largely eliminated during exercise as demonstrated by a decrease in the oscillatory shear index measured throughout the aorta. Although these studies support the hypothesis that exercise training will modify hemodynamic conditions towards a favorable profile to reduce aneurysm growth, the larger scope of the study is to assess the impact of physical activity on aneurym progression as monitored by ultrasound imaging and biologic markers of disease over a three year period. Subject enrollment and exercise and monitoring activities for this study are ongoing, but early experience indicates that exercise training is safe and well-tolerated in patients with early aneurysm disease, and does not lead to paradoxical aneurysm enlargment or increased rupture risk in the short (< 3 years) term.
Figure 8.
Custom software was used to convert magnetic resonance data (left) to a three-dimensional geometric model of the flow domain (center left). The three-dimensional model, in combination with patient-specific blood flow information, was used to simulate blood flow in an aneurysm during rest and exercise. During resting conditions, areas of low flow and flow stagnation exist within the aneurysm even at peak systole (center right); these regions are decreased during simulated exercise (right). Reprinted from White and Dalman, 2008, The Permanente Journal. 12, 10–14, with permission from The Permanente Press.
5. Concluding Remarks
Local aortic hemodynamic conditions may influence the risk for and progression of aneurysm disease. Compared with the suprarenal aorta, the infrarenal environment in resting subjects is characterized by increased peripheral resistance, increased oscillatory wall shear stress and stagnant flow. Several lines of investigations, both clinical and experimental, provide increasing evidence that sedentary hemodynamic conditions contribute to disease susceptibility through underlying influences on inflammatory tone. Experimental models of AAA suggest an inverse relationship between aortic laminar SS and aneurysm expansion. The possibility that hemodynamic conditions in the aorta may be modified physiologically to affect the natural history of AAA disease has broad implications on aneurysm risk assessment and treatment strategies. Current efforts to translate these hemodynamic observations are aimed at examining the associations between physical activity and AAA progression through incorporation of an ongoing supervised exercise clinical trial. Results from this study may further elucidate the biological mechanisms behind the protective effect of exercise-induced hemodynamics and demonstrate a highly effective adjunct to attenuate AAA disease.
Acknowledgments
Portions of this review were supported by NIH grants 2 R01 HL064338-08 and 1 P50 HL083800-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Abbruzzese TA, Guzman RJ, Martin RL, Yee C, Zarins CK, Dalman RL. Matrix metalloproteinase inhibition limits arterial enlargements in a rodent arteriovenous fistula model. Surgery. 1998;124:328–334. [PubMed] [Google Scholar]
- Ailawadi G, Eliason J, Upchurch G., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003a;38:584–588. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- Ailawadi G, Knipp BS, Lu G, Roelofs KJ, Ford JW, Hannawa KK, Bishop K, Thanaporn P, Henke PK, Stanley JC, Upchurch GR., Jr A nonintrinsic regional basis for increased infrarenal aortic MMP-9 expression and activity. J Vasc Surg. 2003b;37:1059–1066. doi: 10.1067/mva.2003.163. [DOI] [PubMed] [Google Scholar]
- Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–981. doi: 10.1161/01.cir.82.3.973. [DOI] [PubMed] [Google Scholar]
- Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RAP, Thompson SG, Walker NM. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–1539. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43:749–756. doi: 10.1016/0026-0495(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Bauman WA, Kahn NN, Grimm DR, Spungen AM. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord. 1999;37:601–616. doi: 10.1038/sj.sc.3100911. [DOI] [PubMed] [Google Scholar]
- Baxter BT, Terrin MC, Dalman RL. Medical management of small abdominal aortic aneurysms. Circulation. 2008;117:1883–1889. doi: 10.1161/CIRCULATIONAHA.107.735274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, Inflammation, and Cardiovascular Disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Blanchard JF, Armenian HK, Friesen PP. Risk factors for abdominal aortic aneurysm: results of a case-control study. Am J Epidemiol. 2000;151:575–583. doi: 10.1093/oxfordjournals.aje.a010245. [DOI] [PubMed] [Google Scholar]
- Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation. 2004;110:16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- Brady AR, Brown LC, Fowkes FGR, Greenhalgh RM, Powell JT, Ruckley CV, Thompson SG. The United Kingdom Small Aneurysm Trial Participants. Long-Term Outcomes of Immediate Repair Compared with Surveillance of Small Abdominal Aortic Aneurysms. N Engl J Med. 2002;346:1445–1452. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–11. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Herfkens RJ, Taylor CA. Comparison of abdominal aortic hemodynamics between men and women at rest and during lower limb exercise. J Vasc Surg. 2003a;37:118–123. doi: 10.1067/mva.2002.107. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamic conditions in healthy subjects aged 50–70 at rest and during lower limb exercise: in vivo quantification using MRI. Atherosclerosis. 2003b;168:323–331. doi: 10.1016/s0021-9150(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Schwandt DF, Topp EL, Anderson JH, Herfkens RJ, Taylor CA. Dynamic exercise imaging with an MR-compatible stationary cycle within the general electric open magnet. Magn Reson Med. 2003c;49:581–585. doi: 10.1002/mrm.10364. [DOI] [PubMed] [Google Scholar]
- Choke E, Thompson MM, Dawson J, Wilson WRW, Sayed S, Loftus IM, Cockerill GW. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler Thromb Vasc Biol. 2006a;26:2077–2082. doi: 10.1161/01.ATV.0000234944.22509.f9. [DOI] [PubMed] [Google Scholar]
- Choke E, Cockerill GW, Dawson J, Wilson RW, Jones A, Loftus IM, Thompson MM. Increased angiogenesis at the site of abdominal aortic aneurysm rupture. Ann N Y Acad Sci. 2006b;1085:315–319. doi: 10.1196/annals.1383.007. [DOI] [PubMed] [Google Scholar]
- Cowan JA, Dimick JB, Henke PK, Rectenwald J, Stanley JC, Upchurch GR. Epidemiology of aortic aneurysm repair in the United States from 1993 to 2003. Ann NY Acad Sci. 2006;1085:1–10. doi: 10.1196/annals.1383.030. [DOI] [PubMed] [Google Scholar]
- Dalman RL. Oxidative stress and abdominal aneurysms: how aortic hemodynamic conditions may influence AAA disease. Cardiovasc Surg. 2003;11:417–419. doi: 10.1016/S0967-2109(03)00075-9. [DOI] [PubMed] [Google Scholar]
- Dalman RL, Tedesco MM, Myers J, Taylor CA. AAA disease: mechanism, stratification, and treatment. Ann N Y Acad Sci. 2006;1085:92–109. doi: 10.1196/annals.1383.008. [DOI] [PubMed] [Google Scholar]
- Darling RC, Messina CR, Brewster DC, Ottinger LW. Autopsy study of unoperated abdominal aortic aneurysms. The case for early resection. Circulation. 1977;56(3Suppl):II161–164. [PubMed] [Google Scholar]
- De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Dillavou ED, Muluk SC, Makaroun MS. Improving aneurysm-related outcomes: nationwide benefits of endovascular repair. J Vasc Surg. 2006;43:446–451. doi: 10.1016/j.jvs.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Doyle BJ, Callanan A, Burke PE, Grace PA, Walsh MT, Vorp DA, McGloughlin TM. Vessel asymmetry as an additional diagnostic tool in the assessment of abdominal aortic aneurysms. J Vasc Surg. 2009;49:443–454. doi: 10.1016/j.jvs.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger MF, Raghavan ML, Marra SP, Cronenwett JL, Kennedy FE. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg. 2002;36:589–597. doi: 10.1067/mva.2002.125478. [DOI] [PubMed] [Google Scholar]
- Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg. 2003;37:724–732. doi: 10.1067/mva.2003.213. [DOI] [PubMed] [Google Scholar]
- Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142:203–211. doi: 10.7326/0003-4819-142-3-200502010-00012. [DOI] [PubMed] [Google Scholar]
- Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S adults. Epidemiology. 2002;13:561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Jr, Anderson KR, Topper JN, Langille BL, Clowes AW, Bercel S, Davies MG, Stenmark KR, Frid MG, Weiser-Evans MC, Aldashev AA, Nemenoff RA, Majesky MW, Landerholm TE, Lu J, Ito WD, Arras M, Scholz D, Imhof B, Aurrand-Lions M, Schaper W, Nagel TE, Resnick N, Dewey CF, Gimbrone MA, Davies PF. Special communication: the critical role of mechanical forces in blood vessel development, physiology and pathology. J Vasc Surg. 1999;29:1104–1151. doi: 10.1016/s0741-5214(99)70252-1. [DOI] [PubMed] [Google Scholar]
- Golledge J, Clancy P, Jamrozik K, Norman PE. Obesity, adipokines, and abdominal aortic aneurysm: Health in Men study. Circulation. 2007;116:2275–2279. doi: 10.1161/CIRCULATIONAHA.107.717926. [DOI] [PubMed] [Google Scholar]
- Golledge J, Tsao PS, Dalman RL, Norman PE. Circulating markers of abdominal aortic aneurysm presence and progression. Circulation. 2008;118:2382–2392. doi: 10.1161/CIRCULATIONAHA.108.802074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon IL, Kohl CA, Arefi M, Complin RA, Vulpe M. Spinal cord injury increases the risk of abdominal aortic aneurysm. Am Surg. 1996;62:249–252. [PubMed] [Google Scholar]
- Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve JM, Les AS, Tang BT, Draney Blomme MT, Wilson NM, Dalman RL, Pelc NJ, Taylor CA. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am J Physiol Heart Circ Physiol. 2006;291:H1700–H1708. doi: 10.1152/ajpheart.00274.2006. [DOI] [PubMed] [Google Scholar]
- Guzman RJ, Abe K, Zarins CK. Flow-induced arterial enlargement is inhibited by suppression of nitric oxide synthase activity in vivo. Surgery. 1997;122:273–279. doi: 10.1016/s0039-6060(97)90018-0. [DOI] [PubMed] [Google Scholar]
- Hall AJ, Busse EF, McCarville DJ, Burgess JJ. Aortic wall tension as a predictive factor for abdominal aortic aneurysm rupture: improving the selection of patients for abdominal aortic aneurysm repair. Ann Vasc Surg. 2000;14:152–157. doi: 10.1007/s100169910027. [DOI] [PubMed] [Google Scholar]
- Hassan T, Timofeev EV, Satto T, Shimizu H, Ezura M, Matsumoto Y, Takayama K, Tominaga T, Takahasi A. A proposed parent vessel geometry based categorization of saccular intracranial aneurysms: computational flow dynamics analysis of the risk factors for lesion rupture. J Neurosurg. 2005;103:662–680. doi: 10.3171/jns.2005.103.4.0662. [DOI] [PubMed] [Google Scholar]
- He CM, Roach MR. The composition and mechanical properties of abdominal aortic aneurysms. J Vasc Surg. 1994;20:6–13. doi: 10.1016/0741-5214(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Hoshina K, Sho E, Sho M, Nakahashi TK, Dalman RL. Wall shear stress and strain modulate experimental aneurysm cellularity. J Vasc Surg. 2003;37:1067–1074. doi: 10.1016/s0741-5214(03)70052-4. [DOI] [PubMed] [Google Scholar]
- Hsiai TK. Mechanosignal transduction coupling between endothelial and smooth muscle cells: role of hemodynamic forces. Am J Physiol Cell Physiol. 2008;294:C659–661. doi: 10.1152/ajpcell.90643.2007. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Taylor CA. Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Ann Rev Biomed Eng. 2008;10:221–246. doi: 10.1146/annurev.bioeng.10.061807.160439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwowski JK, AM, Whitson J, Abbruzzese TA, Zarins CK, Dalman RL. Dose-dependent limitation of arterial enlargement by the matrix metalloproteinase inhibitor RS-113,456. J Surg Res. 1999;87:122–129. doi: 10.1006/jsre.1999.5707. [DOI] [PubMed] [Google Scholar]
- Kent KC, Zwolak RM, Jaff MR, Hollenbeck ST, Thompson RW, Schermerhorn ML, Sicard GA, Riles TS, Cronenwett JL. Screening for abdominal aortic aneurysm: a consensus statement. J Vasc Surg. 2004;39:267–269. doi: 10.1016/j.jvs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Matsubara J, Matsushita M, Nishikimi N, Sakurai T, Nimura Y. Expression of angiogenesis and angiogenic factors in human aortic vascular disease. J Surg Res. 2002;106:239–245. doi: 10.1006/jsre.2002.6468. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H, Platsoucas CD, Tilson MD. Aortic aneurysms: an immune disease with a strong genetic component. Circulation. 2008;117:242–252. doi: 10.1161/CIRCULATIONAHA.107.690982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol. 1996;74:834–841. [PubMed] [Google Scholar]
- Lawrence-Brown MM, Norman PE, Jamrozik K, Semmens JB, Donnelly NJ, Spencer C, Tuohy R. Initial results of ultrasound screening for aneurysm of the abdominal aorta in Western Australia: relevance for endoluminal treatment of aneurysm disease. Cardiovasc Surg. 2001;9:234–240. doi: 10.1016/s0967-2109(00)00143-5. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, Krupski WC, Barone GW, Acher CW, Ballard DJ. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126:441–449. doi: 10.7326/0003-4819-126-6-199703150-00004. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Johnson GR, Wilson SE, Gordon IL, Chute EP, Littooy FN, Krupski WC, Bandyk D, Barone GW, Graham LM, Hye RJ, Reinke DB. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J Vasc Surg. 1997;26:595–601. doi: 10.1016/s0741-5214(97)70057-0. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG. The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med. 2000;160:1425–1430. doi: 10.1001/archinte.160.10.1425. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Nelson DB, Joseph AM. Smokers’ relative risk for aortic aneurysm compared with other smoking-related diseases: a systematic review. J Vasc Surg. 2003;38:329–334. doi: 10.1016/s0741-5214(03)00136-8. [DOI] [PubMed] [Google Scholar]
- Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT, Jr, Matsumura JS, Kohler TR, Lin PH, Jean-Claude JM, Cikrit DF, Swanson KM, Peduzzi PN Open Versus Endovascular Repair (OVER) Veterans Affairs Cooperative Study Group. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302:1535–1542. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- Lee ES, Pickett E, Hedayati N, Dawson DL, Pevec WC. Implementation of an aortic screening program in clinical practice: implications for the Screen For Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act. J Vasc Surg. 2009;49:1107–1111. doi: 10.1016/j.jvs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Li ZY, U-King-Im J, Tang TY, Soh E, See TC, Gillard JH. Impact of calcification and intraluminal thrombus on the computed wall stresses of abdominal aortic aneurysm. J Vasc Surg. 2008;47:928–935. doi: 10.1016/j.jvs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Lindholt JS, Juul S, Fasting H, Henneberg EW. Hospital costs and benefits of screening for abdominal aortic aneurysms. Results from a randomised population screening trial. Eur J Vasc Endovasc Surg. 2002;23:55–60. doi: 10.1053/ejvs.2001.1534. [DOI] [PubMed] [Google Scholar]
- Masuda H, Zhuang YJ, Singh TM, Kawamura K, Murakami M, Zarins CK, Glagov S. Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol. 1999;19:2298–2307. doi: 10.1161/01.atv.19.10.2298. [DOI] [PubMed] [Google Scholar]
- Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002;22:560–565. doi: 10.1161/01.atv.0000013778.72404.30. [DOI] [PubMed] [Google Scholar]
- Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, Shibuya M. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. 2006;108:1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- Nakahashi TK, Hoshina K, Tsao PS, Sho E, Sho M, Karwowski JK, Yeh C, Yang RB, Topper JN, Dalman RL. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002;22:2017–2022. doi: 10.1161/01.atv.0000042082.38014.ea. [DOI] [PubMed] [Google Scholar]
- Niezen RA, Doornbos J, van der Wall EE, de Roos A. Measurement of aortic and pulmonary flow with MRI at rest and during physical exercise. J Comput Assist Tomogr. 1998;22:194–201. doi: 10.1097/00004728-199803000-00006. [DOI] [PubMed] [Google Scholar]
- Ouriel K. The PIVOTAL study: a randomized comparison of endovascular repair versus surveillance in patients with smaller abdominal aortic aneurysms. J Vasc Surg. 2009;49:266–269. doi: 10.1016/j.jvs.2008.11.048. [DOI] [PubMed] [Google Scholar]
- Pedersen EM, Kozerke S, Ringgaard S, Scheidegger MB, Boesiger P. Quantitative abdominal aortic flow measurements at controlled levels of ergometer exercise. Magn Reson Imaging. 1999;17:489–494. doi: 10.1016/s0730-725x(98)00209-4. [DOI] [PubMed] [Google Scholar]
- Raghavan ML, Vorp DA, Federle MP, Makaroun MS, Webster MW. Wall stress distribution on three-dimensionally reconstructed models of human abdominal aortic aneurysm. J Vasc Surg. 2000a;31:760–769. doi: 10.1067/mva.2000.103971. [DOI] [PubMed] [Google Scholar]
- Raghavan ML, Vorp DA. Toward a biomechanical tool to evaluate rupture potential of abdominal aortic aneurysm: identification of a finite strain constitutive model and evaluation of its applicability. J Biomech. 2000b;33:475–482. doi: 10.1016/s0021-9290(99)00201-8. [DOI] [PubMed] [Google Scholar]
- Resnick N, Yahav H, Schubert S, Wolfovitz E, Shay A. Signalling pathways in vascular endothelium activated by shear stress: relevance to atherosclerosis. Curr Opin Lipidol. 2000;11:167–177. doi: 10.1097/00041433-200004000-00010. [DOI] [PubMed] [Google Scholar]
- Sandgren T, Sonesson B, Ryden A, Lanne T. Arterial dimensions in the lower extremities of patients with abdominal aortic aneurysms--no indications of a generalized dilating diathesis. J Vasc Surg. 2001;34:1079–1084. doi: 10.1067/mva.2001.119399. [DOI] [PubMed] [Google Scholar]
- Schalet B, Taylor C, Harris E, Herfkens R, Zarins C. Quantitative assessment of human aortic bood flow during exercise. Surg Forum. 1997;XLVIII:359–362. [Google Scholar]
- Schillinger M, Exner M, Mlekusch W, Domanovits H, Huber K, Mannhalter C, Wagner O, Minar E. Heme oxygenase-1 gene promoter polymorphism is associated with abdominal aortic aneurysm. Thromb Res. 2002;106:131–136. doi: 10.1016/s0049-3848(02)00100-7. [DOI] [PubMed] [Google Scholar]
- Sho E, Sho M, Hoshina K, Kimura H, Nakahashi TK, Dalman RL. Hemodynamic forces regulate mural macrophage infiltration in experimental aortic aneurysms. Exp Mol Pathol. 2004a;76:108–116. doi: 10.1016/j.yexmp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Hemodynamic regulation of CD34+ cell localization and differentiation in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2004b;24:1916–1921. doi: 10.1161/01.ATV.0000142805.20398.74. [DOI] [PubMed] [Google Scholar]
- Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study : The Tromsø Study. Am J Epidemiol. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- Sonesson B, Sandgren T, Länne T. Abdominal aortic aneurysm wall mechanics and their relation to risk of rupture. Eur J Vasc Endovasc Surg. 1999;18:487–493. doi: 10.1053/ejvs.1999.0872. [DOI] [PubMed] [Google Scholar]
- Speelman L, Bohra A, Bosboom EMH, Schurink GWH, van de Vosse FN, Makaroun MS, Vorp DA. Effects of wall calcifications in patient-specific wall stress analyses of abdominal aortic aneurysms. J Biomech Eng. 2007;129:105–109. doi: 10.1115/1.2401189. [DOI] [PubMed] [Google Scholar]
- Stenbaek J, Kalin B, Swedenborg J. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;20:466–469. doi: 10.1053/ejvs.2000.1217. [DOI] [PubMed] [Google Scholar]
- Tang BT, Cheng CP, Draney MT, Wilson NM, Tsao PS, Herfkens RJ, Taylor CA. Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: quantification using image-based computer modeling. Am J Physiol Heart Circ Physiol. 2006;291:H668–H676. doi: 10.1152/ajpheart.01301.2005. [DOI] [PubMed] [Google Scholar]
- Tang PC, Qin L, Zielonka J, Zhou J, Matte-Martone C, Bergaya S, van Rooijen N, Shlomchik WD, Min W, Sessa WC, Pober JS, Tellides G. MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med. 2008;205:3159–3171. doi: 10.1084/jem.20081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Cheng CP, Espinosa LA, Tang BT, Parker D, Herfkens RJ. In vivo quantification of blood flow and wall shear stress in the human abdominal aorta during lower limb exercise. Ann Biomed Eng. 2002;30:402–408. doi: 10.1114/1.1476016. [DOI] [PubMed] [Google Scholar]
- Tobiasch E, Günther L, Bach FH. Heme oxygenase-1 protects pancreatic beta cells from apoptosis caused by various stimuli. J Investig Med. 2001;49:566–571. doi: 10.2310/6650.2001.33721. [DOI] [PubMed] [Google Scholar]
- Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20:E120–126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, De Lisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dernbach E, Reissner A, Vasa M, Zeiher AM, Dimmeler S. Shear stress-induced endothelial cell migration invovles integrin signaling via the fibronectin receptor subunits alpha(5) and beta(1) Arterioscler Thromb Vasc Biol. 2002;22:69–75. doi: 10.1161/hq0102.101518. [DOI] [PubMed] [Google Scholar]
- Vainas T, Lubbers T, Stassen FRM, Herngreen SB, van Dieijen-Visser MP, Bruggeman CA, Kitslaar PJEHM, Schurink GWH. Serum C-reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation. 2003;107:1103–1105. doi: 10.1161/01.cir.0000059938.95404.92. [DOI] [PubMed] [Google Scholar]
- van den Bosch MA, van der Graaf Y, Eikelboom BC, Algra A, Mali WP. Distal aortic diameter and peripheral arterial cclusive disease. J Vasc Surg. 2001;34:1085–1089. doi: 10.1067/mva.2001.118809. [DOI] [PubMed] [Google Scholar]
- Vande Geest JP, Di Martino ES, Bohra A, Makaroun MS, Vorp DA. A biomechanics-based rupture potential index for abdominal aortic aneurysm risk assessment: demonstrative application. Ann N Y Acad Sci. 2006;1085:11–21. doi: 10.1196/annals.1383.046. [DOI] [PubMed] [Google Scholar]
- Verloes A, Sakalihasan N, Koulischer L, Limet R. Aneurysms of the abdominal aorta: familial and genetic aspects in three hundred thirteen pedigrees. J Vasc Surg. 1995;21:646–655. doi: 10.1016/s0741-5214(95)70196-6. [DOI] [PubMed] [Google Scholar]
- Vollmar JF, Paes E, Pauschinger P, Henze E, Friesch A. Aortic aneurysms as late sequelae of above-knee amputation. Lancet. 1989;2:834–835. doi: 10.1016/s0140-6736(89)92999-1. [DOI] [PubMed] [Google Scholar]
- Vorp DA. Biomechanics of abdominal aortic aneurysm. J Biomech. 2007;40:1887–1902. doi: 10.1016/j.jbiomech.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman SM, Mehraban F, Komuves LG, Yang RB, Tomlinson JE, Zhang Y, Spriggs F, Topper JN. Gene expression profile of human endothelial cells exposed to sustained fluid shear stress. Physiol Genomics. 2002;12:13–23. doi: 10.1152/physiolgenomics.00102.2002. [DOI] [PubMed] [Google Scholar]
- Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DEJ, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94:3026–3049. doi: 10.1161/01.cir.94.11.3026. [DOI] [PubMed] [Google Scholar]
- White JJ, Dalman RL. Gaining new insights into early abdominal aortic aneurysm disease. The Permanente Journal. 2008;12:10–14. doi: 10.7812/tpp/07-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Arai H, Zhuge X, Sano H, Murayama T, Yoshimoto M, Heike T, Nakahata T, Nishikawa S-i, Kita T, Yokode M. Role of bone marrow-derived progenitor cells in cuff-induced vascular injury in mice. Arterioscler Thromb Vasc Biol. 2004;24:477–482. doi: 10.1161/01.ATV.0000118016.94368.35. [DOI] [PubMed] [Google Scholar]
- Yeung JJ, Kim HJ, Abbruzzese TA, Vignon-Clementel IE, Draney-Blomme MT, Yeung KK, Perkash I, Herfkens RJ, Taylor CA, Dalman RL. Aortoiliac hemodynamic and morphologic adaptation to chronic spinal cord injury. J Vasc Surg. 2006;44:1254–1265. doi: 10.1016/j.jvs.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Zeilig G, Dolev M, Weingarden H, Blumen N, Shemesh Y, Ohry A. Long-term morbidity and mortality after spinal cord injury: 50 years of follow-up. Spinal Cord. 2000;38:563–566. doi: 10.1038/sj.sc.3101043. [DOI] [PubMed] [Google Scholar]
- Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004;24:470–476. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]