Abstract
Evolution of elastic fibers is associated with establishment of the closed circulation system. Primary roles of elastic fibers are to provide elasticity and recoiling to tissues and organs and to maintain the structural integrity against mechanical strain over a lifetime. Elastic fibers are comprised of an insoluble elastin core and surrounding mantle of microfibrils. Elastic fibers are formed in a regulated, stepwise manner, which includes the formation of a microfibrillar scaffold, deposition and integration of tropoelastin monomers into the scaffold, and cross-linking of the monomers to form an insoluble, functional polymer. In recent years, an increasing number of glycoproteins have been identified and shown to be located on or surrounding elastic fibers. Among them, the short fibulins-3, -4 and -5 particularly drew attention because of their potent elastogenic activity. Fibulins-3, -4 and -5 are characterized by tandem repeats of calcium binding EGF-like motifs and a C-terminal fibulin module, which is conserved throughout fibulin family members. Initial biochemical characterization and gene expression studies predicted that fibulins might be involved in structural support and/or matrix-cell interactions. Recent analyses of short fibulin knockout mice have revealed their critical roles in elastic fiber development in vivo. We review recent findings on the elastogenic functions of short fibulins and discuss the molecular mechanism underlying their activity in vitro and in vivo.
Keywords: elastic fibers, microfibrils, lysyl oxidase, assembly, knockout mouse
1. Introduction
Tissues such as the aorta, lungs and skin need to be resistant to mechanical strain and yet remain extensible throughout life to function. This characteristic feature of elastic tissues is provided by a network of elastic fibers, which allows the tissues to stretch and recoil without damage. Elastic fibers begin to form at midgestation and are completed during postnatal development, with no new elastic fiber formation in the adult (Ritz-Timme et al., 2003). Elastic fibers are generally considered to be non-self renewing extracellular structures. Defects in the elastic fiber formation or accelerated degradation of elastic fibers result in a myriad of pathological conditions, including cutis laxa, arterial tortuosity, pulmonary emphysema and pelvic organ prolapse. It is also known that elastic fibers undergo normal age-related changes, leading to loose skin, stiff vessels and an increase in pulse pressure. Elastic fibers are comprised of polymerized tropoelastin monomers surrounded by a mantle of microfibrils (reviewed in (Wagenseil and Mecham, 2007)). More than 30 elastic fiber-associated proteins have been identified and considerable effort has been put forth to determine biochemical and functional properties of these molecules (Kielty et al., 2002) (Table 1). Elastic fibers are formed in a regulated, stepwise manner, which includes the formation of a microfibrillar scaffold, deposition and integration of tropoelastin monomers into the scaffold, and cross-linking of the monomers to form an insoluble, functional polymer (Wagenseil and Mecham, 2007). As more information on the function of elastic fiber-associated proteins becomes available through protein-protein interaction studies and functional analyses, the molecular mechanisms of elastic fiber assembly will be revealed. Additionally, gene knockout studies in mice have revealed critical roles for elastic fiber-associated proteins in elastic fiber formation. In this review, we will focus on recent publications and update our knowledge on the role of the emerging family of fibulin proteins in the process of elastic fiber assembly. We will discuss how these proteins interact with components of elastic fibers and participate in formation and stabilization of the fiber. Lastly, we will discuss what we have learned from loss-of-function studies in mice and genetic studies in humans.
Table 1. Major components of elastic fibers.
| Core Component |
| Elastin |
| Microfibrils |
| Fibrillin-1, -2 |
| Cross-linking enzymes |
| Lox, Loxl-1, -2, -3, -4 |
| Elastic fiber-associated proteins |
| Fibulin-1, -2, -3, -4, -5 |
| Emilin-1 |
| MAGP-1, -2 |
| LTBP-1, -2, -3, -4 |
2. Short Fibulins
Seven fibulins have been identified to date since the discovery of the fibulin-1 prototype (Argraves et al., 1989; de Vega et al., 2009). Fibulins can be divided into class I and class II based on length and domain structures (Yanagisawa et al., 2009). Class II fibulins, or so-called short fibulins, include fibulin-3 (encoded by the gene EFEMP1, also called S1-5), fibulin-4 (encoded by the gene EFEMP2, also called H411 or MBP1), fibulin-5 (also called EVEC or DANCE) and fibulin-7 (also called TM17, Figure 1A). Fibulin-3 was first found to be overexpressed in senescent human fibroblasts established from a Werner syndrome patient with premature aging (Lecka-Czernik et al., 1995). Fibulin-4 was initially shown to be involved in stress response, cell proliferation and oncogenic activity (Gallagher et al., 1999; Giltay et al., 1999; Heine et al., 1999), and fibulin-5 was originally identified as a secreted molecule involved in cardiovascular development and remodeling (Kowal et al., 1999; Nakamura et al., 1999). Fibulin-7 is the newest member of the family with an atypical domain structure containing a sushi domain in the N-terminal portion, which is found in various proteins involved in complement activation and blood coagulation, and fewer calcium binding EGF-like (cbEGF) motifs compared with the other fibulins (de Vega et al., 2007). Fibulin-7 is expressed in odontoblasts and has been shown to bind various extracellular matrix (ECM) proteins, including fibronectin and fibulin-1, and to bind dental mesenchymal cells (de Vega et al., 2007). Fibulin-7 is phylogenetically distant from the rest of the short fibulins (Figure 1B) and its binding capacity to elastin or potential function in elastic fiber assembly has not been reported. The role of fibulins-3, -4 and -5 in elastic fiber formation was not realized until the generation of knockout mouse models, which unexpectedly exhibited a variety of elastic fiber-related phenotypes.
Figure 1. Human short fibulins.
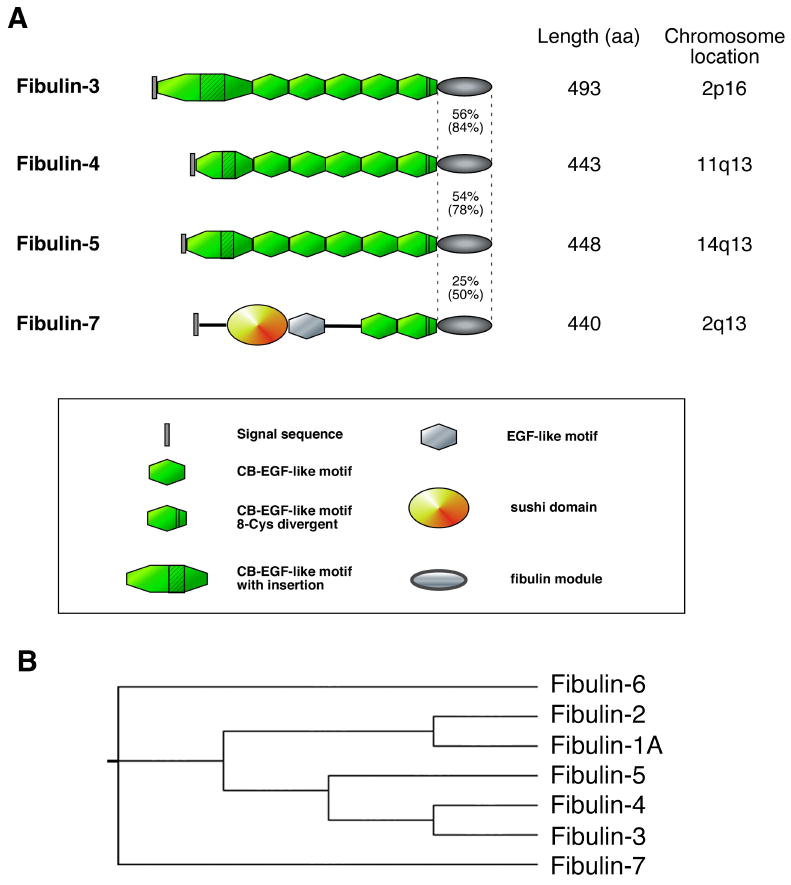
(A) Schematic representation of short fibulins-3, -4, -5 and -7 are shown. Identity and homology (parenthesis) between the C-terminal fibulin modules are indicated. (B) Phylogenetic map of the human fibulin family proteins. GenBank accession numbers used to obtain primary sequences are fibulin-1A (CAQ0836), fibulin-2 (AAH51690), fibulin-3 (NP_001034438), fibulin-4 (CAA10791), fibulin-5 (CAB38568), fibulin-6 (CAC37630) and fibulin-7 (AAH35784).
2.1. Domain structure
Elastogenic short fibulins contain six cbEGF domains, with the first containing a varying length of insertion sequence between the fourth and fifth cysteines, and the sixth being an 8-cysteine divergent type domain. cbEGF domains are found in various secreted and transmembrane proteins and are known to mediate protein-protein interactions (Maurer and Hohenester, 1997). Tandem repeats of cbEGF domains are connected by one amino acid in short fibulins in a manner similar to cbEGF domains in the microfibril protein, fibrillin-1 (Hambleton et al., 2004). Although direct binding of Ca2+ to the cbEGF domain has not been demonstrated in short fibulins, chelating divalent cations in solid-phase binding assays significantly reduces binding between fibulin-5 and elastin (Yanagisawa et al., 2002), and conversely, the presence of Ca2+ significantly enhances the binding between elastin and fibulin-5 (Wachi et al., 2008), indicating that Ca2+ potentiates binding between the two molecules.
Among the short fibulins, only fibulin-5 contains an evolutionally conserved arginine-glycine-aspartic acid (RGD) motif which is known to mediate binding to cell surface integrin receptors (Yanagisawa et al., 2009). It was initially proposed that fibulin-5 facilitates the tethering of polymerized elastic fibers to the cell surface using the RGD motif to connect to the integrin receptors (Nakamura et al., 2002; Yanagisawa et al., 2002). However, fibulin-5 mutant mice in which the RGD motif is mutated to RGE, a mutation known to disrupt RGD-dependent integrin binding, do not develop abnormal elastic fibers nor show altered cell-elastic fiber associations (our unpublished observations), indicating that fibulin-5 binding to integrins is dispensable for elastic fiber assembly and organization. Fibulin-5 binds α5β1, α4β1, αvβ3, αvβ5 and α9β1 integrins on endothelial cells and vascular smooth muscle cells (SMCs) (Lomas et al., 2007; Nakamura et al., 2002). Fibulin-5 facilitates substrate binding of endothelial cells and SMCs in an RGD-dependent manner; however, fibulin-5 attenuates fibronectin-mediated cell spreading and actin fiber formation (Lomas et al., 2007). Fibulin-5 also inhibits fibronectin-mediated generation of reactive oxygen species in an integrin-dependent manner (Schluterman et al., 2010). Thus, since fibulin-5 binds the α5β1 and α4β1 fibronectin receptors, it has been suggested that fibulin-5 acts in a dominant negative manner to inhibit fibronectin receptor-mediated downstream signaling (Lomas et al., 2007; Schluterman et al., 2010).
Fibulins-3, -4 and -5 have a C-terminal fibulin module that is found in all fibulin proteins. Identity and similarity of the fibulin module among human fibulins-3, -4 and -5 are approximately 55% and 80%, respectively. Within this module in fubulin-5, an elastin binding domain was identified (Zheng et al., 2007); however, it is unknown whether the elastin-binding property of fibulins-3 or -4 is also located within the equivalent region of their fibulin modules. Fibulin-5 also uses the C-terminal fibulin module to interact with lysyl-oxidase-like 1 (Loxl1), Loxl2, Loxl4 and extracellular superoxide dismutase (SOD3) (Hirai et al., 2007b; Liu et al., 2004; Nguyen et al., 2004). In contrast, fibulin-4 has been shown to bind lysyl oxidase (Lox) via its N-terminal domain (Horiguchi et al., 2009). Interaction between fibulin-3 and tissue inhibitor of metalloproteinases-3 (TIMP-3) was reported in vitro and the C-terminal fibulin module was shown to be responsible for this interaction (Klenotic et al., 2004). Taken together, the C-terminal fibulin module is involved in interactions with a number of different molecules. These interactions may simply promote tethering of proteins into the ECM, or may be more complex in that they may influence the function of the interacting proteins.
2.2. Gene expression in elastogenic and non-elastogenic tissues
Fibulins-3, -4 and -5 are widely expressed in developing embryos, particularly skeletal and cardiovascular tissues (Ehlermann et al., 2003; Gallagher et al., 2001; Giltay et al., 1999; Kowal et al., 1999). Biological functions of fibulins can be largely divided into elastogenic and non-elastogenic functions. Distribution of fibulins-3, -4 and -5 in elastogenic tissues is distinct and suggests a differential role in tissue-specific elastogenesis. In the aorta and lung, fibulin-5 expression is much higher than that of fibulins-3 or -4, and the difference between fibulin-5 and fibulin-4 expression is more than twelve-fold. In the skin, fibulin-3 shows the highest expression followed by fibulin-5 and fibulin-4, with the difference between fibulin-3 and -4 being three-fold (Kobayashi et al., 2007). In the vasculature, fibulin-5 is expressed both in large and small blood vessels and colocalizes with all layers of elastic lamina in the vessel wall. Fibulin-4 expression is more intense in the outer medial layers towards the adventitia in large blood vessels (Huang J. et al., 2009). Fibulin-3 is predominantly expressed in capillaries and is detected in the basement membrane. At the electron microscope (EM) level, fibulin-5 is localized on the surface of elastic fibers at the interface between elastin and microfibrils, whereas fibulin-4 is localized on microfibrils (Kobayashi et al., 2007; Yanagisawa et al., 2002).
In non-elastogenic tissues, high expression of fibulin-3 is observed in olfactory ensheathing cells (OEC) (Vukovic et al., 2009), inner and outer segments of photoreceptors and nerve fiber bundles (Marmorstein et al., 2002). Fibulin-3 supports proliferation of OEC, whereas it negatively regulates migration of OEC in vitro (Vukovic et al., 2009). Fibulin-3 is not expressed in normal retinal pigment epithelium (RPE) or Bruck's membrane; however, increased expression of fibulin-3 has been detected in sub-retinal pigment epithelial deposits in the eyes of individuals with Doyne honeycomb retinal dystrophy (MIM#601548) (Marmorstein et al., 2002). Consistent with this observation, a point mutation in fibulin-3 (R345W) was shown to be responsible for autosomal dominant Doyne honeycomb retinal dystrophy (Stone et al., 1999), which causes abnormal retention of fibulin-3 in endoplasmic reticulum and leads to upregulation of stress response and VEGF expression (Roybal et al., 2005). Fibulin-4 is expressed in chondrocytes and elevated levels of autoantibodies to fibulin-4 have been detected in osteoarthritis patients (Xiang et al., 2006). Interestingly, fibulin-4 has been shown to be upregulated upon LPS stimulation in peritoneal macrophages (Heine et al., 1999), suggesting a potential link between the immune response and elevation of fibulin-4 in osteoarthritis patients. Fibulin-5 gene expression is significantly downregulated in the vasculature after birth and is reactivated in the neointima following vascular injury (Kowal et al., 1999). Since fibulin-5 is expressed in endothelial cells and vascular SMCs and functions to inhibit proliferation and migration of these cells in vitro (Preis et al., 2006; Spencer et al., 2005), reactivation of fibulin-5 may counteract the overproliferation of SMCs that is responsible for neointima formation. Taken together, short fibulins exert regulatory functions on various cell types in an elastogenic activity-dependent or -independent manner, adding complexity to the understanding of their biological functions in vivo.
3. Process of elastogenesis
3.1. Secretion and coacervation of tropoelastin
Tropoelastin is a 70-kDa protein and synthesized by elastogenic cells, including skin fibroblasts, lung alveolar cells, chondrocytes and vascular SMCs. Tropoelastin can bind cell surface galactosaminoglycans via its C-terminus (Broekelmann et al., 2005) or to cell surface αvβ3 integrins via a C-terminal GRKRK motif (Bax et al., 2009). Whereas assembly of fibronectin fibrils or collagen fibrils occurs near cell surface and involves matrix-cell interactions (Mao and Schwarzbauer, 2005), requirement of cell surface binding of tropoelastin in elastic fiber assembly has not been determined. Recent time-lapse imaging experiments using tropoelastin timer reporters showed that tropoelastin forms aggregates which align on the cell surface, indicating the possibility of cell involvement in early steps of elastic fiber micro-assembly (Czirok et al., 2006; Kozel et al., 2006).
Tropoelastin has the ability to undergo self-aggregation and phase separation in vitro in a process called coacervation. This process occurs in a temperature and sodium chloride concentration dependent manner and provides optimal condition for the subsequent cross-linking (Vrhovski et al., 1997). Fibulin-5 preferentially binds tropoelastin, but not to polymerized elastin in vitro (Zheng et al., 2007) and it has been shown that recombinant fibulin-5 accelerates coacervation of tropoelastin in vitro (Hirai et al., 2007b; Wachi et al., 2008). In contrast, using elastin-like polypeptides, fibulin-5 was shown to inhibit the maturation phase of coacervation, in which the tropoelastin coacervates coalesce into large aggregates and the phase separation becomes irreversible, without affecting the speed of coacervation (Cirulis et al., 2008). Similarly, fibulin-4 was shown to inhibit maturation of elastin-like polypeptide but was less potent than fibulin-5. Consistent with this in vitro maturation data, EM observations of skin from mice deficient in the fibulin-5 gene (Fbln-/-5) showed the accumulation of large elastin globules that were not incorporated into the microfibrils (Figure 2, (Choi et al., 2009)). These observations support the in vivo function of fibulin-5 in inhibiting excessive maturation of tropoelasin coacervates thereby allowing for the integration of micro-assemblies of tropoelastin into the microfibrillar scaffold where subsequent cross-linking would occur to form the mature fiber. Interestingly, the N-terminal half of fibrillin-1 and microfibril-associated glycoprotein-1 (MAGP-1) accelerates maturation of elastin-like peptides. Thus, elastic fiber-associated proteins likely play critical regulatory roles in the early steps of elastogenesis involving the coacervation and maturation of tropoelastin.
Figure 2. Electron micrographs of dermal elastic fibers in wild-type and Fbln5-null mouse skin.
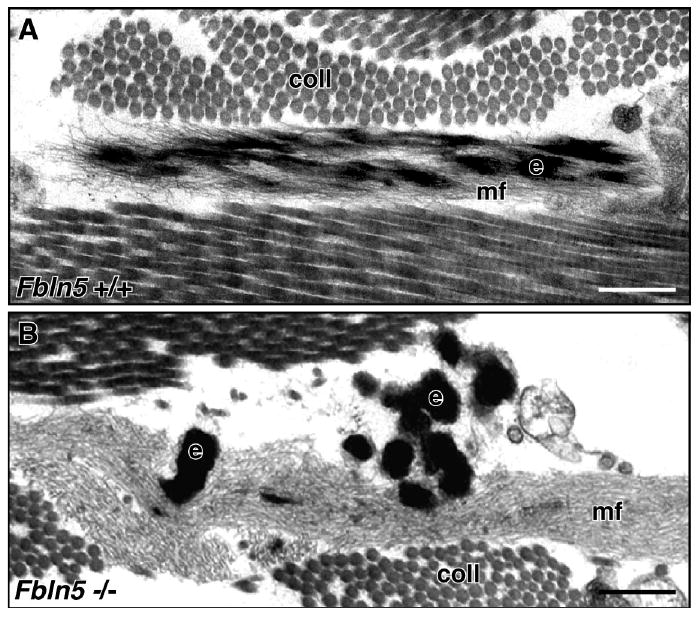
(A) Elastic fibers in wild-type dermis show a core of elastin (e) integrated within a bundle of microfibrils (mf). (B) In contrast, the elastic fibers in Fbln5-null dermis are composed of large elastin aggregates (e) located outside extensive bundles of microfibrils (mf). Scale bar = 0.25 μm, coll = collagen. Adapted and used with permission from Choi et al. Matrix Biol. 28:211-220, 2009.
3.2. Scaffold formation
In vivo, elastic fiber assembly always occurs in the presence of microfibrils. The microfibrils are primarily composed of fibrillin polymers with associated glycoproteins (reviewed in (Ramirez and Dietz, 2007)) and serve as a scaffold for elastin deposition. Fibrillins-1 and -2 are the major components of microfibrils and are large cysteine-rich glycoproteins with a modular domain structure that consists of multiple repeats of cbEGF and eight cysteine domains. Whereas elastic fiber assembly is dependent on the presence of microfibrils and elastin is never observed in the absence of microfibrils, elastin-independent functions of microfibrils exist. These include structural support of microfibril-rich tissues such as skeletal, cardiovascular and ocular tissues, and regulation of growth factor bioavailability in the ECM (reviewed in (Ramirez et al., 2007)). Fibrillin-1 indirectly tethers TGF-β by binding to latent TGF-β binding proteins (LTBPs)-1 and -4, which form a large latent complex by covalently binding to a smaller latent complex containing the TGF-β dimer and latency associated proteins (LAPs). Thus, mutations in the fibrillin-1 gene (FBN1) can not only disrupt the structural integrity of microfibrils but may also lead to dysregulation of TGF-β-mediated signaling, resulting in Marfan syndrome in humans (MIM#154700). Fibrillin-2 is expressed during embryogenesis and decreases after birth. The absolute amount of fibrillin-2, however, is much lower than fibrillin-1 in aortic tissues, and it is suggested that fibrillin-2 plays a minor role in elastogenesis in large blood vessels (Kelleher et al., 2004). Mutations in FBN2 are responsible for congenital contractural arachnodactyly in humans (MIM# 612570) and mice (Chaudhry et al., 2001; Putnam et al., 1995).
The requirement of microfibrils in elastic fiber assembly was confirmed by observations of tissues from double knockout mice of the fibrillin-1 and fibrillin-2 genes. Absence of the two major components of microfibrils abolished elastogenesis in the aortic wall and affected maturation of vascular SMCs, resulting in embryonic lethality at midgestation (Carta et al., 2006). It has also been shown that alterations in fibrillin-1 alone can have profound effects on elastic fiber formation. In mice homozygous for hypomorphic alleles of Fbn1 (Fbn1mgR/mgR), the surface of elastic fibers in the aorta was blunt and lacked elastin extensions that connected the elastic fibers to adjacent SMCs (Bunton et al., 2001). This resulted in activation of SMCs and upregulation of MMP-9 in the vessel wall, contributing to aortic dissection and rupture. Tight skin mice, in which a duplication of the fibrillin-1 gene exists, produce abnormal fibrillin-1 that acts in a dominant negative manner, exhibit an altered ratio of fibulin-2 and fibulin-5 expression in the hypodermis and show compromised assembly of elastic fibers in the skin (Lemaire et al., 2004). Fibrillin-1 microfibril deposition is dependent on the formation of a fibronectin matrix. Fibrillin-1 colocalizes with fibronectin fibrils and siRNA-mediated knockdown of fibronectin completely abolishes fibrillin-1 microfibrils (Sabatier et al., 2009). Disruption of fibronectin engagement with the β1 integrin or inhibition of the α5β1 downstream kinase, Rho-associated kinase (ROCK), also disrupts fibrillin-1 microfibril assembly (Kinsey et al., 2008). Thus, it is perceivable that non-optimized conditions for fibrillin-1 fibrillinogenesis result in defective elastic fibers. Overexpression of chondroitin sulfate, which constitutes a side chain of versican, leads to abnormal deposition of fibrillin-1 in human keloid lesions and disrupts elastic fiber formation (Ikeda et al., 2009). Interaction between the central domain of fibrillin-1 and the C-terminal lectin binding domain of versican has been reported (Isogai et al., 2002) and overexpression of versican significantly reduces the formation of elastic fibers within the neointima in a rat carotid artery injury model (Huang et al., 2006). Thus, disturbance of microfibril assembly has profound effects on the subsequent elastic fiber assembly.
3.3. Cross-linking
Cross-linking of tropoelastin monomers is a critical step to generate an insoluble elastin polymer and is initiated by the lysyl oxidase family of enzymes (Sato et al., 2007). Lysyl oxidases are copper-dependent amine oxidases with five family members, including the prototype lysyl oxidase (Lox) and lysyl oxidase-like (Loxl) proteins (Loxl1-4). Lysyl oxidases mediate the generation of peptidyl α-aminoadipic-δ-semialdehyde (allysine) through oxidative deamination of peptidyl lysine (reviewed in (Kagan and Wande, 2003; Lucero and Kagan, 2006)). Allysine then spontaneously reacts with an unmodified lysine or another allysine to form bi-, tri- and tetrafunctional cross-links, which include elastin-specific desmosine and isodesmosine (Rosenbloom et al., 1993). Lox and Loxl1 are closely related within the Lox family and have been the most extensively studied (Hornstra et al., 2003). Lox and Loxl proteins are secreted as proenzymes and are compartmentalized in the ECM. Proteolytic activation is required to release the C-terminal enzymatically active mature protein, and this is mediated by bone morphogenic protein-1 (BMP-1) or tolloid-like-1 (Tll1). Fibronectin binds Lox and positively regulates Lox activity (Fogelgren et al., 2005). Other studies have shown that fibronectin binds BMP-1 outside its enzymatically active site and enhances its proteolytic activity (Huang G et al., 2009). Thus, correct localization of Lox in the ECM and efficient proteolytic activation of the enzyme, perhaps through its interaction with fibronectin, is critical for its biological function. Conditions that alter enzymatic activity of Lox also lead to elastic fiber defects in vivo. Copper is an essential cofactor for Lox and mutations in the gene encoding P-type ATPase ATP7A, a copper-specific transporter, is responsible for Menkes disease in humans (MIM# 300011) and mice (Levinson et al., 1994; Vulpe et al., 1993). Lack of ATP7A or under copper-deficient conditions, Lox activity is reduced and collagen and elastin cross-linking is severely affected.
Knockout studies of Lox and Loxl1 in mice revealed differential roles of these enzymes in vivo. Lox-/- mice exhibited perinatal lethality due to aortic aneurysm rupture and diaphragm hernia (Hornstra et al., 2003; Maki et al., 2002), whereas Loxl1-/- mice developed remodeling defects of elastic fibers in the adult, including genital prolapse (Liu et al., 2004). Lox-/- embryos showed 39% of normal desmosine levels in the aorta and approximately 60% of the immature collagen cross-links in the whole body compared to wild-type embryos. Loxl1-/- mice showed approximately 60% of normal desmosine in the skin and lung, indicating that Lox can mediate the cross-linking of both collagen and elastin during embryogenesis, whereas Loxl1 preferentially mediates elastin cross-linking in the postnatal life. Other Lox family proteins may have also compensated for the loss of single Lox protein.
3.4. Modulators of elastic fiber assembly
Increasing numbers of elastic fiber-associated proteins have been identified and shown to affect elastogenesis through interactions with key elastogenic molecules or by yet unknown mechanisms. LTBPs-1 through -4 share structural homology with fibrillin-1, and LTBPs-1, -2 and -4 physically interact with fibrillin-1 and colocalize with microfibrils (Hirani et al., 2007; Isogai et al., 2003). It has recently been shown that fibrillin-1 microfibrils are necessary for matrix deposition of LTBPs-1, -2 and -4 (Ono et al., 2009; Vehvilainen et al., 2009). LTBP-2 binds fibulin-5 and positively regulates the deposition of fibulin-5 onto fibrillin-1 microfibrils in vitro (Hirai et al., 2007a). In contrast, LTBP-1 competes with fibulins-2, -4 and -5 for binding to fibrillin-1, and likewise, LTBP-4 competes with fibulins-4 and -5, although less effectively than LTBP-1 (Ono et al., 2009). Deletion of Ltbp4 in mice showed defective assembly of elastic fibers in the terminal air sacs in the lungs, which was independent of TGF-β activity. Since the phenotype of Ltbp4-null mice was remarkably similar to that seen in Fbln5-/- mice, it was suggested that LTBP-4 might potentiate the function of fibulin-5 (Dabovic et al., 2009). Elastin microfibril interface-located protein (emilin)-1 is found at the interface between the elastin core and surrounding microfibrils and binds to tropoelastin and fibulin-5 in vitro (Zanetti et al., 2004). Emilin-1-deficient fibroblasts show abnormal elastic fibers with decreased fibrous structure and altered distribution of fibulin-5 in vitro. MAGP-2 colocalizes with microfibrils and overexpression of MAGP-2 increases the assembly of elastic fibers without increasing the levels of tropoelastin or other elastic fiber-associated proteins, including fibrillin-1, fibulins-2 and -5, or emilin-1 (Lemaire et al., 2007). These observations strongly suggest the potential involvement of elastic fiber-associated proteins during the process of elastogenesis in vitro and in vivo. Further investigations on temporal expressions and protein-protein interactions with tropoelastin, fibulins and fibrillins will provide important insights into the modulator functions of these molecules.
4. Fibulins function as multiple adaptor proteins during elastic fiber assembly
4.1. Biochemical basis of the elastogenic activity of fibulins
Severe elastinopathic phenotypes in mice deficient in the short fibulin genes revealed their critical roles in elastogenesis in vivo. Fibulin-5 has been shown to exert strong elastogenic activity in various experimental systems. For example, recombinant fibulin-5 increases formation of elastic fibers in in vitro elastogenesis assays using primary human skin fibroblasts (Hirai et al., 2007b). Overexpression of Fbln5 increases deposition of elastic fibers and the level of the elastin-specific cross-link, desmosine, in elastogenic cells (Katsuta et al., 2008). Fibulin-5 also facilitates deposition of fibrillin-1 in non-elastogenic retinal pigment epithelial cells engineered to express tropoelastin (Nonaka et al., 2009). Adenovirus-mediated gene transfer of Fbln5 induces de novo assembly of elastic fibers in the skin of Fbln5-/- mice (Zheng et al., 2007). A critical question, therefore, is what is the molecular basis of the potent elastogenic activity of fibulin-5? Identification of fibulin-interacting proteins involved in the core elastic fiber-assemble machinery has provided a clue to this question. Fibulin-5 binds tropoelastin, but not polymerized elastin, via N-terminal cbEGF domains and a C-terminal elastin-binding region (Zheng et al., 2007). The central region of the molecule, including the 4th and 5th cbEGF domains, has also been shown to contain binding sites for tropoelastin (Choudhury et al., 2009). Conversely, the entire tropoelastin molecule is required to bind fibulin-5 (Wachi et al., 2008). Fibulin-4 binds tropoelastin less effectively than fibulin-5 and the tropoelastin binding site is within the C-terminal fibulin domain (Choudhury et al., 2009). These data support the notion that fibulins-4 and -5 may be involved in relatively early stages of elastogenesis.
Biochemical interactions and colocalization between fibulins-4 and -5 and lysyl oxidase proteins have also been shown in cell cultures (Choudhury et al., 2009; Horiguchi et al., 2009; Liu et al., 2004). Fibulin-5 preferentially binds Loxl1 via its C-terminal domain (Liu et al., 2004) and fibulin-4 preferentially binds Lox via its N-terminal domain (Choudhury et al., 2009; Horiguchi et al., 2009). These data indicate a possible regulatory role of fibulins-4 and -5 in determining substrate specificity for the cross-linking enzymes. In Fbln5-/- skin, the normal, fibrous pattern of Loxl1 immunostaining was dramatically decreased at the light microscopic level (Liu et al., 2004). Further analysis by immunogold labeling at the EM level showed that in wild-type mice, the N-terminal Loxl1 pro-domain was barely detected in the dermis and only the C-terminal mature enzyme colocalized with the elastic fibers. In contrast, both the N- and C-terminal portions of Loxl1 were clearly detected in the defective elastin globules formed in the Fbln5-/- skin, suggesting that fibulin-5 may facilitate processing of proLoxl1 to enzymatically active mature form (Choi et al., 2009). Fibulin-4 forms a ternary complex with Lox and tropoelastin and Lox enhances fibulin-4 binding to tropoelastin at low concentration, whereas a high concentration of Lox reduces binding of fibulin-4 to tropoelastin (Choudhury et al., 2009). Fibulin-4 can also facilitate the binding of tropoelastin to Lox (Horiguchi et al., 2009).
Colocalization of fibulin-5 and microfibrils has been demonstrated in cell culture systems (El-Hallous et al., 2007; Hirai et al., 2007b; Hu et al., 2006; Zheng et al., 2007) and fibulins-4 and -5, but not fibulin-3, can bind to fibrillin-1 in vitro (Choudhury et al., 2009; El-Hallous et al., 2007; Freeman et al., 2005). Binding domains for fibulins-4 and -5 are found at the N-terminal domain of fibrillin-1 with partial overlap (Choudhury et al., 2009; El-Hallous et al., 2007). Thus, fibulins are thought to mediate binding between fibrillin-1 and tropoelastin by acting as an adaptor protein (El-Hallous et al., 2007). A later study, however, showed that fibrillin-1 inhibits fibulin-5 binding to tropoelastin and fibulin-4 binding to Lox and tropoelastin in vitro (Choudhury et al., 2009). Clearly, these results demonstrate that there are highly dynamic interactions involving fibulins-4 and -5 with the Lox proteins, tropoelastin and fibrillin-1; however, how the fibulins facilitate and regulate the deposition and assembly of elastin into functional elastic fibers remains to be completely elucidated.
4.2 A model of elastogenesis
Based on the biochemical characterization of fibulins-4 and -5 and the elucidation of critical interactions of these fibulins with core components of elastic fibers, we propose an in vivo model of elastogenesis (Figure 3). Following the secretion of tropoelastin by elastogenic cells, tropoelastin undergoes coacervation near the cell surface. Lack of fibulins-4 and -5 may result in reduction of coacervation and/or dysregulated maturation of coacervates generating abnormally large elastin globules that are not optimal for incorporation into the microfibril scaffold or subsequent cross-linking. Fibulins-4 and -5 bind tropoelastin and Lox proteins and regulate spatial distribution and substrate specificity. The former function is achieved by the physical interaction of fibulins with the microfibrillar component, fibrillin-1, in the ECM. The ability of fibulins to bind fibrillin-1 facilitates correct deposition of Lox-tropoelastin and Loxl1-tropoelastin onto microfibrils, ensuring a proper ratio between fibulins and Lox proteins to allow for maximal cross-linking of tropoelastin. In addition to regulating the distribution of Lox and Loxl1, fibulins-4 and -5 may also affect the processing of lysyl oxidase proteins. Once tropoelastin is deposited onto the microfibrils together with the cross-linking enzyme(s), the initial reaction of oxidative deamination can occur and the formation of cross-links proceeds. The loss of fibulins, therefore, would principally affect the early stages of deposition and correct assembly of elastin on the microfibril bundles.
Figure 3.
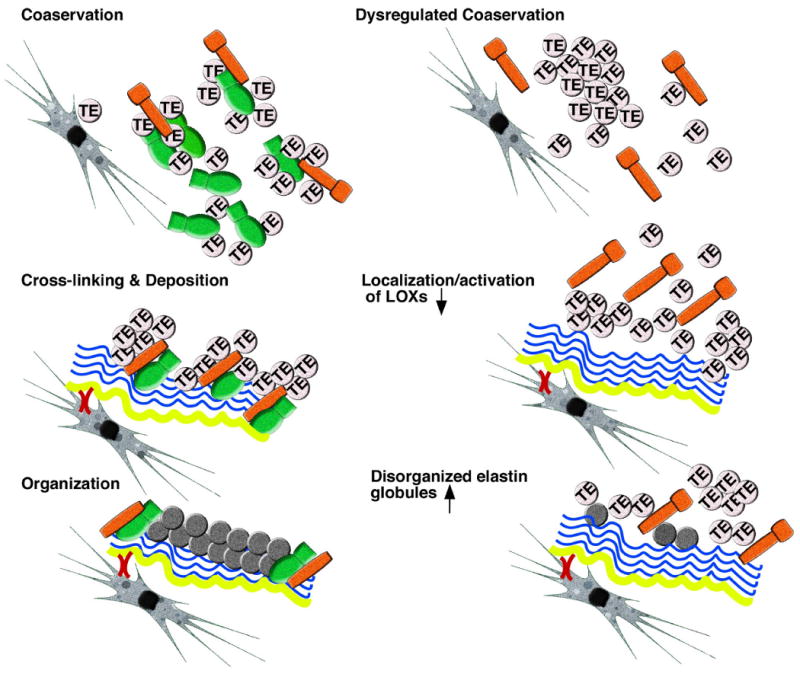
A working model of elastic fiber formation. (Left) The normal elastogenesis process, including coacervation, cross-linking and deposition, and organization of elastic fibers is shown. 1) Following secretion of tropoelastin (TE) from elastogenic cells, coacervation takes place near the cells. Fibulins-4 and -5 (green) regulate this process. Inactive Lox proteins (orange) are present in the coacervate. 2) Fibronectin fibrils (yellow) are tethered to cells and provide a scaffold for microfibril assembly (blue). Microfibrils, in turn, provide a scaffold for deposition of tropoelastin. Fibulins bind to Lox proteins and compartmentalize them to the microfibrils and/or potentiate proteolytic activation of Lox proteins. 3) Lox-mediated cross-linking and polymerization of tropoelastin takes place. Fibulins-4 and -5 most likely remain on the periphery of assembled elastic fibers and/or microfibrils. (Right) Hypothetical condition without fibulins-4 and -5 is shown. 1) In the absence of fibulins-4 and -5, coacervation is decreased and/or maturation is dysregulated, leading to inefficient formation of tropoelastin coacervates and a compromised condition for subsequent cross-linking. 2) Lox/Loxl1-tropoelastin complexes cannot be incorporated onto the microfibril bundle and Lox proteins may not be properly cleaved to become active. The association between tropoelastin and Lox proteins may be decreased without fibulins-4 and -5. 3) As a consequence, aggregated tropoelastin globules are formed and a significant reduction of mature elastic fibers is observed.
5. Differential role of fibulins in elastogenesis in vivo
5.1. Insight from knockout mouse models
Differences in biochemical properties, spatial and temporal gene expression and protein distribution among the short fibulins contribute to distinct phenotypes when perturbed in vivo. Knockout mice of elastogenic short fibulins have been generated and shown to exhibit non-overlapping phenotypes in vivo (McLaughlin et al., 2007; McLaughlin et al., 2006; Nakamura et al., 2002; Yanagisawa et al., 2002). Fbln3-/- mice have normal embryonic development but exhibit reduced reproductivity and show an early aging phenotype in the adult. Although no abnormalities were observed in the prominent elastic tissues, a strain-dependent severe elastic fiber defect was seen in fascia throughout the body, leading to inguinal hernias and pelvic prolapse (McLaughlin et al., 2007; Rahn et al., 2009). These observations indicate a critical function of fibulin-3 in the formation of elastic fibers in the thin, supportive connective tissues. Fbln5-/- mice also live until adulthood but develop systemic elastic fiber defects, involving all major elastogenic tissues such as the skin, lungs, aorta and pelvic organs (Drewes et al., 2007; Nakamura et al., 2002; Yanagisawa et al., 2002). Although the abnormal elastic fiber phenotype in Fbln5-/- mice is principally due to an assembly defect, dysregulated protease activation may also contribute to abnormal elastic fibers in tissues where active remodeling takes place, by increasing the degradation of mature elastic fibers (Wieslander et al., 2009). Fbln4-/- mice exhibit the most severe phenotype with perinatal lethality from aortic aneurysm rupture and a marked reduction in desmosine, indicating that fibulin-4 is the most critical molecule for elastic fiber formation in vivo, known to date (McLaughlin et al., 2006). Interestingly, mice with a vascular SMC-specific knockout of Fbln4 develop aortic aneurysms exclusively in the ascending aorta in adulthood (Horiguchi et al., 2009; Huang J et al., 2009). This aneurysm phenotype has not been observed in other mouse models involving the components of elastic fibers, except for mice deficient for the fibrillin-1 gene. Fbln4-/- mice also develop diaphragm hernia, which indicates an involvement of collagen fibers. Although morphological abnormalities of collagen fibers have not been reported in Fbln4-/- mice, it is possible that the quality of collagen fibers may be affected in addition to defective elastic fibers possibly due to a loss of binding between fibulin-4 and Lox, leading to incorrect localization of Lox and/or impaired proteolytic activation of Lox (Horiguchi et al., 2009). Alternatively, fibulin-4 may have novel functions independent of elastic/collagen fibers, or work in concert with elastic/collagen fibers to affect overall tissue development in vivo (Huang J et al., 2009).
Among all fibulins, fibulin-2 has the highest affinity to tropoelastin in vivo (Kobayashi et al., 2007; Timpl et al., 2003); however, single knockout mice for Fbln2 show no appreciable defects in elastogenic tissues (Sicot et al., 2008). It has been suggested that loss of fibulin-2 can be compensated for by other fibulins. Indeed, double knockout mice for Fbln2 and Fbln5 develop a marked thinning of the internal elastic lamina of the aorta compared to the single Fbln5 null mouse. Since fibulin-2 is highly expressed in the subendothelial region (Tsuda et al., 2001), these results suggest that fibulins-2 and -5 can cooperatively function to form the internal elastic lamina and emphasize the importance of the distinct localizations of fibulin proteins with respect to their functions in vivo (Chapman et al., 2009).
5.2 Fibulins and human elastic fiber diseases
Congenital cutis laxa is a rare connective tissue disorder characterized by loose skin with or without internal organ involvement. Congenital cutis laxa can be divided into autosomal recessive (ARCL), autosomal dominant (ADCL), and X-linked type. Mutations in ELN and ATP7A are responsible for ADCL and X-linked type cutis laxa, respectively. ARCL is further divided into type I (ARCL1 [MIM#219100]), type IIA (ARCL2A [MIM#219200]), and type IIB (ARCL2B [MIM#612940]). Causal mutations in genes encoding for vacuolar-type proton pump (ATP6V0A2) and Pyroline-5-carboxylate reductase-1 have been identified to cause ARCL2A and ARCL2B, respectively. Mutations in FBLN5 are responsible for ARCL1, which include homozygous missense mutations, p.S227P or p.C217R (Claus et al., 2008; Elahi et al., 2006; Loeys et al., 2002). Both mutations lead to decreased binding of fibulin-5 to tropoelastin and disrupt its association with fibrillin-1. The S227P mutation is located in the 4th cbEGF domain and this mutation significantly reduces synthesis and secretion of the mutant protein, as well as induces the endoplasmic reticulum stress response (Hu et al., 2006). Although mutations in ELN cause ADCL, a heterozygous tandem duplication of FBLN5 was found in a case of ADCL. The patient had mild symptoms and the large mutant protein was suggested to act in a dominant negative manner (Markova et al., 2003). Mutations in FBLN4, which include a missense mutation (p.E57K) (Hucthagowder et al., 2006) and compound heterozygous mutations (p.R279C/c. 1070_1073dupCCGC) (Dasouki et al., 2007), were also reported in ARCL1 with severe systemic manifestations. In both cases, aortic aneurysms and skeletal abnormalities accompanied elastic fiber defects and the incorporation of fibulin-4 into the ECM was severely impaired. In all individuals with the FBLN4 and FBLN5 mutations, the cutis laxa phenotype was due to the absence of fibulins-4 and -5 in the ECM and the resulting failure of elastin to be correctly incorporated into microfibrillar scaffold.
Apart from cutis laxa, heterozygous missense variations of FBLN5 were reported to be associated with age-related macular degeneration (ARMD) (Lotery et al., 2006; Stone et al., 2004). ARMD is a major cause of blindness in adult and fibulin-5 was detected in the pathological basal deposits in eyes of ARMD (Mullins et al., 2007). Some mutant proteins, including fibulin-5 (G412E, G267S, I169 T, and Q124P) showed impaired secretion (Lotery et al., 2006), suggesting a possible relationship between compromised elastic fiber formation and the underlying mechanism of macular diseases.
6. Conclusions and perspectives
Short fibulins possess potent elastogenic activity in vitro and in vivo and play essential roles in elastic fiber formation. As more information on protein-protein interactions and preferential binding partners for fibulins and other elastic fiber-associated proteins becomes available, the gaps in our current understanding of the process of elastic fiber assembly will be filled. In addition, how short fibulins are differentially expressed in the various elastogenic tissues and whether a compensatory mechanism exists among short fibulins are important issues to be solved. Increased knowledge on tissue-specific elastogenesis must be obtained in order to build optimal elastic fibers in various tissues and organs that have distinct biomechanical properties. Understanding the molecular mechanisms of elastic fiber formation is thus pivotal for reconstitution of elastic fibers in vitro and regeneration of elastic fibers in vivo. Important aspects of future fibulin research may include discovery of small molecule drugs that facilitate production of elastogenic fibulins or potentiate their functions. In this regards, development of more elaborate in vitro elastogenic assays will be required. Conversely, identification of molecules that inhibit fibulin-mediated elastogenesis will provide useful tools to further delineate the molecular mechanisms of elastic fiber formation in vitro.
Acknowledgments
This work was supported by grants from National Institutes of Health (HL071157 to HY), American Heart Association (HY), Welch Foundation (HY), National Marfan Foundation (HY), Canadian Institutes of Health (MOP57663 and MOP86713 to ECD) and Natural Sciences and Engineering Research Council of Canada (RGPIN 35710-08 to ECD). ECD is a Canada Research Chair.
Abbreviations
- SMC
smooth muscle cell
- TGF
transforming growth factor
- ECM
extracellular matrix
- Lox
lysyl oxidase
- Loxl1
lysyl oxidase-like 1
- LTBP
latent TGF-beta binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argraves WS, Dickerson K, Burgess WH, Ruoslahti E. Fibulin, a novel protein that interacts with the fibronectin receptor beta subunit cytoplasmic domain. Cell. 1989;58:623–9. doi: 10.1016/0092-8674(89)90097-4. [DOI] [PubMed] [Google Scholar]
- Bax D, Rodgers U, Bilek M, Weiss AS. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J Biol Chem. 2009;284:28616–23. doi: 10.1074/jbc.M109.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, et al. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J Biol Chem. 2005;280:40939–47. doi: 10.1074/jbc.M507309200. [DOI] [PubMed] [Google Scholar]
- Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ Res. 2001;88:37–43. doi: 10.1161/01.res.88.1.37. [DOI] [PubMed] [Google Scholar]
- Carta L, Pereira L, Arteaga-Solis E, Lee-Arteaga SY, Lenart B, Starcher B, et al. Fibrillins 1 and 2 Perform Partially Overlapping Functions during Aortic Development. J Biol Chem. 2006;281:8016–23. doi: 10.1074/jbc.M511599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SL, Sicot FX, Davis EC, Huang J, Sasaki T, Chu ML, et al. Fibulin-2 and fibulin-5 cooperatively function to form the internal elastic lamina and protect from vascular injury. Arterioscler Throm Vasc Biol. 2010;30:68–74. doi: 10.1161/ATVBAHA.109.196725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry SS, Gazzard J, Baldock C, Dixon J, Rock MJ, Skinner GC, et al. Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum Mol Genet. 2001;10:835–43. doi: 10.1093/hmg/10.8.835. [DOI] [PubMed] [Google Scholar]
- Choi J, Bergdahl A, Zheng Q, Starcher B, Yanagisawa H, Davis EC. Analysis of dermal elastic fibers in the absence of fibulin-5 reveals potential roles for fibulin-5 in elastic fiber assembly. Matrix Biol. 2009;28:211–220. doi: 10.1016/j.matbio.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, McGovern A, Ridley C, Cain SA, Baldwin A, Wang MC, et al. Differential regulation of elastic fiber formation by fibulin-4 and -5. J Biol Chem. 2009;284:24553–67. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulis JT, Bellingham CM, Davis EC, Hubmacher D, Reinhardt DP, Mecham RP, et al. Fibrillins, fibulins, and matrix-associated glycoprotein modulate the kinetics and morphology of in vitro self-assembly of a recombinant elastin-like polypeptide. Biochemistry. 2008;47:12601–13. doi: 10.1021/bi8005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus S, Fischer J, Megarbane H, Megarbane A, Jobard F, Debret R, et al. A p.C217R mutation in fibulin-5 from cutis laxa patients is associated with incomplete extracellular matrix formation in a skin equivalent model. J Invest Dermatol. 2008;128:1442–50. doi: 10.1038/sj.jid.5701211. [DOI] [PubMed] [Google Scholar]
- Czirok A, Zach J, Kozel BA, Mecham RP, Davis EC, Rongish BJ. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J Cell Physiol. 2006;207:97–106. doi: 10.1002/jcp.20573. [DOI] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, et al. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. J Cell Physiol. 2009;219:14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, et al. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A:2635–41. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- de Vega S, Iwamoto T, Nakamura T, Hozumi K, McKnight DA, Fisher LW, et al. TM14 is a new member of the fibulin family (fibulin-7) that interacts with extracellular matrix molecules and is active for cell binding. J Biol Chem. 2007;282:30878–88. doi: 10.1074/jbc.M705847200. [DOI] [PubMed] [Google Scholar]
- de Vega S, Iwamoto T, Yamada Y. Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci. 2009;66:1890–902. doi: 10.1007/s00018-009-8632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes PG, Yanagisawa H, Starcher B, Hornstra IK, Csiszar K, Marinis SI, et al. Pelvic organ prolapse in Fibulin-5 knockout mice: pregnancy changes in elastic fiber homeostasis in mouse vagina. Am J Pathol. 2007;170:578–89. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlermann J, Weber S, Pfisterer P, Schorle H. Cloning, expression and characterization of the murine Efemp1, a gene mutated in Doyne-Honeycomb retinal dystrophy. Gene Expr Patterns. 2003;3:441–7. doi: 10.1016/s1567-133x(03)00084-x. [DOI] [PubMed] [Google Scholar]
- El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, et al. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. J Biol Chem. 2007;282:8935–46. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- Elahi E, Kalhor R, Banihosseini SS, Torabi N, Pour-Jafari H, Houshmand M, et al. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. J Invest Dermatol. 2006;126:1506–9. doi: 10.1038/sj.jid.5700247. [DOI] [PubMed] [Google Scholar]
- Fogelgren B, Polgar N, Molnarne Szauter K, Ujfaludi Z, Laczko R, Fong KSK, et al. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690–97. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, et al. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. Biochem J. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher WM, Argentini M, Sierra V, Bracco L, Debussche L, Conseiller E. MBP1: a novel mutant p53-specific protein partner with oncogenic properties. Oncogene. 1999;18:3608–16. doi: 10.1038/sj.onc.1202937. [DOI] [PubMed] [Google Scholar]
- Gallagher WM, Greene LM, Ryan MP, Sierra V, Berger A, Laurent-Puig P, et al. Human fibulin-4: analysis of its biosynthetic processing and mRNA expression in normal and tumour tissues. FEBS Letters. 2001;489:59–66. doi: 10.1016/s0014-5793(00)02389-9. [DOI] [PubMed] [Google Scholar]
- Giltay R, Timpl R, Kostka G. Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biology. 1999;18:469–80. doi: 10.1016/s0945-053x(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Hambleton S, Valeyev NV, Muranyi A, Knott V, Werner JM, McMichael AJ, et al. Structural and functional properties of the human notch-1 ligand binding region. Structure. 2004;12:2173–83. doi: 10.1016/j.str.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Heine H, Delude RL, Monks BG, Espevik T, Golenbock DT. Bacterial lipopolysaccharide induces expression of the stress response genes hop and H411. J Biol Chem. 1999;274:21049–55. doi: 10.1074/jbc.274.30.21049. [DOI] [PubMed] [Google Scholar]
- Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. EMBO J. 2007a;26:3283–95. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, et al. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. J Cell Biol. 2007b;176:1061–71. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 2007;26:213–23. doi: 10.1016/j.matbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, et al. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci U S A. 2009;106:19029–34. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. Journal of Biological Chemistry. 2003;278:14387–93. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Hu Q, Loeys BL, Coucke PJ, De Paepe A, Mecham RP, Choi J, et al. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15:3379–86. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- Huang G, Zhang Y, Kim B, Ge G, Annis DS, Mosher DF, et al. Fibronectin binds and enhances the activity of bone morphogenetic protein 1. J Biol Chem. 2009;284:25879–88. doi: 10.1074/jbc.M109.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, et al. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.207852. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Merrilees MJ, Braun K, Beaumont B, Lemire J, Clowes AW, et al. Inhibition of versican synthesis by antisense alters smooth muscle cell phenotype and induces elastic fiber formation in vitro and in neointima after vessel injury. Circ Res. 2006;98:370–7. doi: 10.1161/01.RES.0000202051.28319.c8. [DOI] [PubMed] [Google Scholar]
- Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4 : A Novel Gene for an Autosomal Recessive Cutis Laxa Syndrome. Am J Hum Genet. 2006;78:1075–80. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Naitoh M, Kubota H, Ishiko T, Yoshikawa K, Yamawaki S, et al. Elastic fiber assembly is disrupted by excessive accumulation of chondroitin sulfate in the human dermal fibrotic disease, keloid. Biochem Biophys Res Commun. 2009;390:1221–8. doi: 10.1016/j.bbrc.2009.10.125. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Aspberg A, Keene DR, Ono RN, Reinhardt DP, Sakai LY. Versican interacts with fibrillin-1 and links extracellular microfibrils to other connective tissue networks. J Biol Chem. 2002;277:4565–72. doi: 10.1074/jbc.M110583200. [DOI] [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–7. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Wande L. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. Journal of Cellular Biochemistry. 2003;88:660–72. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Katsuta Y, Ogura Y, Iriyama S, Goetinck PF, Klement JF, Uitto J, et al. Fibulin-5 accelerates elastic fibre assembly in human skin fibroblasts. Exp Dermatol. 2008;17:837–42. doi: 10.1111/j.1600-0625.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol. 2004;62:153–88. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. Elastic fibres. J Cell Sci. 2002;115:2817–28. doi: 10.1242/jcs.115.14.2817. [DOI] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, et al. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Klenotic PA, Munier FL, Marmorstein LY, Anand-Apte B. Tissue Inhibitor of Metalloproteinases-3 (TIMP-3) is a binding partner of Epithelial Growth Factor-containing Fibulin-like Extracellular Matrix Protein 1 (EFEMP1): Implications for macular degenerations. J Biol Chem. 2004;279:30469–73. doi: 10.1074/jbc.M403026200. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Kostka G, Garbe JH, Keene DR, Bächinger HP, Hanisch FG, et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J Biol Chem. 2007;282:11805–16. doi: 10.1074/jbc.M611029200. [DOI] [PubMed] [Google Scholar]
- Kowal RC, Richardson JA, Miano JM, Olson EN. EVEC, a novel epidermal growth factor-like repeat-containing protein upregulated in embryonic and diseased adult vasculature. Circ Res. 1999;84:1166–76. doi: 10.1161/01.res.84.10.1166. [DOI] [PubMed] [Google Scholar]
- Kozel BA, Rongish BJ, Czirok A, Zach J, Little CD, Davis EC, et al. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. J Cell Physiol. 2006;207:87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B, Lumpkin CK, Jr, Goldstein S. An overexpressed gene transcript in senescent and quiescent human fibroblasts encoding a novel protein in the epidermal growth factor-like repeat family stimulates DNA synthesis. Mol Cell Biol. 1995;15:120–8. doi: 10.1128/mcb.15.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire R, Bayle J, Mecham RP, Lafyatis R. Microfibril-associated MAGP-2 stimulates elastic fiber assembly. J Biol Chem. 2007;282:800–8. doi: 10.1074/jbc.M609692200. [DOI] [PubMed] [Google Scholar]
- Lemaire R, Korn JH, Schiemann WP, Lafyatis R. Fibulin-2 and fibulin-5 alterations in tsk mice associated with disorganized hypodermal elastic fibers and skin tethering. J Invest Dermatol. 2004;123:1063–9. doi: 10.1111/j.0022-202X.2004.23471.x. [DOI] [PubMed] [Google Scholar]
- Levinson B, Vulpe C, Elder B, Martin C, Verley F, Packman S, et al. The mottled gene is the mouse homologue of the Menkes disease gene. Nat Genet. 1994;6:369–73. doi: 10.1038/ng0494-369. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, et al. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Human Molecular Genetics. 2002;11:2113–8. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- Lomas AC, Mellody KT, Freeman LJ, Bax DV, Shuttleworth CA, Kielty CM. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. Biochem J. 2007;405:417–28. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Baas D, Ridley C, Jones RP, Klaver CC, Stone E, et al. Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum Mutat. 2006;27:568–74. doi: 10.1002/humu.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM. Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006;63:2304–16. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, et al. Inactivation of the Lysyl Oxidase Gene Lox Leads to Aortic Aneurysms, Cardiovascular Dysfunction, and Perinatal Death in Mice. Circulation. 2002;106:2503–09. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–99. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Markova D, Zou Y, Ringpfeil F, Sasaki T, Kostka G, Timpl R, et al. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am J Hum Genet. 2003;72:998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein LY, Munier FL, Arsenijevic Y, Schorderet DF, McLaughlin PJ, Chung D, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:13067–72. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer P, Hohenester E. Structural and functional aspects of calcium binding in extracellular matrix proteins. Matrix Biol. 1997;15:569–80. doi: 10.1016/s0945-053x(97)90033-0. discussion 81. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Bakall B, Choi J, Liu Z, Sasaki T, Davis EC, et al. Lack of fibulin-3 causes early aging and herniation, but not macular degeneration in mice. Hum Mol Genet. 2007;16:3059–70. doi: 10.1093/hmg/ddm264. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, et al. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26:1700–9. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Olvera MA, Clark AF, Stone EM. Fibulin-5 distribution in human eyes: relevance to age-related macular degeneration. Exp Eye Res. 2007;84:378–80. doi: 10.1016/j.exer.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–5. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ruiz-Lozano P, Lindner V, Yabe D, Taniwaki M, Furukawa Y, et al. DANCE, a novel secreted RGD protein expressed in developing, atherosclerotic, and balloon-injured arteries. J Biol Chem. 1999;274:22476–83. doi: 10.1074/jbc.274.32.22476. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Itoh S, Jeney V, Yanagisawa H, Fujimoto M, Ushio-Fukai M, et al. Fibulin-5 Is a Novel Binding Protein for Extracellular Superoxide Dismutase. Circ Res. 2004;95:1067–74. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- Nonaka R, Onoue S, Wachi H, Sato F, Urban Z, Starcher BC, et al. DANCE/fibulin-5 promotes elastic fiber formation in a tropoelastin isoform-dependent manner. Clin Biochem. 2009;42:713–21. doi: 10.1016/j.clinbiochem.2008.12.020. [DOI] [PubMed] [Google Scholar]
- Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bachinger HP, Sasaki T, et al. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J Biol Chem. 2009;284:16872–81. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preis M, Cohen T, Sarnatzki Y, Ben Yosef Y, Schneiderman J, Gluzman Z, et al. Effects of fibulin-5 on attachment, adhesion, and proliferation of primary human endothelial cells. Biochem Biophys Res Commun. 2006;348:1024–33. doi: 10.1016/j.bbrc.2006.07.156. [DOI] [PubMed] [Google Scholar]
- Putnam EA, Zhang H, Ramirez F, Milewicz DM. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet. 1995;11:456–8. doi: 10.1038/ng1295-456. [DOI] [PubMed] [Google Scholar]
- Rahn DD, Acevedo JF, Roshanravan S, Keller PW, Davis EC, Marmorstein LY, et al. Failure of pelvic organ support in mice deficient in fibulin-3. Am J Pathol. 2009;174:206–15. doi: 10.2353/ajpath.2009.080212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Dietz HC. Fibrillin-rich microfibrils: Structural determinants of morphogenetic and homeostatic events. J Cell Physiol. 2007;213:326–30. doi: 10.1002/jcp.21189. [DOI] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY, Rifkin DB, Dietz HC. Extracellular microfibrils in development and disease. Cell Mol Life Sci. 2007;64:2437–46. doi: 10.1007/s00018-007-7166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz-Timme S, Laumeier I, Collins M. Aspartic acid racemization: evidence for marked longevity of elastin in human skin. Br J Dermatol. 2003;149:951–9. doi: 10.1111/j.1365-2133.2003.05618.x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: The elastic fiber. FASEB Journal. 1993;7:1208–18. [PubMed] [Google Scholar]
- Roybal CN, Marmorstein LY, Vander Jagt DL, Abcouwer SF. Aberrant Accumulation of Fibulin-3 in the Endoplasmic Reticulum Leads to Activation of the Unfolded Protein Response and VEGF Expression. Invest Ophthalmol Vis Sci. 2005;46:3973–79. doi: 10.1167/iovs.05-0070. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, et al. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–58. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Wachi H, Ishida M, Nonaka R, Onoue S, Urban Z, et al. Distinct Steps of Cross-linking, Self-association, and Maturation of Tropoelastin Are Necessary for Elastic Fiber Formation. J Mol Biol. 2007;369:841–51. doi: 10.1016/j.jmb.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Schluterman MK, Chapman SL, Korpanty G, Ozumi K, Fukai T, Yanagisawa H, et al. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Disease Models and Mech. doi: 10.1242/dmm.003707. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicot FX, Tsuda T, Markova D, Klement JF, Arita M, Zhang RZ, et al. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol Cell Biol. 2008;28:1061–7. doi: 10.1128/MCB.01876-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JA, Hacker SL, Davis EC, Mecham RP, Knutsen RH, Li DY, et al. Altered vascular remodeling in fibulin-5-deficient mice reveals a role of fibulin-5 in smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 2005;102:2946–51. doi: 10.1073/pnas.0500058102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–53. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- Stone EM, Lotery AJ, Munier FL, Heon E, Piguet B, Guymer RH, et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nat Genet. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- Timpl R, Sasaki T, Kostka G, Chu ML. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol. 2003;4:479–89. doi: 10.1038/nrm1130. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Wang H, Timpl R, Chu ML. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev Dyn. 2001;222:89–100. doi: 10.1002/dvdy.1172. [DOI] [PubMed] [Google Scholar]
- Vehvilainen P, Hyytiainen M, Keski-Oja J. Matrix association of latent TGF-beta binding protein-2 (LTBP-2) is dependent on fibrillin-1. J Cell Physiol. 2009;221:586–93. doi: 10.1002/jcp.21888. [DOI] [PubMed] [Google Scholar]
- Vrhovski B, Jensen S, Weiss AS. Coacervation characteristics of recombinant human tropoelastin. Eur J Biochem. 1997;250:92–8. doi: 10.1111/j.1432-1033.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- Vukovic J, Ruitenberg MJ, Roet K, Franssen E, Arulpragasam A, Sasaki T, et al. The glycoprotein fibulin-3 regulates morphology and motility of olfactory ensheathing cells in vitro. Glia. 2009;57:424–43. doi: 10.1002/glia.20771. [DOI] [PubMed] [Google Scholar]
- Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Wachi H, Nonaka R, Sato F, Shibata-Sato K, Ishida M, Iketani S, et al. Characterization of the molecular interaction between tropoelastin and DANCE/fibulin-5. J Biochem. 2008;143:633–9. doi: 10.1093/jb/mvn014. [DOI] [PubMed] [Google Scholar]
- Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today. 2007;81:229–40. doi: 10.1002/bdrc.20111. [DOI] [PubMed] [Google Scholar]
- Wieslander CK, Rahn DD, McIntire DD, Acevedo JF, Drewes PG, Yanagisawa H, et al. Quantification of pelvic organ prolapse in mice: vaginal protease activity precedes increased MOPQ scores in fibulin 5 knockout mice. Biol Reprod. 2009;80:407–14. doi: 10.1095/biolreprod.108.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Sekine T, Nakamura H, Imajoh-Ohmi S, Fukuda H, Yudoh K, et al. Fibulin-4 is a target of autoimmunity predominantly in patients with osteoarthritis. J Immunol. 2006;176:3196–204. doi: 10.4049/jimmunol.176.5.3196. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, et al. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–71. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Schluterman MK, Brekken RA. Fibulin-5, an integrin-binding matricellular protein: its function in development and disease. J Cell Com Signaling. 2009;3:337–347. doi: 10.1007/s12079-009-0065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M, Braghetta P, Sabatelli P, Mura I, Doliana R, Colombatti A, et al. EMILIN-1 deficiency induces elastogenesis and vascular cell defects. Molecular & Cellular Biology. 2004;24:638–50. doi: 10.1128/MCB.24.2.638-650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Davis EC, Richardson JA, Starcher BC, Li T, Gerard RD, et al. Molecular analysis of fibulin-5 function during de novo synthesis of elastic fibers. Mol Cell Biol. 2007;27:1083–95. doi: 10.1128/MCB.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


