Abstract
Objective
The current study was designed to examine the cross-sectional association between hostility and measures of abdominal fat (visceral, subcutaneous) in middle-aged African-American and white women. Because fat-patterning characteristics are known to differ by race,we were particularly interested in examining whether these associations were similar for women of both racial/ethnic groups.
Methods
Participants were 418 (45% African-American, 55% white) middle-aged women from the Chicago site of the Study of Women’s Health Across the Nation (SWAN). Visceral and Subcutaneous fat were measured by Computed Tomographic Scans and hostility was assessed via questionnaire. Multivariate linear regression models were conducted to test associations among race/ethnicity, hostility and measures of abdominal fat.
Results
In models adjusted for race/ethnicity and total percent fat, higher levels of hostility were associated with a greater amount of visceral fat (B=1.8, s.e.=.69, p=.01). This association remained significant after further adjustments for age, education, and multiple coronary heart disease (CHD) risk factors. Hostility was not associated with subcutaneous fat (p=.8). Although there were significant racial/ethnic differences in hostility (p<.001) and the amount of total body (p<.001), subcutaneous (p<.001) and visceral fat (p<.001), the associations between hostility and measures of abdominal fat did not differ for African-American compared to white women (race/ethnicity*hostility interaction p=.67 for visceral, p=.85 for subcutaneous).
Conclusions
Hostility may affect CHD risk in women via the accumulation of visceral fat. Despite significant black-white differences in fat patterning and overall CHD risk, the association between hostilty and visceral fat appears to be similar for both African-American and white women.
Keywords: Hostility, visceral fat, African-American, White, race, psychosocial risk factors, cardiovascular disease (CVD), women’s health
Background
Hostility is a personality construct that is characterized by cynical or mistrustful attitudes and beliefs about the world (1). Across studies, hostility has been associated with coronary heart disease (CHD) risk factors (2, 3) and outcomes (4, 5), including intima-media thickening of the carotid arteries (6), sudden cardiac death, and myocardial infarction (4). While there have been some null or inconsistent findings (see (7) for a review), the bulk of the evidence suggests that hostility may be an independent risk factor for both CHD and all-cause mortality (4, 8) .
Although the majority of early studies examining the association between hostility and CHD were conducted in samples comprised primarily of men (7, 8), an emerging body of research suggests that hostility may be associated with adverse CHD outcomes in women as well (9-11). With few exceptions (6, 12, 13), however, studies in women have largely focused on clinical outcomes (9-11); thus, less is known about the association between hostile attitudes and risk markers for CHD in women prior to the onset of clinical disease.
Visceral fat (i.e. fat around the internal organs) has been identified as an important pre-clinical risk factor for CHD (14), particularly in women (15, 16). Compared to total body or subcutaneous fat, visceral fat is believed to be more metabolically active and consequently is viewed as the most atherogenic, or “toxic” component of fat (17). In women, the accumulation of visceral fat has been consistently associated with indices of the metabolic syndrome--hyperinsulemia, dyslipidemia, hypertension – and other established markers of early CHD (16, 18).
To our knowledge, only one study has examined the association between hostility and visceral fat in women. In a sample of 157 postmenopausal women free of clinical disease, Raikkonen and colleagues (19) observed a positive association between self-reported hostility and visceral fat that remained significant after adjusting for a number of cardiovascular risk factors. This suggests that the development of visceral fat may be an important pathway through which hostility contributes to CHD morbidity and mortality. However, the sample in that study was predominantly white; thus, it is unknown whether hostility is similarly related to visceral fat among women of other racial/ethnic groups.
Across samples, African-American women report significantly higher levels of hostility and mistrust compared with white women (2, 20). African-American women also have more total body and subcutaneous fat than white women, as well as higher rates of overall CHD morbidity and mortality (21-23). Despite this, most studies have found that for a given level of total body fat, African-American women have lower levels of visceral fat than their white counterparts (21, 24) -- although the associations between visceral fat and disease risk appear to be equally strong for women of both racial/ethnic groups (16, 22).
In the current study, we examined the associations among race/ethnicity, hostility, and visceral fat in a population-based cohort of middle-aged African-American and white women. In order to determine whether hostility was uniquely associated with visceral fat (rather than abdominal fat more generally), we also examined the associations among race/ethnicity, hostility and subcutaneous fat. We hypothesized that women reporting higher levels of hostility would have greater amounts of visceral, but not subcutaneous, fat compared to women with lower levels of hostility. Further, given the previously reported black-white differences in levels of hostility, visceral fat, and subcutaneous fat, we explored whether the hypothesized associations differed by race/ethnicity. Finally, in order to determine whether hostility was an independent contributor to visceral fat, we examined whether any associations among race/ethnicity, hostility, and visceral fat were independent of standard CHD risk factors and physical activity.
Methods
Participants
Participants in the current study were from the Chicago, Illinois, site of the Study of Women’s Health Across the Nation (SWAN). As previously described (25), SWAN is a multiethnic, multi-site, longitudinal cohort study of the menopausal transition. The Chicago cohort consists of African-American and white women from three contiguous neighborhoods on Chicago’s south side. This area was chosen because it features an approximately equal distribution of socioeconomic status across the two racial/ethnic groups. A complete census was conducted in this area in the early 1990′s as part of an unrelated study of aging and health (26). Using information from this census on age, sex and race, Chicago SWAN recruited a random sample of women from each racial/ethnic group. Women were invited to participate in the SWAN cohort if they were aged 42-52, had an intact uterus and at least one ovary, and reported a menstrual period in the preceding three months. Women who were pregnant, breastfeeding, or reported exogenous hormone use in the three months preceding the baseline examination were ineligible. Of those eligible for participation, 72% agreed to participate. The baseline SWAN exam began in 1996-1997.
Beginning in 2002, the Chicago site collected additional measures on fat patterning as part of an ancillary “Fat Patterning Study”, designed to examine the association between the menopausal transition and the development of visceral fat. The current analyses are based on the baseline Fat Patterning Study assessment, which took place over the course of three years (2002-2005), and corresponded with the fifth through tenth annual SWAN exam (SWAN follow-up 04-09)1.
Women were only eligible for the Fat Patterning Study if they were still transitioning through menopause (i.e. not post-menopausal), had not had a hysterectomy or oophorectomy (subsequent to their enrollment in the parent SWAN study), were not pregnant or planning to become pregnant and were free of diabetes, chronic liver disease, and/or renal disease. Women with a self-reported history of alcohol/drug abuse or anorexia nervosa were ineligible. Because of equipment limitations, women were also unable to participate if they had breast implants, a hip replacement, or weighed 299 pounds or more. Of those 386 SWAN participants eligible for participation in the fat patterning study 77% (n=297) enrolled.
Because the Fat Patterning Study did not begin until year five of the parent SWAN study, many participants in the original SWAN cohort had already completed the menopausal transition. Thus, beginning in 2003 and using the eligibility criteria detailed above, we recruited an additional 138 women from the neighborhood census who were screened during the original Chicago SWAN recruitment period, but were too young to participate in 1996. Although these women were on average, five years younger, they did not differ from previously recruited women on race/ethnicity, education, hostility, body mass index (BMI kg/m2) or age-adjusted total or visceral fat. The final cohort consisted of 435 women (199 African-American and 236 white). Due to equipment malfunctions, 4 women were missing data on either intra-abdominal or total body fat and another 13 were missing data on hostility. Thus, the current analyses are based on a final sample of 418 women (189 African-American, 229 white).
Procedure
Beginning with the baseline SWAN exam and annually thereafter, participants completed a standard protocol that included self- and interviewer-administered questionnaires, measured height and weight, clinical tests, and a fasting blood and urine collection. Interviews included detailed assessments of demographic, psychosocial, and behavioral characteristics. In addition to the standard SWAN protocol, participants in the Fat Patterning Study also underwent Dual Energy X-ray Absorptiometry (DXA) scans to measure overall body fat, and Computed Tomographic (CT) scans of the abdomen, to assess the amount of visceral fat. The baseline fat patterning assessment corresponded to years five through ten of the parent SWAN study. The 138 additional participants recruited into the study to increase the number of pre- and perimenopausal participants (Fat-Patterning-Only study participants) completed the same protocol as the women in the SWAN cohort as part of a separate assessment.
Study procedures were approved by the Institutional Review Board at Rush University Medical Center and all participants provided written informed consent.
Measures
Hostility
Hostility was assessed with the 13-item cynicism subscale of the Cook-Medley Hostility Scale (1). The cynicism subscale measures cynical, distrustful attitudes and hostile feelings and behaviors, rather than overt expressions of anger. Prior research has found an association between high scores on the cynicism subscale and greater CHD morbidity and mortality (27). Sample items from the cynicism subscale include: “It is safer to trust nobody” and “I think most people would lie to get ahead”. Items have a true/false response format, with a point assigned for each “true” response. A summary score is created by summing across the 13 items, with higher scores indicative of greater cynicism or hostility (range, 0-13). Hostility was assessed only once in the full SWAN cohort because it has been found to be relatively stable in middle and later life (28-30), with test-retest reliability on the Cook-Medley ranging from .88 over three years (29) to .74 over 10 years (30). Hostility was assessed at the baseline SWAN visit (1996-1997) for women from the SWAN cohort, and at the fat patterning baseline assessment for the 138 Fat-Patterning-Only study participants. Cronbach’s alpha for the full cohort was excellent (alpha=.94).
Visceral and Subcutaneous Fat
Computed Tomography (CT) scans were used to measure both visceral and subcutaneous fat in the abdominal cavity. Each participant was examined in the supine position with both arms on her chest. A trained technician performed the CT scans using a General Electric Lightspeed VCT scanner (General Electric Medical Systems, Milwaukee, WI). After a scout view, the scan obtained a single 10-mm thick image of the abdomen at the L4-L5 vertebral space. Images were then placed on optical disks and transferred to the reading center at the University of Colorado Health Sciences Center for analysis. A trained radiologist unaware of participants’ demographic or clinical characteristics used a graph pen (cursor) to delineate the area within the muscle wall surrounding the abdominal cavity (31, 32). This area was considered the ‘Total abdominal fat area’. Visceral fat was defined as all adipose tissue within this area with an attenuation range between -190 and -30 Hounsfeld Units (31). Subcutaneous fat was calculated by subtracting the visceral fat area from the total abdominal fat area (32). Scans were read using software developed by the reading center (RSI Inc, Boulder, CO) that has also been used in several large cohort studies (15, 33). All abdominal CT scans were obtained during the first 12 days of the participants’ menstrual cycle.
Total Body Fat
Although widely used, BMI kg/m2 is a relatively crude measure of total body fat, because it includes both fat and fat-free (lean tissue + bone) mass. In order to assess total body fat mass separate from other components, whole body DXA scans were performed using a General Electric Lunar Prodigy scanner (GE-Lunar, Madison, WI). By employing two different X-ray energy sources, DXA scans are able to separate body mass into fat mass, lean tissue mass and bone mineral content. All participants were examined in a hospital gown, after removing all clothing, shoes and metal objects (e.g. jewelry, belts, underwire bras). Each participant was examined in the supine position with both arms by her side. Scans were analyzed using GE-Lunar enCORE software (Madison, WI). DXA scans were obtained on the same day as the CT scan, during the first 12 days of the participants’ menstrual cycle. For data analyses, total body fat was quantified as the percent of fat in the total body in order to represent the amount of fat for a given body size. Total percent fat was calculated as total fat mass/(total fat mass + fat-free mass).
BMI kg/m2 was also assessed at the time of the DXA scan for descriptive purposes. BMI was calculated as (measured) weight in kilograms divided by (measured) height in meters squared and obesity was defined as BMI ≥30 kg/m2.
Descriptives/Covariates
Race/Ethnicity was self-reported as either non-Hispanic African-American or non-Hispanic white (referent). Age and education were self-reported in years and modeled continuously in all analyses. Continuous values of sex hormone binding globulin (SHBG) were used as a biological measure of menopausal status and were assessed from fasting blood specimens. Blood was drawn on the morning following an overnight fast, during days two to five of the menstrual cycle. All samples were maintained at 4°C until separated and then were frozen at −80°C and shipped on dry ice to central laboratory. SHBG was then measured by a competitive chemiluminescent assay. We also assessed standard CHD risk factors consistently associated with visceral fat in other studies such as cigarette smoking, systolic blood pressure (SBP), high density lipoprotein cholesterol (HDL-c) and physical activity (34). For descriptive purposes, we assessed additional CHD risk factors less consistently associated with visceral fat, such as diastolic blood pressure (DBP), total cholesterol, and low density lipoprotein (LDL) cholesterol. Current cigarette smoking was self-reported as either yes or no. SBP and DBP measurements were obtained by a trained technician and standardized for cuff size, position and rest period. Two blood pressure readings were taken for each participant and the average score was used in all analyses. Measurements of total cholesterol, LDL and HDL-c were taken from blood specimens obtained between the second and fifth days of each participant’s menstrual cycle, following a 12-hour fast. Lipid and lipoprotein fractions were analyzed on EDTA-treated plasma (35, 36), and HDL-c was isolated using heparin-2m manganese chloride (36). Blood samples at both sites were analyzed at the same central laboratory (Medical Research Laboratories, Highland Heights, KY), that conforms to the quality control standards of the National Heart, Lung and Blood Institute and the Centers for Disease Control (37). Self-reported physical activity was assessed using an adapted version of the Kaiser Physical Activity Survey, and was modeled continuously in all analyses (38). Depressive symptoms were included as a covariate in exploratory analyses, and were measured at each timepoint in SWAN with the Centers for Epidemiologic Studies Depression Scale (CES-D) (39).
Timing of Assessments
The timing of assessments differed for women in the original SWAN cohort compared to women in the Fat-Patterning-only study. For women in the original SWAN cohort, all demographic variables (date of birth, race/ethnicity, and education) were assessed at the SWAN baseline exam (1996-1997), along with hostility. Fat patterning characteristics (visceral fat, subcutaneous fat, total fat and BMI), SHBG, and CHD risk factors were assessed at the Fat Patterning Study baseline (2002-2005). Because physical activity is not measured every year in the SWAN cohort, we chose the assessment of physical activity that was concurrent with or immediately prior to the fat patterning assessment for SWAN women (this corresponded to SWAN years five, seven, eight, or ten/SWAN follow-up 04, 06, 07 or 09). For women in the Fat Patterning only study, all assessments were completed at the fat patterning baseline assessment. In exploratory analyses examining the role of depressive symptoms, we chose the CES-D score concurrent with the assessment of hostility for all women in the cohort. This was done to maintain methodological consistency.
Primary Analyses
Descriptive statistics were used to characterize the study sample on age, education, hostility, CHD risk factors, physical activity, and amount of total body, subcutaneous, and visceral fat. T-tests and chi-square tests were conducted to examine differences in these characteristics by race/ethnicity. A series of multiple regression analyses were conducted to assess the cross-sectional relationship between hostility and visceral fat, with hostility modeled continuously. In order to assess the independent effects of hostility on visceral (rather than overall) fat, total percent fat was included in all models as a covariate. The initial model adjusted for age, race/ethnicity and total percent fat only, in order to examine fairly basic associations among race/ethnicity, hostility and visceral fat. Following this initial model, a race/ethnicity*hostility interaction term was added to test for black-white differences in the association between hostility and visceral fat. The final model added additional terms for education as a demographic covariate, SHBG (as a marker of menopausal status), smoking, SBP, HDL-c, and physical activity. Non-significant interaction terms were eliminated from the final model. Following these models, a second series of multiple regression analyses were conducted using the exact same sequence, with subcutaneous fat as the outcome. Because the length of time between the measurement of hostility and the assessment of visceral fat varied considerably for women in the current study, all models included a control variable designed to represent the length of time between the assessment of hostility and visceral fat. This variable ranged from 0 (for women in the fat-patterning-only study) to 10 (for women in the original SWAN cohort whose fat patterning assessment took place during their 10th SWAN visit). Although models with and without this variable produced the same results, it was retained in all analyses for methodological purposes.
Secondary Analyses
Because BMI kg/m2 is often used as a less expensive surrogate of overall body fat in studies of abdominal adiposity, we were interested in determining whether associations among race/ethnicity, hostility and visceral fat would be comparable in models using BMI versus DXA-assessed total body fat. Thus, following our primary analyses above, we re-ran our models, using BMI kg/m2 as our primary measure of overall, or total, body fat.
Exploratory Analyses
We have previously observed a significant association between depressive symptoms and visceral fat in this cohort (40). Thus, a series of exploratory analyses were conducted to examine both the independent and additive effects of hostility and depressive symptoms on abdominal fat measures. Using a median split to create high/low categories for each variable, we created a three-category “negative affect index” score (0, 1 or 2), based on whether women were low on both scales or had elevated scores on hostility, depressive symptoms, or both.
We then we-ran our primary models as detailed above, with 1) one set of models including depressive symptoms as a covariate and 2) a second set of models using the negative affect index as the primary predictor. All analyses were conducted using SPSS, version 15 (SPSS, Inc, Chicago, IL).
Results
Participant Characteristics
Participants ranged in age from 42 to 61, with an average age of 50. The sample was fairly well-educated, with approximately 89% of the women reporting between 14 and 19 years of education. Values on SBP ranged from 95 to 174 mm/Hg, with an average of 121.22 mm/Hg. HDL-c values ranged from 27 to 120 mg/dL, with a mean of 59.22 mg/dL. Approximately twenty percent of women were current smokers, and the average score on the physical activity scale was a 7.7 (SD=1.62). The average hostility score was 3.90 (SD=3.15). Characteristics of the study sample by race/ethnicity are presented in Table 1. There were no signifcant racial/ethnic differences in age or years of education, but African-American women had higher SBP (p<.0001) and DBP (p=.001) levels, were less physically active (p<.0001), and had higher levels of hostility (p<.0001) than their white counterparts. Compared to white women, African-American women had lower levels of total (p=.003) and HDL-c (p=.02) cholesterol, but did not differ on levels of LDL cholesterol.
Table 1.
Demographic Characteristics, Coronary Heart Disease Risk Factors and levels of Hostility by Race/Ethnicity
| Variable | African-Americans (N=189; 45%) |
Whites (N=229; 55%) |
p-value* |
|---|---|---|---|
| Demographics | |||
| Age | 50.6 ± 4.01 | 50.5 ± 3.68 | .83 |
| Education (in years) | 15.8 ± 1.94 | 16.10 ± 2.06 | .12 |
| SBP mm/Hg | 124.90 ± 15.42 | 118.23 ± 11.82 | <.0001 |
| DBP mm/Hg | 79.39 ± 9.10 | 76.46 ± 8.82 | .001 |
| Total Cholesterol | 193.91 ± 35.4 | 203.90 ± 34.3 | .004 |
| HDL mg/dL | 57.33 ± 14.15 | 60.84 ± 15.98 | .02 |
| LDL mg/dL | 119.28 ± 34.64 | 119.28 ± 29.48 | .49 |
| Current Smoker, % | 21.8 | 17.7 | .32 |
| Physical Activity | 7.21 ± 1.57 | 8.15 ± 1.54 | <.0001 |
| Hostility | 5.03± 3.23 | 2.97 ± 2.73 | <.0001 |
Note: Values are mean ± SD or percentage.
T-tests and Chi-squared tests for racial/ethnic differences.
Racial/Ethnic differences were most apparent when examining fat patterning characteristics, as shown in Table 2. African-American women had higher BMIs, more total percent fat, and a greater amount of unadjusted subcutaneous fat compared to white women (all p-values <.0001). However, African-American and white women had similar levels of unadjusted visceral fat (p=.92). After adjusting for total percent fat, African-American women continued to have a greater amount of subcutaneous fat (p<.0001), but had less visceral fat than their white counterparts (p=.004).
Table 2.
Fat Patterning Characteristics by Race
| Variable | African-Americans (N=189; 45%) |
Whites (N=229; 55%) |
p-value* |
|---|---|---|---|
| BMI (kg/m2) | 30.61 ± 7.55 | 27.34 ± 5.85 | <.0001 |
| % Obese | 50.3 | 29.1 | <.0001 |
| Total percent fat (kg) | 44.96 ± 7.69 | 41.74 ± 8.63 | <.0001 |
| Subcutaneous Fat (cm2) (unadjusted) |
440.06 ± 166.59 | 351.36 ± 147.78 | <.0001 |
| Subcutaneous Fat (cm2) (adjusted for Total percent fat) |
410.90 ± 5.80† | 375.09 ± 5.23† | <.0001 |
| Visceral Fat (cm2) (unadjusted) |
96.39 ± 48.84 | 95.87 ± 58.06 | .92 |
| Visceral Fat (cm2) (adjusted for Total percent fat) |
89.29 ± 3.17† | 101.65 ± 2.85† | .004 |
Note: Except where noted, values are mean ± SD or percentage.
T-tests or ANOVAs for racial/ethnic differences.
The standard error (rather than standard deviation) is presented for the adjusted means.
Associations between Hostility and Visceral Fat
In an initial linear regression model adjusting for age, total percent fat, length of time since hostility assessment, and race/ethnicity, higher levels of cynical hostility were associated with a greater amount of visceral fat (B=1.84, s.e.=.69, p=.01). Thus, for every one-point increase on the cynical hostility scale, women had a 1.84 cm2 greater amount of visceral fat. This association did not differ by race/ethnicity (p-value for race/ethnicity*hostility interaction =.67).
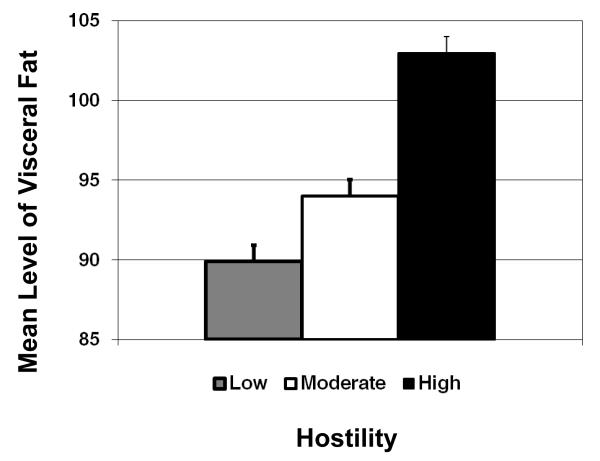
The association between hostility and visceral fat for the full sample is graphically illustrated in Figure 1, with hostility scores categorized into approximate tertiles (low, moderate and high) for descriptive purposes. There was a dose-response association between hostility and visceral fat, where each higher level of hostility was associated with a greater amount of visceral fat (Figure 1). The association between hostility and visceral fat was slightly reduced, but remained significant after adjusting for education, SHBG, SBP, HDL-c, smoking and physical activity (B =1.44, s.e.=.65, p=.03). In order to determine the relative contribution of hostility to visceral fat compared to other important risk factors, Table 3 presents the fully adjusted model with both unstandardized (scale dependent) and standardized (scale independent) regression estimates.
Figure 1.
Mean level of visceral fat by tertiles of hostility.
Table 3.
Fully adjusted Multivariate Regression Model Predicting Visceral Fat from Hostility, R2=52
| B | S.E. | Beta | p-value | |
|---|---|---|---|---|
| Race/Ethnicity | ||||
| African-American | −20.75 | 4.25 | −.19 | <.0001 |
| White(referent) | -- | -- | -- | |
| Total percent fat | 2.63 | .27 | .41 | <.0001 |
| Age | 2.82 | .70 | .20 | <.0001 |
| Education | −1.31 | .99 | −.05 | .19 |
| SHBG | −.34 | .06 | −.21 | <.0001 |
| SBP | .48 | .16 | .12 | .002 |
| HDL | −.68 | .14 | −.19 | <.0001 |
| Smoking | 1.81 | 5.26 | .01 | .73 |
| Physical Activity | −4.18 | 1.31 | −.13 | .002 |
| Hostility | 1.44 | .65 | .08 | .03 |
Note: Model is adjusted for length of time since hostility assessment.
Associations between Hostility and Subcutaneous Fat
Hostility was not associated with subcutaneous fat in the initial model adjusting for age, race/ethnicity, total percent fat and length of time since hostility assessment (B =−.33, s.e.= 1.30, p=.80). There was also no evidence of a race/ethnicity*hostility interaction (p=.85). Adding education, SHBG, SBP, HDL-c, smoking and physical activity to the model did not significantly alter these results (Β= −.68, s.e.= 1.32, p=.61).
Secondary Analyses
In secondary analyses examining associations among race/ethnicity, hostility and visceral fat with BMI kg/m2 substituted for DXA-assessed total body fat, hostility was associated with visceral fat in minimally-adjusted models (B =1.7, s.e.=.7, p=.02), and the race/ethnicity*hostility interaction remained non-significant (p=.50). In fully-adjusted models, hostility was significantly associated with visceral fat (B =1.31, s.e.=.65, p=.04), and this association was slightly weaker than that observed in fully adjusted models using DXA-assessed total body fat (Table 3).
Associations between hostility and subcutaneous fat remained non-significant in models using BMI kg/m2 as a measure of overall body fat. Hostility was not associated with subcutaneous fat in minimally-adjusted models (B =−.99, s.e.= 1.7, p=.58), and the race/ethnicity*hostility interaction remained non-significant (p=.43). There were also no significant hostility and subcutaneous fat associations in fully-adjusted models (p=.61).
Exploratory Analyses: Combined role of Hostility and Depressive Symptoms
The correlation between hostility and depressive symptoms was moderate in this cohort, at r=.24 (p<.001). In the model including depressive symptoms and adjusting for total percent fat, age, race/ethnicity, and length of time since hostility/depressive symptoms assessment, higher levels of cynical hostility remained significantly associated with a greater amount of visceral fat (B=1.5, s.e.=.71, p=.04). The race/ethnicity*hostility interaction remained non-significant (p=.64). In the fully adjusted model with depressive symptoms, the association between hostility and visceral fat was reduced to marginal significance (p=.065). The association between depressive symptoms and visceral fat was also marginal in this model (p=.066) and the standardized regression coefficients for hostility (β=.07) and depressive symptoms (β=.069) were comparable.
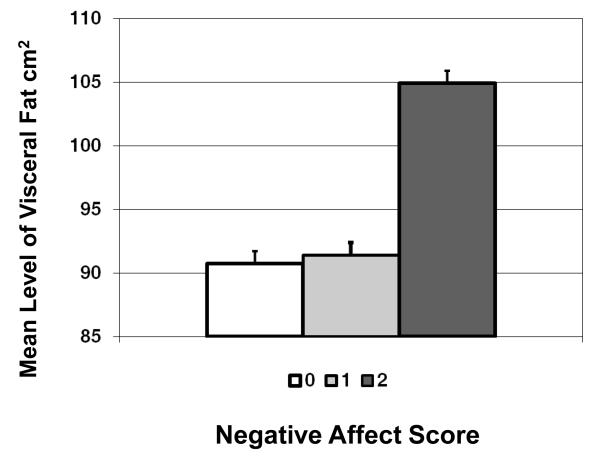
Twenty-eight percent of women were elevated on both hostility and depressive symptoms, 41% were elevated on only one scale and 31% were low on both scales. In models adjusted for total percent fat, age, race/ethnicity, and length of time since hostility/depressive symptoms assessment, the three-category negative affect index was significantly associated with higher levels of visceral fat (p=.01), and there was no race/ethnicity *negative affect interaction (p=.44). As illustrated in Figure 2, women who had elevated scores on both hostility and depressive symptoms (negative affect index =2) had higher levels of visceral fat than women who were low on both scales. There were no significant differences (p=.9) between women who were low on both (negative affect score =0) and women who were only elevated on one (negative affect score =1). The association between the negative affect index and visceral fat remained significant in fully-adjusted models (p=.04).
Figure 2.
Mean level of visceral fat by combined negative affect score.
All exploratory analyses examining the independent and additive effects of hostility and depressive symptoms on subcutaneous fat yielded nonsignificant results (data not shown).
Discussion
The current study found a significant cross-sectional association between levels of hostility and visceral fat in a population-based cohort of middle-aged African-American and white women. This association was linear in nature (Figure 1), with higher levels of baseline hostility predicting a greater amount of visceral fat. The association between hostility and visceral fat was independent of the total amount of body fat, as well as a number of potential confounding factors including CHD risk factors and physical activity. Levels of hostility were not associated with subcutaneous fat, suggesting that hostility uniquely affects the amount of visceral fat, rather than abdominal fat more generally.
Consistent with previous studies, African-American women had higher levels of hostility (2, 20), higher BMIs (41), a greater amount of total percent fat (22), and more subcutaneous fat than their white counterparts (24). Also consistent with previous studies, African-American women had a lower amount of total-body adjusted visceral fat compared to white women (21, 24). Despite these differences, the overall associations between hostility and visceral fat did not differ for African-American compared to white women. Thus, although fat patterning characteristics differed for African-American and white women, these results suggest that hostility affects fat similarly for women of both racial/ethnic groups.
The observed findings linking hostility to visceral fat in middle-aged African-American and white women are similar to those reported by Raikkonen et. al (19) in slightly older white women. They are also consistent with findings in predominantly male samples examining the association between hostility and less sensitive measures of visceral fat, such as waist, waist-to-hip ratio (WHR) and BMI (5, 42). In fact, because waist, WHR and BMI tend to be more strongly associated with subcutaneous, rather than visceral fat (43), these previous studies may have underestimated the overall impact of hostility on visceral fat. The lower sensitivity of these measures may also explain why some previous studies have found non-significant associations between hostility and waist, WHR and/or BMI (3), particularly in African-American samples (20).
These findings suggest that increased accumulation of visceral fat may be one potential pathway by which hostility is associated with an elevated risk of CHD in women. The accumulation of visceral fat is believed by some to be the first in a cascade of events, preceding the development of insulin resistance, hypertension, dyslipidemia and other components of the metabolic syndrome that enhance risk for CHD (17). Less is known, however, about factors that promote the development of visceral fat, and how hostility might be involved.
Although several studies have documented an association between depressive symptoms and visceral fat (40, 44), exploratory analyses in this cohort indicate that while the impact of hostility is slightly reduced when depressive symptoms are accounted for, depressive symptoms do not completely explain the observed association between hostility and higher levels of visceral fat. In fact, the two variables had independent and comparable effects on visceral fat when in the same model. When combined into one index, hostility and depressive symptoms appeared to exert an additive effect on visceral fat, with the highest level of visceral fat observed in women who were elevated on both hostility and depressive symptoms, compared to women who were elevated on only one component, or low on both.
There are several potential mechanisms through which higher levels of hostility might independently (or additively) affect the development of visceral fat. Increased levels of the stress hormone cortisol have been consistently linked to the deposition of visceral fat in prior studies (45). Further, although much of the research in this area has focused on depressive symptoms, at least one prior study has shown an association between higher levels of hostility and increased daytime cortisol secretion (46) -- suggesting a plausible link between hostility, increased cortisol secretion, and visceral fat. Additional research in this area is warranted.
The autonomic nervous system (ANS) might also play a role. There is a considerable body of research linking hostility to ANS activity (13, 47), and animal models have documented important associations between ANS function and the differential enervation of visceral vs. subcutaneous abdominal fat (48). Future studies should focus on examining the association between ANS activity and visceral fat deposition in humans, and whether ANS dysregulation might be an additional pathway through which hostility, and negative affect more broadly, might influence the development of visceral fat.
Several limitations of the current study must be noted. To begin with, the observed associations are cross-sectional in nature -- additional research is needed to determine whether these associations persist over time. Second, African-American and white women in the current sample were fairly well-educated (the majority of women reported between 14 and 19 years of education), and it is unclear how well these findings would generalize to women from less well-educated backgrounds. Finally, because of the study design, there was a differential time lag between the assessment of hostility and the assessment of visceral fat for a sizable percentage of women in our cohort. Women from the original Chicago SWAN cohort had a five to ten year lag between the assessment of hostility and the assessment of visceral fat, while women in the Fat Patterning Only study had assessments of hostility that were concurrent with their assessments of visceral fat. Because research suggests that hostility is a fairly stable personality construct, particularly during the middle and older adult years (28, 29), it is likely that assessments of hostility at a previous timepoint (i.e. five-ten years before) would be comparable to a concurrent assessment. In exploratory analyses, we used a measure of depressive symptoms that was concurrent with our measure of hostility. Using a measure of depressive symptoms closer to the visceral fat assessment did not substantively change our findings (data not shown). It is reasonable to assume that this would be true of hostility as well. Further, we statistically controlled for the amount of time between assessments of hostility and visceral fat, and in additional analyses tested the hostility*cohort interaction (non-significant, data not shown). However it is still possible that the differential time lag between assessments of hostility may have somehow biased the results.
Despite these limitations, this study has notable strengths. To our knowledge, it is the first to examine the association between hostility and visceral fat in a biracial cohort. The sample is population-based, with approximately equal numbers of African-American and white women, living in a similar environment in an urban community. We also utilized well-validated measures of hostility and other covariates, as well as state-of-the-art assessment of visceral, subcutaneous, and total percent fat.
In summary, current findings indicate that hostility is associated with higher levels of visceral, but not subcutaneous, fat in middle-aged African-American and white women. Exploratory analyses revealed that this association is not completely explained by depressive symptoms, although hostility and depressive symptoms appear to exert an additive effect on visceral fat. The mechanisms underlying these associations have yet to be determined, however, findings suggest that visceral fat may be one pathway through which hostility -- and negative affect more broadly -- increases CHD risk in middle-aged women. Therapeutic interventions aimed at reducing the impact of hostility and other negative emotional states on visceral fat may ultimately prove beneficial in reducing women’s long-term CHD risk.
ACKNOWLEDGMENTS
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The SWAN Fat Patterning Study is supported by the National Heart, Lung and Blood Institute (Grant HL067128) and the Charles J. and Margaret Roberts Trust. Dr. Lewis was supported by a Minority Postdoctoral Supplement (Grant HL067128) and received additional support from the National Institute of Mental Health (Grant MH075625). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
We thank the study staff at each site and all the women who participated in SWAN and the SWAN Fat Patterning study.
Glossary
Acronyms
- CHD
Coronary Heart Disease
- SWAN
Study of Women’s Health Across the Nation
- BMI
Body Mass Index
- DXA
Dual Energy X-ray Absorptiometry
- CT
Computed Tomographic
- SBP
Systolic Blood Pressure
- HDL-c
High density lipoprotein cholesterol
- DBP
diastolic blood pressure
- LDL
low density lipoprotein
- SHBG
Sex Hormone Binding globulin
- WHR
Waist-to-Hip Ratio
- ANS
Autonomic Nervous System
Footnotes
Because of the size of the original Chicago SWAN cohort, it takes several years for the Chicago site to complete a single SWAN visit. For example, in 2002, some Chicago women were completing SWAN follow-up visit 04, others 05, and others 06. In 2005, women were completing follow-up visits 07, 08 or 09.
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 - 1999; Joel Finkelstein, PI 1999- present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark –Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair Susan Johnson, Chair
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr. The Cook-Medley hostility scale: item content and ability to predict survival. Psychosom Med. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Allen J, Markovitz J, Jacobs DR, Jr., Knox SS. Social Support and Health Behavior in Hostile Black and White Men and Women in CARDIA. Psychosom Med. 2001;63:609–618. doi: 10.1097/00006842-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Bunde J, Suls J. A quantitative analysis of the relationship between the Cook-Medley Hostility Scale and traditional coronary artery disease risk factors. Health Psychol. 2006;25:493–500. doi: 10.1037/0278-6133.25.4.493. [DOI] [PubMed] [Google Scholar]
- 4.Chida Y, Steptoe A. The Association of Anger and Hostility With Future Coronary Heart Disease: A Meta-Analytic Review of Prospective Evidence. Journal of the American College of Cardiology. 2009;53:936–946. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 5.Everson SA, Kauhanen J, Kaplan GA, Goldberg DE, Julkunen J, Tuomilehto J, Salonen JT. Hostility and Increased Risk of Mortality and Acute Myocardial Infarction: The Mediating Role of Behavioral Risk Factors. Am. J. Epidemiol. 1997;146:142–152. doi: 10.1093/oxfordjournals.aje.a009245. [DOI] [PubMed] [Google Scholar]
- 6.Everson-Rose SA, Lewis TT, Karavolos KK, Matthews KA, Sutton-Tyrrell K, Powell LH. Cynical hostility and carotid atherosclerosis in African American and white women: The Study of Women’s Health Across the Nation (SWAN) Heart Study. American Heart Journal. 2006;152:982.e7–982.e13. doi: 10.1016/j.ahj.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 8.Rosenman RH, Brand RJ, Sholtz RI, Friedman M. Multivariate prediction of coronary heart disease during 8.5 year follow-up in the Western Collaborative Group Study. Am J Cardiol. 1976;37:903–10. doi: 10.1016/0002-9149(76)90117-x. [DOI] [PubMed] [Google Scholar]
- 9.Olson MB, Krantz DS, Kelsey SF, Pepine CJ, Sopko G, Handberg E, Rogers WJ, Gierach GL, McClure CK, Merz CNB, for the WSG Hostility Scores Are Associated With Increased Risk of Cardiovascular Events in Women Undergoing Coronary Angiography: A Report from the NHLBI-Sponsored WISE Study. Psychosom Med. 2005;67:546–552. doi: 10.1097/01.psy.0000170830.99263.4e. [DOI] [PubMed] [Google Scholar]
- 10.Chaput LA, Adams SH, Simon JA, Blumenthal RS, Vittinghoff E, Lin F, Loh E, Matthews KA. Hostility Predicts Recurrent Events among Postmenopausal Women with Coronary Heart Disease. Am. J. Epidemiol. 2002;156:1092–1099. doi: 10.1093/aje/kwf158. [DOI] [PubMed] [Google Scholar]
- 11.Lahad A, Heckbert SR, Koepsell TD, Psaty BM, Patrick DL. Hostility, aggression and the risk of nonfatal myocardial infarction in postmenopausal women. Journal of Psychosomatic Research. 1997;43:183–195. doi: 10.1016/s0022-3999(96)00369-8. [DOI] [PubMed] [Google Scholar]
- 12.Matthews KA, Owens JF, Kuller LH, Sutton-Tyrrell K, Jansen-McWilliams L. Are hostility and anxiety associated with carotid atherosclerosis in healthy postmenopausal women? Psychosom Med. 1998;60:633–638. doi: 10.1097/00006842-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Sherwood A, Hughes JW, Kuhn C, Hinderliter AL. Hostility Is Related to Blunted {beta}-Adrenergic Receptor Responsiveness Among Middle-Aged Women. Psychosom Med. 2004;66:507–513. doi: 10.1097/01.psy.0000132876.95620.04. [DOI] [PubMed] [Google Scholar]
- 14.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289:1257–61. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicklas BJ, Penninx BWJH, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, Pahor M, Jingzhong D, Harris TB. Association of Visceral Adipose Tissue with Incident Myocardial Infarction in Older Men and Women: The Health, Aging and Body Composition Study. Am. J. Epidemiol. 2004;160:741–749. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 16.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–20. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 17.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int. J. Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 18.Faria AN, Filho FF Ribeiro, Ferreira SR Gouveia, Zanella MT. Impact of Visceral Fat on Blood Pressure and Insulin Sensitivity in Hypertensive Obese Women. Obesity Res. 2002;10:1203–1206. doi: 10.1038/oby.2002.164. [DOI] [PubMed] [Google Scholar]
- 19.Raikkonen K, Matthews KA, Kuller LH, Reiber C, Bunker CH. Anger, hostility, and visceral adipose tissue in healthy postmenopausal women. Metabolism. 1999;48:1146–51. doi: 10.1016/s0026-0495(99)90129-4. [DOI] [PubMed] [Google Scholar]
- 20.Surwit RS, Williams RB, Siegler IC, Lane JD, Helms M, Applegate KL, Zucker N, Feinglos MN, McCaskill CM, Barefoot JC. Hostility, Race, and Glucose Metabolism in Nondiabetic Individuals. Diabetes Care. 2002;25:835–839. doi: 10.2337/diacare.25.5.835. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res. 2005;13:66–74. doi: 10.1038/oby.2005.9. [DOI] [PubMed] [Google Scholar]
- 22.Lovejoy JC, Smith SR, Rood JC. Comparison of Regional Fat Distribution and Health Risk Factors in Middle-Aged White and African American Women: The Healthy Transitions Study. Obesity Res. 2001;9:10–16. doi: 10.1038/oby.2001.2. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, for the American Heart Association Statistics Committee and Stroke Statistics S Heart Disease and Stroke Statistics--2008 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 24.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R. Visceral Fat, Waist Circumference, and BMI: Impact of Race/ethnicity. Obesity. 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 25.Sowers M, Crawford S, Sternfeld B, Marganstein D, E.B. G, Greendale GA, Evans D, Neer R, Matthews KA, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition, Menopause: Biology and Pathobiology. Academic Press; New York: 2000. pp. 175–188. [Google Scholar]
- 26.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP) J Alzheimers Dis. 2003;5:349–55. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 27.Barefoot JC, Larsen S, von der Lieth L, Schroll M. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. American Journal of Epidemiology. 1995;142:477–84. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- 28.Barefoot JC, Beckham JC, Haney TL, Siegler IC, Lipkus IM. Age Differences in Hostility Among Middle-Aged and Older Adults. Psychology and Aging. 1993;8:3–9. doi: 10.1037//0882-7974.8.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Raikkonen K, Matthews KA, Kuller LH. Trajectory of psychological risk and incident hypertension in middle-aged women. Hypertension. 2001;38:798–802. [PubMed] [Google Scholar]
- 30.Shekelle RB, Gale M, Ostfeld AM, Paul O. Hostility, risk of coronary heart disease, and mortality. Psychosom Med. 1983;45:109–114. doi: 10.1097/00006842-198305000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Yoshizumi T, Nakamura T, Yamane M, Waliul Islam AHM, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal Fat: Standardized Technique for Measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 32.Seidell JC, Oosterlee A, Thijssen MA, Burema J, Deurenberg P, Hautvast JG, Ruijs JH. Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am J Clin Nutr. 1987;45:7–13. doi: 10.1093/ajcn/45.1.7. [DOI] [PubMed] [Google Scholar]
- 33.Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–6. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 34.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults, : Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 35.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the lipid clinics’ methodology. J of Clin Chem. 1981 [Google Scholar]
- 36.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 37.Myers GL, Cooper GR, Winn CL, Smith SJ. The Centers for Disease Control-National Heart, Lung and Blood Institute Lipid Standardization Program. An approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–35. [PubMed] [Google Scholar]
- 38.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 39.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 40.Everson-Rose SA, Lewis TT, Karavolos K, Dugan SA, Wesley D, Powell LH. Depressive symptoms and increased visceral fat in middle-aged women. Psychosom Med. doi: 10.1097/PSY.0b013e3181a20c9c. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis TT, Everson-Rose SA, Sternfeld B, Karavolos K, Wesley D, Powell LH. Race, education, and weight change in a biracial sample of women at midlife. Arch Intern Med. 2005;165:545–51. doi: 10.1001/archinte.165.5.545. [DOI] [PubMed] [Google Scholar]
- 42.Niaura R, Todaro JF, Stroud L, Spiro A, 3rd, Ward KD, Weiss S. Hostility, the metabolic syndrome, and incident coronary heart disease. Health Psychol. 2002;21:588–93. doi: 10.1037//0278-6133.21.6.588. [DOI] [PubMed] [Google Scholar]
- 43.Bonora E, Micciolo R, Ghiatas AA, Lancaster JL, Alyassin A, Muggeo M, DeFronzo RA. Is it possible to derive a reliable estimate of human visceral and subcutaneous abdominal adipose tissue from simple anthropometric measurements? Metabolism. 1995;44:1617–25. doi: 10.1016/0026-0495(95)90084-5. [DOI] [PubMed] [Google Scholar]
- 44.Vogelzangs N, Kritchevsky SB, Beekman ATF, Newman AB, Satterfield S, Simonsick EM, Yaffe K, Harris TB, Penninx BWJH. Depressive Symptoms and Change in Abdominal Obesity in Older Persons. Arch Gen Psychiatry. 2008;65:1386–1393. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasquali R, Vicennati V, Cacciari M, Pagotto U. The Hypothalamic-Pituitary-Adrenal Axis Activity in Obesity and the Metabolic Syndrome. Ann NY Acad Sci. 2006;1083:111–128. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- 46.Pope MK, Smith TW. Cortisol excretion in high and low cynically hostile men. Psychosom Med. 1991;53:386–392. doi: 10.1097/00006842-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Sloan RP, Bagiella E, Shapiro PA, Kuhl JP, Chernikhova D, Berg J, Myers MM. Hostility, Gender, and Cardiac Autonomic Control. Psychosom Med. 2001;63:434–440. doi: 10.1097/00006842-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. J Clin Invest. 2002;110:1243–50. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]