Abstract
To add to the existing evidence, which comes mostly from within White populations, we conducted a nested case-control study within the Multiethnic Cohort to examine the association between circulating sex hormones and breast cancer risk among postmenopausal women. Of the women who had a plasma sample, 129 women developed breast cancer during follow-up. Two controls per case, matched on area (Hawaii, Los Angeles), ethnicity/race (Japanese American, White, Native Hawaiian, African American, Latina), birth year, date of blood draw and time fasting, were selected from the women who had not developed breast cancer. Levels of estradiol, estrone, androstenedione, dehydroepiandrosterone (DHEA) and testosterone were quantified with radioimmunoassay after organic extraction and Celite column chromatography separation. Estrone sulfate, dehydroepiandrosterone sulfate (DHEAS), and sex hormone binding globulin (SHBG) were quantified by direct immunoassays. As estimated with conditional logistic regression, the sex hormones were positively associated and SHBG was negatively associated with breast cancer risk. All associations, except those with DHEAS and testosterone showed a significant linear trend. The odds ratio associated with a doubling of estradiol was 2.29 (95% confidence interval (CI) 1.58–2.35) and the odds ratio associated with a doubling of testosterone was 1.35 (95% CI 0.98–1.82). The associations in Japanese American women, who constituted 54% of our sample, were similar or slightly qualitatively stronger than in those of other ethnicity/race. This study supports previous evidence of an association between sex hormones and breast cancer risk and suggests that the associations are similar in Japanese American women.
Keywords: estrogens, androgens, blood, breast cancer, postmenopausal, nested case-control
Introduction
Many risk factors for postmenopausal breast cancer are thought to be mediated by a hormonal mechanism (Clemons & Goss 2001, Henderson & Feigelson 2000). The investigation of circulating sex hormones has provided more direct evidence. A pooled analysis published in 2002 (Key et al. 2002) of nine prospective studies of women not using hormone replacement therapy (HRT) (Barrett-Connor et al. 1990, Berrino et al. 1996, Cauley et al. 1999, Dorgan et al. 1997, Dorgan et al. 1996, Garland et al. 1992, Gordon et al. 1990, Hankinson et al. 1998, Helzlsouer et al. 1994, Kabuto et al. 2000, Thomas et al. 1997, Toniolo et al. 1995, Zeleniuch-Jacquotte et al. 1997), found that all estrogenic and androgenic steroid sex hormones examined were associated with increased breast cancer risk, and that sex hormone binding globulin (SHBG) was associated with decreased risk. Since the publication of this pooled analysis, these findings have been supported for the most part by analyses in some of the studies with extended follow-up (Cummings et al. 2005, Eliassen et al. 2006, Missmer et al. 2004, Sieri et al. 2009, Zeleniuch-Jacquotte et al. 2004) and additional large prospective studies (Beattie et al. 2006, Cummings et al. 2002, Gunter et al. 2009, Kaaks et al. 2005, Manjer et al. 2003, Onland-Moret et al. 2003).
With a single exception, all of these studies were conducted in largely Caucasian populations. Kabuto et al. (2000) investigated the association with estradiol, SHBG and dehydroepiandrosterone sulfate (DHEAS) in Japanese women but had only 26 postmenopausal cases and did not investigate testosterone levels. The odds ratio (OR) for the association with a doubling of estradiol was 0.90 (95% confidence interval (CI) 0.42–1.92) (reported in (Key et al. 2002)) suggesting that the association may be different in women of Japanese ancestry. Two case-control studies conducted in China, on the other hand, found that breast cancer risk in Asian postmenopausal women was positively associated with estrogen and testosterone levels (Wang et al. 2009, Yu et al. 2003). This preliminary report examines the association between estrogens (estradiol, estrone, estrone sulfate), androgens (androstenedione, DHEA, DHEAS, testosterone), and SHBG and breast cancer among postmenopausal women not using HRT within the Multiethnic Cohort (MEC) study, in which a large proportion of the participants are Japanese American.
Materials and methods
Study design and population
We conducted a case-control study nested within the MEC. This cohort includes over 215,000 men and women between the ages of 45 and 75 years living in Hawaii and Los Angeles who returned a baseline questionnaire between 1993 and 1996 (Kolonel et al. 2000). From information in the questionnaire, participants were assigned to a single ethnic/racial group and if more than one group was reported, assignment was based on the following priority ranking: African American, Native Hawaiian, Latino, Japanese American, and White. More than 95% of all non-Hawaiians reported only one ethnic group. Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2). Diet was assessed with a food frequency questionnaire inquiring about frequency of consumption and portion size for over 180 food items consumed over the past year. Menopausal status was inferred from reported cessation of menstrual periods, oophorectomy, and initiation of HRT.
Between 2001 and 2006, 67,594 MEC participants agreed to donate a biospecimen and complete a short questionnaire that updated information including HRT use and menopausal status. Fasting blood was drawn in the early morning at a clinical laboratory or in the participant’s home, processed, and separated. Plasma was divided into 2 mL aliquots and stored at −80 C. The MEC and this study were approved by the Institutional Review Boards of the University of Southern California and the University of Hawaii. All participants provided informed consent at time of blood collection.
Cases and controls
Cases were women who had contributed a blood specimen before being diagnosed with a first primary breast cancer to the end of follow-up (December, 2006). Diagnoses were identified by linkage with the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registries that cover the entire population of the two states. Deaths were identified by linkage with state vital statistics databases and the National Death Index. Two control women per case were randomly selected from a pool of subcohort members alive and not diagnosed with breast cancer at the age of the diagnosis of the case, and matched to the case by area (Hawaii, Los Angeles), ethnicity, birth year (± 1 year), date of blood draw (± 6 months), time of blood draw (± 2 hours), hours fasting before blood draw (0–<6, 6–<8, 8–<10, ≥10), and type of biospecimen (serum, plasma). Some women only had serum available and were excluded because the volume of serum that could be extracted from the 0.5 mL straws in which it was stored was too low to reliably quantify the low hormone levels found in postmenopausal women.
Of the 327 cases who had a fasting blood sample available in the biorepository, 124 were currently using HRT, 70 only had serum samples, and one had been matched to controls with serum samples by mistake. Of the 264 controls matched to the remaining 132 cases, one did not have a plasma sample, and two did not have sufficient sample for assays. Thus, 129 cases with two matched controls and three cases with one matched control had assay results for the analytes of primary interest (estrogens, testosterone, and DHEA). One control did not have sufficient sample to assay SHBG and DHEAS.
Laboratory assays
Assays for sex hormones and SHBG were performed at the Reproductive Endocrine Research Laboratory at the University of Southern California (FZS). Each batch included matched case-control sets and pooled quality control samples, which were in random order and the identity of which were blinded to laboratory personnel, plus laboratory standards. Levels of the unconjugated steroid hormones were determined by radioimmunoassay after organic extraction and partition chromatography on Celite columns (Stanczyk et al. 2007). Levels of the conjugated steroid hormones were determined using direct immunoassays (Ranadive et al. 1998). Levels of SHBG were determined using a chemiluminescent immunometric assay on an Immulite analyzer (Siemens Medical Solutions, Los Angeles, CA). The limits of detection were 5 pg/mL for estrone, 0.01 ng/mL for estrone sulfate, 2 pg/mL for estradiol, 0.03 ng/mL for androstenedione, 0.04 ng/mL for DHEA, 15 μg/dL for DHEAS, 1.5 ng/dL for testosterone, and 1 nmol/L for SHBG. Seven women were below the limit for estradiol and 57 women were below the limit for DHEAS. These women were assumed to have levels equal to half of the detection limit. Bioavailable testosterone and estradiol were estimated with a validated algorithm using the measured concentrations of the sex hormones and SHBG and an assumed albumin concentration of 43 g/L (Rinaldi et al. 2002, Sodergard et al. 1982, Vermeulen et al. 1999). The intra-batch coefficients of variation were [10%] for estrone, [8.5%] for estrone sulfate, [9%] for estradiol, [6%] for androstenedione, [4%] for DHEA, [4.5%] for DHEAS, [3.5%] for testosterone, and [8%] for SHBG.
Statistical analysis
Because levels of hormones and SHBG were positively skewed, they were logarithmically (base 2) transformed when used as continuous variables. Odds ratios estimating the association between quartiles of hormone concentrations based on the distribution in controls and breast cancer risk were computed using conditional logistic regression. Odds ratios estimating the association between a doubling of hormone concentrations and breast cancer risk were also computed; these analyses also constituted our test for linear trend. Included in the models were two of the matching factors as continuous variables, age at blood draw and hours fasting, to control for any residual differences in these variables between cases and controls. We also evaluated a number of risk factors as potential confounders (listed in Table 1). None were retained as confounders because when added to the models, they changed the ORs associated with a doubling of hormone concentrations no more than 10%. Analyses stratified by HRT use (past, never) and time between blood collection and diagnosis (<1, ≥1 year) were done. These analyses used hormone levels in tertiles; the product between the stratification variable and hormone levels as continuous variables was introduced into the models to examine the significance of the effect modification. An analysis stratified by ethnicity (Japanese American, other) also used hormone levels in tertiles and as continuous variables. The analyses were done using the LOGISTIC procedure with a STRATA statement in SAS version 9.1 (SAS Institute, Cary, NC). All statistical tests were two-sided with a 0.05 level of significance.
Table 1.
Characteristics of the cases and controls
| Characteristic | Cases | Controls |
|---|---|---|
| N (%) | N (%) | |
| Number of subjects | 132 (100) | 261 (100) |
| Ethnicity/racea | ||
| Japanese American | 72 (54.5) | 142 (54.4) |
| White | 30 (22.7) | 60 (23.0) |
| Native Hawaiian | 17 (12.9) | 34 (13.0) |
| African American | 8 (6.1) | 15 (5.7) |
| Latina | 5 (3.8) | 10 (3.8) |
| Number of childrenb | ||
| 0 | 17 (12.9) | 28 (10.7) |
| 1 | 7 (5.3) | 24 (9.2) |
| 2–3 | 70 (53.0) | 134 (51.3) |
| >=4 | 38 (28.8) | 74 (28.4) |
| Aged ≥ 25 years at first birth, parous womenb | 40 (34.8) | 65 (27.9) |
| Hawaii a | 113 (85.6) | 224 (85.8) |
| >12 years of educationb | 91 (68.9) | 172 (65.9) |
| Aged <13 years at menarche b | 70 (53.0) | 145 (55.6) |
| Past oral contraceptive use | 67 (50.8) | 119 (45.6) |
| Past use of HRTb | 68 (51.5) | 132 (50.6) |
| 1st degree family history of breast cancer | 10 (7.6) | 34 (13.0) |
| ≥1 alcoholic drink/day | 12 (9.1) | 23 (8.8) |
| Median (IQR) | Median (IQR) | |
| Age at blood drawa (years) | 68.0(61.4 – 75.7) | 68.1 (61.2 – 75.2) |
| Years between blood draw and diagnosisa | 0.9(0.5 – 1.9) | 1.0 (0.5 – 1.9) |
| Years between baseline and blood draw | 9.2(8.6 – 10.4) | 9.4 (8.9 – 10.4) |
| Hours fasting before blood drawa | 13.1(12.0 – 14.1) | 12.9 (11.8 – 14.1) |
| Body mass indexb (kg/m2) | 24.7(22.4 – 27.6) | 24.0 (21.5 – 27.5) |
| Physical activityb (MET-hours/week) | 4.2(1.4 – 8.9) | 3.6 (2.0 – 8.2) |
IQR, Interquartile range (25th percentile to 75th percentile); MET, metabolic equivalent.
Matching variables.
Summary statistics based on slightly fewer than 132 cases and 261 controls due to missing values.
Results
Characteristics of the 132 cases and 261 controls are shown in Table 1. The median age of the participants was 68 years and over half of the women were Japanese American. About half of both cases and controls used HRT in the past. Levels of the sex hormones were higher in cases than controls and level of SHBG was lower in cases than controls (Table 2). Among controls, levels of the analytes were often significantly correlated. For example, the Pearson correlations of estradiol with estrone, testosterone and SHBG were 0.51, 0.33, and −0.27, respectively.
Table 2.
Geometric mean (95% confidence interval) concentrations of sex hormones and SHBG in cases and controls
| Sex hormone/SHBG | Cases | Controls |
|---|---|---|
| SHBG (nmol/L) | 37.6 (34.7 – 40.9) | 43.6 (40.7 – 46.7) |
| Estrone (pmol/L) | 132.0 (121.9 – 142.9) | 111.9 (105.7 – 118.4) |
| Estrone sulfate (pmol/L) | 1363 (1218 – 1525) | 1168 (1092 – 1250) |
| Estradiol (pmol/L) | 36.7 (33.4 – 40.5) | 28.3 (26.4 – 30.4) |
| Free estradiol (pmol/L) | 0.96 (0.87 – 1.07) | 0.70 (0.64 – 0.76) |
| Androstenedione (nmol/L) | 2.02 (1.87 – 2.19) | 1.73 (1.63 – 1.84) |
| DHEA (nmol/L) | 7.61 (6.82 – 8.49) | 6.30 (5.79 – 6.86) |
| DHEAS (nmol/L) | 1068 (927 – 1229) | 916 (825 – 1018) |
| Testosterone (nmol/L) | 0.83 (0.76 – 0.90) | 0.75 (0.71 – 0.80) |
| Free testosterone (pmol/L) | 17.2 (15.9 – 18.6) | 14.4 (13.5 – 15.3) |
DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin.
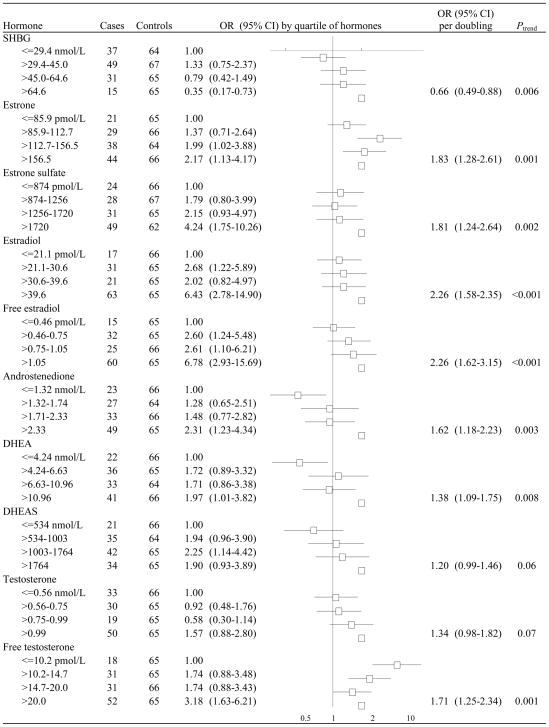
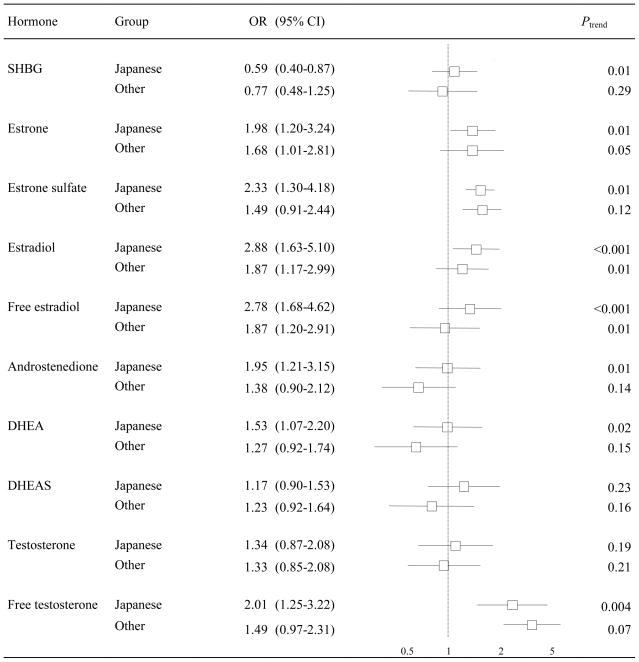
The sex hormones were positively associated with breast cancer risk and SHBG was inversely associated with breast cancer risk (Figure 1). All associations, except those with DHEAS and testosterone, showed a significant trend, the strongest of which were observed for estradiol and free estradiol (Figure 2). Women who had estradiol levels in the highest quartile had an approximately six-fold increase in breast cancer risk relative to women in the lowest quartile (OR 6.42, 95% CI 2.78–14.90). We did not find that past HRT use significantly modified any of these associations (all P for heterogeneity > 0.05). Generally the trends observed in the overall group were reflected in both past and never users of HRT but were qualitatively stronger among the latter group (data not shown). The associations between the estrogens and breast cancer risk were slightly, but nonsignificantly, stronger if blood was taken one year or more before diagnosis whereas the association between SHBG and breast cancer risk was slightly, but nonsignificantly, stronger if blood was taken less than one year before diagnosis (data not shown). As depicted in Figure 2, when the analysis was conducted among Japanese American women, all associations except those with DHEAS and testosterone showed a significant trend and tended to be stronger than those in the group of other ethnicity/race. For example, the OR for a doubling of estradiol was 2.88 (95% CI 1.63–5.10) in Japanese American women whereas it was 1.87 (95% CI 1.17–2.99) in the overall sample.
Figure 1.
Odds ratios (OR) and 95% confidence intervals (CI) for the association of hormone concentrations with the risk of breast cancer.
Figure 2.
Odds ratios (OR) and 95% confidence intervals (CI) for the association between a doubling of hormone concentrations and the risk of breast cancer among Japanese American women and women of other race/ethnicity.
Discussion
In this study, estrogenic and androgenic hormones were positively associated and SHBG was inversely associated with breast cancer risk. The pooled analysis done in 2002 (Key et al. 2002) and four of the five prospective studies of circulating hormones done since then (Cummings et al. 2002, Gunter et al. 2009, Kaaks et al. 2005, Manjer et al. 2003) have also found such associations. In the current study, however, stronger associations were observed for some analytes such as estradiol, estrone sulfate, and SHBG. For example, the OR corresponding to a doubling of estradiol was 2.26 (95% CI 1.58–3.25) whereas the summary OR in the pooled analysis was 1.29 (95% CI 1.15–1.44) with ORs ranging from 0.76 to 1.69 in the individual studies.
The impact of the short time between blood collection and diagnosis (median of 1 year), which could be construed as a limitation of our study, should be considered. Because mammary tumors express aromatase, they have the capability of producing estrogens (Pasqualini et al. 1996) and reverse causality could be a concern. However, in breast cancer patients, breast tissue estrogens do not reflect circulating estrogens (Geisler 2003). Furthermore, time between blood collection and diagnosis has been assessed in the prospective studies of sex hormones and breast cancer risk studies and most found that less time between blood and diagnosis had little effect on the association between most hormones and breast cancer risk (Dorgan et al. 1996, Hankinson et al. 1998, Kabuto et al. 2000, Key et al. 2002, Sieri et al. 2009, Zeleniuch-Jacquotte et al. 1997), but other studies found some associations to be weaker (Dorgan et al. 1997, Hankinson et al. 1998, Onland-Moret et al. 2003, Toniolo et al. 1995) and other studies yet found some associations to be stronger (Dorgan et al. 1996, Kabuto et al. 2000, Zeleniuch-Jacquotte et al. 2004). Finally, in one study that had two blood samples a mean of 31 months apart, the rate of change in hormone levels was not significantly different between cases and controls, although for androstenedione there was a suggestive difference in rates (Zeleniuch-Jacquotte et al. 2004).
Some of our associations may have been stronger than in other studies because our indirect assays were quite valid and reliable, perhaps leading to less attenuation of risk estimates due to nondifferential exposure misclassification. Estradiol measurements done in the laboratory in which our assays were performed correlate very well with the gold standard method (GC-MS/MS), as do other indirect assays, whereas direct assays without an extraction step to eliminate cross-reactivity with other hormone metabolites seem to correlate less well (Lee et al. 2006). In other reliability studies, however, direct assays correlate well with indirect assays (Rinaldi et al. 2001). Furthermore, the coefficients of variation of the assays done in this study are not noticeably lower than what has been reported in other studies that also ensure that case-control sets are in the same batch to eliminate the effect of inter-batch variability. Also possibly contributing to the strength of our associations is that over half of our sample was Japanese American women and in this study, the associations between most sex hormones and breast cancer risk were stronger than in the women in of other ethnicity/race. This difference, however, may not be enough to wholly explain our stronger results in comparison to previous studies. Several factors, including chance, may have contributed to our stronger associations between levels of estradiol, estrone sulfate, and SHBG and breast cancer risk.
The only other prospective study done among postmenopausal Asian women, conducted in Japan, found no significant association between estradiol, DHEAS, and SHBG; the point estimate for estradiol was below 1.0 but the CIs were wide due to a small sample size (Kabuto et al. 2000). Two case-control studies conducted in China, however, found a positive association between postmenopausal breast cancer risk and levels of estrogens and testosterone (Wang et al. 2009, Yu et al. 2003). The null association could have been due to chance or could have been influenced by the population under study having low levels of estradiol. It is difficult to compare the levels of sex hormones between studies due to differences in assay techniques.
The Japanese American women in the current study likely have higher levels of sex hormones than in these other studies of Asian women. Studies directly comparing Eastern and Western Japanese populations with the same assay techniques have observed lower sex hormone levels in former (Shimizu et al. 1990). In the MEC, Japanese American women have sex hormone levels similar to Whites but once BMI and other factors are taken into account, their estradiol levels are higher than Whites (Setiawan et al. 2006). The adoption of a Westernized lifestyle may be responsible for the sex hormone levels of the women of Japanese ethnicity/race in our study. Ninety-two percent of them were born in the United States and Japanese Americans in the MEC seem to have partially adopted a Westernized lifestyle with respect to dietary patterns although it is still likely to differ from Whites (Park et al. 2005). Indicators of a less Westernized lifestyle, such as a dietary pattern high in vegetable and soy intake, are associated with higher SHBG concentrations (Wu et al. 2009) and lower testosterone concentrations (Setiawan et al. 2006). Japanese American women in the baseline MEC have similar rates of breast cancer incidence relative to White women and, in fact, once other known risk factors are taken into account, they are at slightly higher risk (Pike et al. 2002, Woolcott et al. 2009). In Japanese American women, it is possible that hormone levels mediate the association between the adoption of a Westernized lifestyle and breast cancer risk.
Some other limitations of our study should be noted. We had a small sample size; although we had sufficient power to detect associations of the magnitude observed in other studies and had a larger number of women of Asian ancestry than other published prospective studies, our ability to detect differences in effect between subgroups was limited. As follow-up continues, we will be able to pursue analyses in other ethnic/racial groups. We also had blood at only one point in time. Other studies have observed that hormone levels up to five years apart are moderately correlated (Hankinson et al. 1998, Kim & Zeleniuch-Jacquotte 1997, Toniolo et al. 1995, Zeleniuch-Jacquotte et al. 2004). Our covariate information was derived from the baseline questionnaire, which was done an average of 9.3 years before the blood was drawn. This limitation only affects the time-dependent covariates, such as BMI, and are unlikely to change the conclusions in this study because most other studies have found that covariates confound the associations between circulating sex hormones and breast cancer risk very little (Cummings et al. 2005, Dorgan et al. 1996, Endogenous Hormones and Breast Cancer Collaborative Group 2003, Hankinson et al. 1998, Kaaks et al. 2005, Kabuto et al. 2000, Key et al. 2002, Onland-Moret et al. 2003, Zeleniuch-Jacquotte et al. 2004).
In conclusion, this study supports previous evidence that sex hormone levels are associated with an increased risk of breast cancer, and SHBG levels are associated with a decreased risk among postmenopausal women. It suggests that these associations are similar in Japanese American women; in these women, it is possible that hormone levels mediate the association between the adoption of a Westernized lifestyle and breast cancer risk.
Acknowledgments
Funding
This work was supported in part by National Cancer Institute (grants P01 CA33619 and R37 CA54281), and by the National Institutes of Health, Department of Health and Human Services (contracts N01-PC-35137 and N01-PC-35139). CGW was supported by a postdoctoral fellowship on grant R25 CA90956.
We thank Kami White and Anne Tome for database management, and all participants and staff involved in the study.
Footnotes
Declaration of interest
[To check - The authors had no conflict of interest that would be perceived as prejudicing the impartiality of this work.]
Author contributions
CGW carried out the statistical analysis, interpreted the results, and wrote the manuscript. LRW, and MTG also helped to interpret the results. YBS and LRW provided statistical expertise. BEH and LNK are the Principal Investigators of the Multiethnic Cohort Study and with MTG, LRW, and LLM were involved in the concept and procurement of funding for this particular project. FZS carried out the hormone assays and helped to interpret this data. All authors provided feedback on the initial draft of the manuscript and approved the final manuscript.
References
- Barrett-Connor E, Friedlander NJ, Khaw KT. Dehydroepiandrosterone sulfate and breast cancer risk. Cancer Research. 1990;50:6571–6574. [PubMed] [Google Scholar]
- Beattie MS, Costantino JP, Cummings SR, Wickerham DL, Vogel VG, Dowsett M, Folkerd EJ, Willett WC, Wolmark N, Hankinson SE. Endogenous sex hormones, breast cancer risk, and tamoxifen response: an ancillary study in the NSABP Breast Cancer Prevention Trial (P-1) Journal of the National Cancer Institute. 2006;98:110–115. doi: 10.1093/jnci/djj011. [DOI] [PubMed] [Google Scholar]
- Berrino F, Muti P, Micheli A, Bolelli G, Krogh V, Sciajno R, Pisani P, Panico S, Secreto G. Serum sex hormone levels after menopause and subsequent breast cancer. Journal of the National Cancer Institute. 1996;88:291–296. doi: 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- Cauley JA, Lucas FL, Kuller LH, Stone K, Browner W, Cummings SR. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of Osteoporotic Fractures Research Group. Annals of Internal Medicine. 1999;130:270–277. doi: 10.7326/0003-4819-130-4_part_1-199902160-00004. [DOI] [PubMed] [Google Scholar]
- Clemons M, Goss P. Estrogen and the risk of breast cancer. New England Journal of Medicine. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Lee JS, Lui LY, Stone K, Ljung BM, Cauleys JA. Sex hormones, risk factors, and risk of estrogen receptor-positive breast cancer in older women: a long-term prospective study. Cancer Epidemiology Biomarkers & Prevention. 2005;14:1047–1051. doi: 10.1158/1055-9965.EPI-04-0375. [DOI] [PubMed] [Google Scholar]
- Dorgan JF, Longcope C, Stephenson HE, Falk RT, Miller R, Franz C, Kahle L, Campbell WS, Tangrea JA, Schatzkin A. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiology Biomarkers & Prevention. 1996;5:533–539. [PubMed] [Google Scholar]
- Dorgan JF, Stanczyk FZ, Longcope C, Stephenson HE, Chang L, Miller R, Franz C, Falk RT, Kahle L. Relationship of serum dehydroepiandrosterone (DHEA), DHEA sulfate, and 5-androstene-3 beta, 17 beta-diol to risk of breast cancer in postmenopausal women. Cancer Epidemiology Biomarkers & Prevention. 1997;6:177–181. [PubMed] [Google Scholar]
- Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer: does the association vary by a woman’s predicted breast cancer risk? Journal of Clinical Oncology. 2006;24:1823–1830. doi: 10.1200/JCO.2005.03.7432. [DOI] [PubMed] [Google Scholar]
- Endogenous Hormones and Breast Cancer Collaborative Group. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. Journal of the National Cancer Institute. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- Garland CF, Friedlander NJ, Barrett-Connor E, Khaw KT. Sex hormones and postmenopausal breast cancer: a prospective study in an adult community. American Journal of Epidemiology. 1992;135:1220–1230. doi: 10.1093/oxfordjournals.aje.a116228. [DOI] [PubMed] [Google Scholar]
- Geisler J. Breast cancer tissue estrogens and their manipulation with aromatase inhibitors and inactivators. J Steroid Biochem Mol Biol. 2003;86:245–253. doi: 10.1016/s0960-0760(03)00364-9. [DOI] [PubMed] [Google Scholar]
- Gordon GB, Bush TL, Helzlsouer KJ, Miller SR, Comstock GW. Relationship of serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing postmenopausal breast cancer. Cancer Research. 1990;50:3859–3862. [PubMed] [Google Scholar]
- Gunter MJ, Hoover DR, Yu H, Wassertheil-Smoller S, Rohan TE, Manson JE, Li J, Ho GY, Xue X, Anderson GL, et al. Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute. 2009;101:48–60. doi: 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute. 1998;90:1292–1299. doi: 10.1093/jnci/90.17.1292. [DOI] [PubMed] [Google Scholar]
- Helzlsouer KJ, Alberg AJ, Bush TL, Longcope C, Gordon GB, Comstock GW. A prospective study of endogenous hormones and breast cancer. Cancer Detect Prev. 1994;18:79–85. [PubMed] [Google Scholar]
- Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, Dossus L, Lukanova A, Bingham S, Khaw KT, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocrine-Related Cancer. 2005;12:1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- Kabuto M, Akiba S, Stevens RG, Neriishi K, Land CE. A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiology Biomarkers & Prevention. 2000;9:575–579. [PubMed] [Google Scholar]
- Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. Journal of the National Cancer Institute. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Kim MY, Zeleniuch-Jacquotte A. Correcting for measurement error in the analysis of case-control data with repeated measurements of exposure. American Journal of Epidemiology. 1997;145:1003–1010. doi: 10.1093/oxfordjournals.aje.a009056. [DOI] [PubMed] [Google Scholar]
- Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. American Journal of Epidemiology. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Ettinger B, Stanczyk FZ, Vittinghoff E, Hanes V, Cauley JA, Chandler W, Settlage J, Beattie MS, Folkerd E, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. Journal of Clinical Endocrinology & Metabolism. 2006;91:3791–3797. doi: 10.1210/jc.2005-2378. [DOI] [PubMed] [Google Scholar]
- Manjer J, Johansson R, Berglund G, Janzon L, Kaaks R, Agren A, Lenner P. Postmenopausal breast cancer risk in relation to sex steroid hormones, prolactin and SHBG (Sweden) Cancer Causes & Control. 2003;14:599–607. doi: 10.1023/a:1025671317220. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. Journal of the National Cancer Institute. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- Onland-Moret NC, Kaaks R, van Noord PA, Rinaldi S, Key T, Grobbee DE, Peeters PH. Urinary endogenous sex hormone levels and the risk of postmenopausal breast cancer. British Journal of Cancer. 2003;88:1394–1399. doi: 10.1038/sj.bjc.6600890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Murphy SP, Wilkens LR, Yamamoto JF, Sharma S, Hankin JH, Henderson BE, Kolonel LN. Dietary patterns using the Food Guide Pyramid groups are associated with sociodemographic and lifestyle factors: the multiethnic cohort study. J Nutr. 2005;135:843–849. doi: 10.1093/jn/135.4.843. [DOI] [PubMed] [Google Scholar]
- Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. Journal of Clinical Endocrinology & Metabolism. 1996;81:1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- Pike MC, Kolonel LN, Henderson BE, Wilkens LR, Hankin JH, Feigelson HS, Wan PC, Stram DO, Nomura AM. Breast cancer in a multiethnic cohort in Hawaii and Los Angeles: risk factor-adjusted incidence in Japanese equals and in Hawaiians exceeds that in whites. Cancer Epidemiology Biomarkers & Prevention. 2002;11:795–800. [PubMed] [Google Scholar]
- Ranadive GN, Mistry JS, Damodaran K, Khosravi MJ, Diamandi A, Gimpel T, Castracane VD, Patel S, Stanczyk FZ. Rapid, convenient radioimmunoassay of estrone sulfate. Clinical Chemistry. 1998;44:244–249. [PubMed] [Google Scholar]
- Rinaldi S, Dechaud H, Biessy C, Morin-Raverot V, Toniolo P, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Secreto G, Ciampi A, et al. Reliability and validity of commercially available, direct radioimmunoassays for measurement of blood androgens and estrogens in postmenopausal women. Cancer Epidemiology Biomarkers & Prevention. 2001;10:757–765. [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Dechaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiology Biomarkers & Prevention. 2002;11:1065–1071. [PubMed] [Google Scholar]
- Setiawan VW, Haiman CA, Stanczyk FZ, Le ML, Henderson BE. Racial/ethnic differences in postmenopausal endogenous hormones: the multiethnic cohort study. Cancer Epidemiology Biomarkers & Prevention. 2006;15:1849–1855. doi: 10.1158/1055-9965.EPI-06-0307. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ross RK, Bernstein L, Pike MC, Henderson BE. Serum oestrogen levels in postmenopausal women: comparison of American whites and Japanese in Japan. British Journal of Cancer. 1990;62:451–453. doi: 10.1038/bjc.1990.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieri S, Krogh V, Bolelli G, Abagnato CA, Grioni S, Pala V, Evangelista A, Allemani C, Micheli A, Tagliabue G, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiology Biomarkers & Prevention. 2009;18:169–176. doi: 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. Journal of Steroid Biochemistry. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Lee JS, Santen RJ. Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiology Biomarkers & Prevention. 2007;16:1713–1719. doi: 10.1158/1055-9965.EPI-06-0765. [DOI] [PubMed] [Google Scholar]
- Thomas HV, Key TJ, Allen DS, Moore JW, Dowsett M, Fentiman IS, Wang DY. A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. British Journal of Cancer. 1997;76:401–405. doi: 10.1038/bjc.1997.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniolo PG, Levitz M, Zeleniuch-Jacquotte A, Banerjee S, Koenig KL, Shore RE, Strax P, Pasternack BS. A prospective study of endogenous estrogens and breast cancer in postmenopausal women. Journal of the National Cancer Institute. 1995;87:190–197. doi: 10.1093/jnci/87.3.190. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. Journal of Clinical Endocrinology & Metabolism. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Wang B, Mi M, Wang J, Wei N, Zhang Q, Zhu J, Yang S, Guo B, Xu J, Yang X. Does the increase of endogenous steroid hormone levels also affect breast cancer risk in Chinese women? A case-control study in Chongqing, China. International Journal of Cancer. 2009;124:1892–1899. doi: 10.1002/ijc.24132. [DOI] [PubMed] [Google Scholar]
- Woolcott CG, Maskarinec G, Pike MC, Henderson BE, Wilkens LR, Kolonel LN. Breast cancer risk and hysterectomy status: the Multiethnic Cohort study. Cancer Causes & Control. 2009;20:539–547. doi: 10.1007/s10552-008-9262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AH, Yu MC, Tseng CC, Stanczyk FZ, Pike MC. Dietary patterns and breast cancer risk in Asian American women. Am J Clin Nutr. 2009;89:1145–1154. doi: 10.3945/ajcn.2008.26915. [DOI] [PubMed] [Google Scholar]
- Yu H, Shu XO, Shi R, Dai Q, Jin F, Gao YT, Li BD, Zheng W. Plasma sex steroid hormones and breast cancer risk in Chinese women. International Journal of Cancer. 2003;105:92–97. doi: 10.1002/ijc.11034. [DOI] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Bruning PF, Bonfrer JM, Koenig KL, Shore RE, Kim MY, Pasternack BS, Toniolo P. Relation of serum levels of testosterone and dehydroepiandrosterone sulfate to risk of breast cancer in postmenopausal women. American Journal of Epidemiology. 1997;145:1030–1038. doi: 10.1093/oxfordjournals.aje.a009059. [DOI] [PubMed] [Google Scholar]
- Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, Afanasyeva Y, Kato I, Kim MY, Rinaldi S, Kaaks R, Toniolo P. Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. British Journal of Cancer. 2004;90:153–159. doi: 10.1038/sj.bjc.6601517. [DOI] [PMC free article] [PubMed] [Google Scholar]