Abstract
B-lineage acute lymphoblastic leukemia (ALL) arises by transformation of a progenitor (pre-B) cell. Cure rates in adults remain low and treatment is complicated by support provided by the microenvironment to the leukemic cells, indicating an urgent need to better understand the factors that promote their survival. B cell activating factor (BAFF) and its receptor BAFF-R are important for survival and growth of mature normal and malignant B-cells but are not expressed on pre-B cells. Unexpectedly, all cells in the primary Philadelphia-chromosome positive and negative ALL samples tested were positive for high BAFF-R cell surface expression. The BAFF-R was fully competent to bind BAFF and stimulation of the receptor activated both the classical and the non-canonical NFκB pathways. Recombinant BAFF supported survival of the ALL cells in the absence of stroma, and it significantly attenuated the rate of apoptosis caused by exposure to nilotinib, a drug used therapeutically to treat Philadelphia-chromosome positive ALLs. Surprisingly, BAFF mRNA and protein were also expressed in the same cells but BAFF was not shed into the medium. Our report is the first showing universal expression of the BAFF-R by pre-B ALL cells and opens the possibility of blocking its function as an adjuvant therapeutic strategy.
Keywords: BAFF-R, BR3, TACI, NFκB, Ph-positive, Bcr/Abl, drug resistance, stromal support, OP9
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy affecting children, and is a major cause of mortality from hematopoietic malignancies in adults (1). The t(9;22)(q34;q11) translocation, leading to the generation of the Philadelphia (Ph) chromosome, is the most common cytogenetic abnormality in adult ALL and is associated with a poor prognosis (2). The translocation moves ABL on chromosome 9 to BCR on chromosome 22 and results in the formation of a BCR-ABL fusion gene (3). The high incidence of such unfavorable genetic alterations accounts for the low overall cure rate in adult patients with ALL and the identification of new and more effective therapies remains an urgent goal. To this end, a thorough understanding of the survival signals and microenvironment contributing to the establishment of the leukemic clone and its resistance to therapy is required.
ALL develops by transformation of normal B cell progenitors in the bone marrow. Pre-B ALL cells depend on bone marrow stroma for in vitro proliferation and survival (4, 5). Bone marrow stromal cells support leukemic cell growth by the production of soluble factors such as CXCL12, IL-7 and Wnt proteins, as well as via cell-cell adhesion mediated by molecules such as VLA-4/VCAM1 (6-8). Previously we and others have shown that stroma exerts a protective effect that contributes to the poor response of leukemic cells to chemotherapeutic drugs (9-11), and the examination of the molecular nature of the protection provided by stroma remains the focus of significant interest.
Several studies have shown that malignant B cells from patients with chronic lymphocytic leukemia (CLL), non-Hodgkins lymphoma-B and multiple myeloma express abnormal levels of B-cell activating factor (BAFF), which protects these cells from spontaneous or drug-induced apoptosis (12-14). BAFF belongs to the tumor necrosis factor (TNF) ligand family and is displayed either on the cell surface or is released in a soluble form after cleavage from the plasma membrane by a furin-like protease (15, 16). BAFF is crucial for the survival, maturation, and differentiation of normal B cells (17, 18).
The three known receptors for BAFF on B cells include BCMA (B-cell maturation antigen), TACI (transmembrane activator, calcium-modulator, and cyclophilin ligand interactor), and BAFF-R/BR3 (BAFF receptor/BLyS receptor 3). Both BAFF and the related cytokine APRIL bind to TACI and to BCMA, whereas only BAFF binds to the BAFF-R. The BAFF-R/BR3 pathway in particular is crucial to the differentiation of late primary B-cells and survival of mature B cells (19, 20). Interestingly, neoplastic B-lineage lymphocytes express not only BAFF but also the receptors for BAFF, which, when ligated, can promote their cell survival in vitro (21).
BAFF-R expression was reported to be restricted to more mature B cells, starting at the T1 transitional B cell stage (22). Thus, the BAFF-R is not present on murine pro-B or pre-B cells (20). In agreement with the proposed lack of significance of this receptor/cytokine for early B-cell development, mice lacking the BAFF-R or BAFF have normal pro- and pre-B cell compartments (18, 19). Since Ph-positive ALL cells typically represent transformed pre-B cells that do not produce surface IgM, the BAFF-R /BAFF pathway would not be anticipated to play a role in their survival or growth. However, the expression of both BAFF and its receptor BAFF-R in leukemias and lymphomas involving more mature B-lineage cells prompted us to examine a possible role of BAFF and its receptors in ALL. We here present evidence that pre-B ALL is characterized by the abnormal expression of a functional BAFF/BAFF-R pathway.
Materials and Methods
Human ALL cell culture
ALL cells used in this study are listed in Supplementary Table 1. US7 and US7R were from one patient before and after the patient developed drug resistance. US6 has a t(7;14). TXL-2 and TXL-3 were from patients at diagnosis. Primary viably frozen human ALL cells were engrafted through tail vein injections into female NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ mice (Jackson Labs). Leukemia cells harvested from spleens were plated on irradiated OP9 feeder layers and grown in αMEM medium (Gibco, Rockville, MD), 20% FBS, 1% L-glutamine and 1% penicillin/streptomycin. Murine OP9 (CRL-2749), U937 and 293 were purchased from the ATCC (Manassas, USA). Viability of ALL cells collected from the medium was determined by examining and manually counting total and Trypan blue excluding lymphoblasts under a microscope.
Expression studies
RNA was isolated using Trizol from ALL cells harvested without stroma. First strand synthesis used oligo(dT) as primer. Primers for RT/PCR of BAFF-R, TACI, BCMA, BAFF and β2-microglobulin have been described (13). We used ELISA to detect soluble human BAFF (Apotech Corporation, Epalinges, Switserland) in ALL culture supernatant concentrated using Amicon filters (Millipore, MA).
We used a Becton Dickinson FACS-SCAN instrument for FACS analysis. Isotype-matched antibody was used to verify staining specificity. ALL cells were Fc-blocked by treatment with 1 μg of human IgG/105 cells for 15 minutes at room temperature prior to staining. An Annexin apoptosis detection kit (BD Pharmingen, San Jose, USA) was used for detecting apoptotic cells.
For detection of NF-kB (p65 and p52), a nuclear extraction kit (Imgenex, San Diego, CA) was used to separate nuclear and cytoplasmic fractions. For Western blot BAFF, BAFF-R and Pim-2 detection, 20 μg of cell lysates prepared in radioimmunoprecipitation assay buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.28 TIU (typsin inhibitor units)/mL aprotinin, 50 μg/mL leupeptin, 1 mM benzamidine, and 0.7 μg/mL pepstatin was used.
Antibodies used in this study are described in detail in Supplementary Table 2.
Isolation of mononuclear cells from ALL and normal control bone marrow
Viably frozen bone marrow from a normal donor and a patient with ALL were obtained from the National Disease Research Interchange (Philadelphia, PA). Samples were thawed, RBC lysis was performed and the cells pelleted. Cells were suspended in PBS-/- and mononuclear cells isolated by centrifugation on a Ficoll gradient (according to Miltenyi Biotec, Auburn, CA). PB mononuclear cells from US.7R had been viably frozen directly from the patient. Mononuclear cells were incubated with CD19, IgM, CD10 and BAFF-R antibodies. Cells were gated for the CD19+, IgM-, CD10+ fraction and then examined for BAFF-R expression.
Statistical analysis
Results are shown as mean ±SD of at least 3 samples each. For statistical comparison between groups, the Student t test was used, with a p value less than 0.05 considered significant.
Results
Human ALL cells express the BAFF receptor BR-3
Primary cells from different B-lineage ALL patients were passaged in NOD.Cg-PrkdcscidIl2rgtm1Wjll/SzJ mice using previously published protocols (23). To culture these cells ex vivo, we used OP9 stromal cells because we found that murine embryonic fibroblast (MEFs), which we previously utilized to support the ex vivo growth of murine ALL cells, failed to support the long-term growth of these human ALL cells.
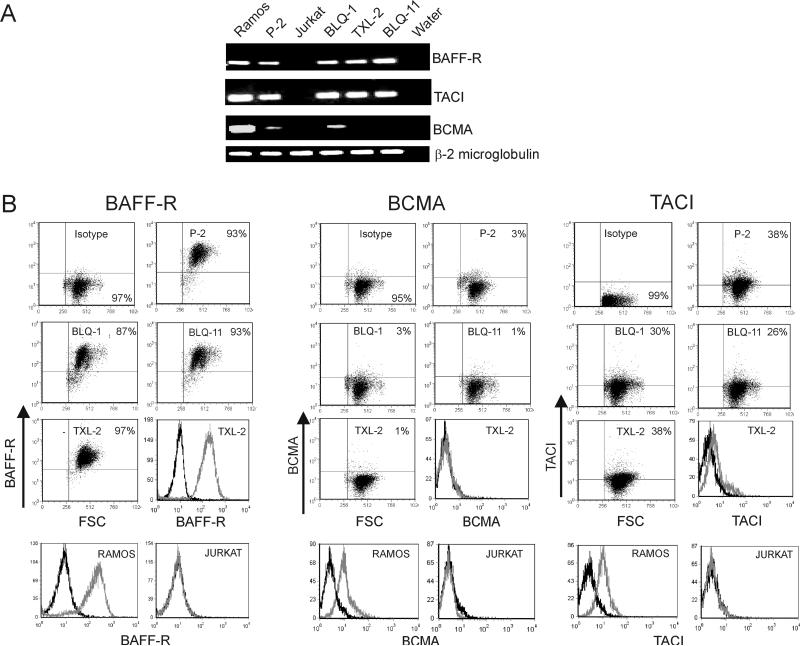
In initial experiments, we examined the human ALL cells for mRNA expression using semi-quantitative RT-PCR for BAFF-R, TACI and BCMA. ). Ramos and Jurkat cells were used as positive and negative controls. To our surprise, as shown in Figure 1A, all of the four human ALL cells tested were positive for BAFF-R and TACI. However, they showed very weak or no expression of BCMA. To examine whether the detected mRNA resulted in production of substantial amounts of cell surface receptors, flow cytometric analysis was done on 12 different ALL samples. Surprisingly, all showed high expression of BAFF-R (Figure 1B, Supplementary Table 1). Remarkably, within the individual samples, more than 90% of the cells were positive. Of the different ALL samples tested for the expression of TACI and BCMA, all were weakly positive for TACI (Figure 1B, Supplementary Table 1) but negative for BCMA (Figure 1B, Supplementary Table 1). Although 25-40% of the cells expressed TACI, the intensity of expression was much lower compared to the BAFF-R expression on these cells (Figure 1B). Both Ph-positive and Ph-negative ALL samples expressed BAFF-R (Supplementary Table 1).
Figure 1. Human ALL cells cultured in vitro express BAFF receptors.
(A) Semi-quantitative RT-PCR on RNA from human ALL cells cultured on OP9 stroma, using the primers indicated to the right. β2 microglobulin serves as control. Ramos and Jurkat; positive and negative controls respectively. (B) FACS analysis on the indicated ALL cells (P-2, BLQ-1, BLQ-11 and TXL-2) cultured on OP9 stroma using anti BAFF-R, BCMA and TACI antibodies. Ramos and Jurkat; positive and negative controls, respectively. Histograms show overlay of isotype control (black line) and receptor antibodies (gray line).
Pre-B ALL cells isolated from fresh bone marrow and peripheral blood of ALL patients express BAFF-R
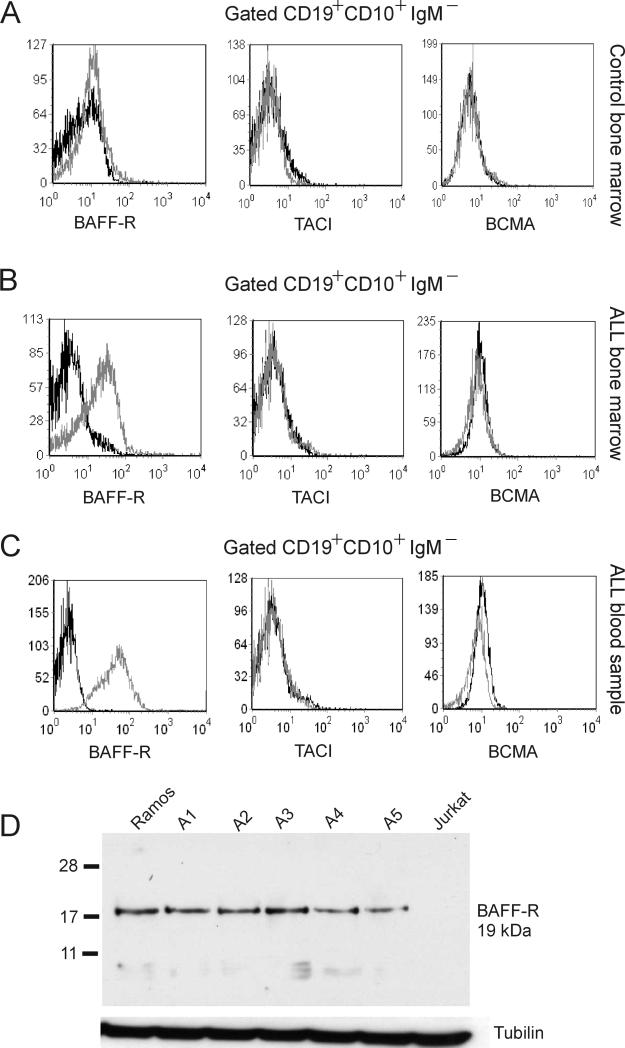
The samples that tested positive for BAFF-R had all been passaged in mice and then plated on stroma. We therefore considered the possibility that passage in mice had induced BAFF-R expression in the ALL cells. To address this, we obtained uncultured fresh bone marrow and peripheral blood and isolated mononuclear cells from these ALL samples. We also obtained bone marrow from a healthy donor. We gated for CD19+ CD10+ IgM- cells to identify pre-B cells and tested these for the expression of BAFF-R, TACI and BCMA using flow cytometry. Consistent with earlier reports, normal pre-B cells were negative for expression of BAFF-R, BCMA and TACI. (Figure 2A). About 60% of the population of mononuclear cells in the ALL patient's bone marrow consisted of CD10+, CD19+, IgM- cells (not shown). More than 50% of gated pre-B ALL cells from the bone marrow of this patient (Figure 2B) and from the peripheral blood of a different patient (Figure 2C) were positive for BAFF-R and uniformly negative for BCMA and TACI expression (Figure 2B, C).
Figure 2. BAFF-R is expressed on non-cultured primary ALL cells.
(A-C) We gated for CD19+ CD10+ IgM- cells (pre-B cells) from bone marrow mononuclear cells of a (A) healthy donor, (B) an ALL patient and from (C) peripheral blood of ALL patient US.7R, then assessed the gated population for expression of BAFF-R, TACI and BCMA. Gray line, receptor antibody; black line, isotype control. (D) Western blot analysis for BAFF-R. A1, A2 and A5, unrelated Ph-positive ALLs; A3 and A4, unrelated Ph-negative ALLs. Ramos and Jurkat; positive and negative controls. Tubulin, loading control.
To confirm the expression of the BAFF-R by non-cultured human ALL cells, we used Western blot analysis on lysates made from independent peripheral blood lymphocytes of 2 Ph-positive and 3 Ph-negative ALLs. As shown in Figure 2D, all five samples tested were positive for BAFF-R and expression levels were comparable to those of the positive control Jurkat cells.
BAFF receptor expressed by ALL cells shows high affinity towards its ligand BAFF
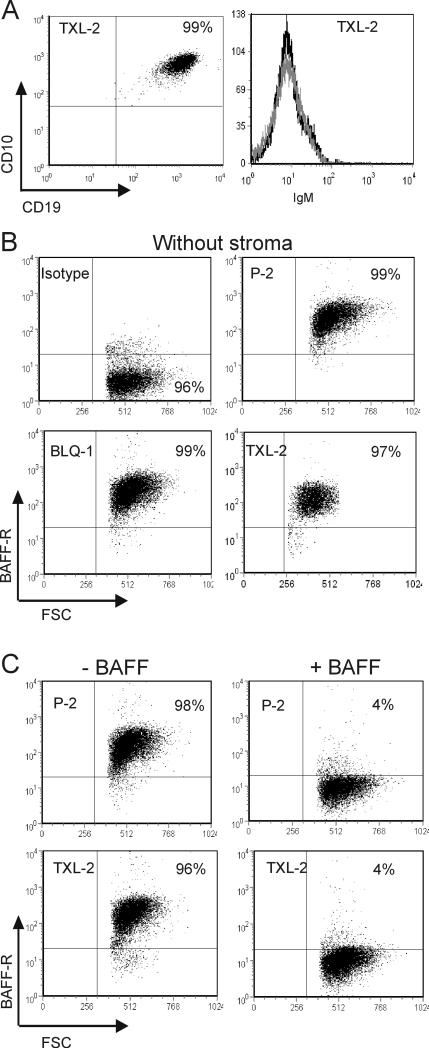
Early B cells can differentiate into more mature stages, when co-cultured with feeder stromal layers (24, 25). Acute myeloid leukemia cells (AML) (26) and CLL cells (27) were reported to differentiate in vitro, and hence we considered the possibility that the presence of stromal feeder layers had changed the immunophenotype of the cells. However, all the human ALL cells tested retained their phenotype (CD19+ CD10+ IgM-) after in vitro culture (TXL-2, Figure 3A; similar data for the other samples; not shown). We also examined the expression of BAFF-R on different ALL cells growing in the absence of stroma for 5 days using flow cytometry. As shown in Figure 3B, cells remained positive for BAFF-R also in the absence of stroma.
Figure 3. BAFF-R phenotype of human ALL cells growing with OP9 stroma.
(A) FACS analysis of TXL-2 ALL cells on OP9 stroma using CD19, CD10 and IgM antibodies. Dot plot shows expression of CD19, CD10 and histogram shows lack of IgM by these cells. (B) P-2, BLQ-1 and BLQ-11 cells were cultured without stroma for 5 days, then analyzed by FACS for BAFF-R expression. (C) P-2 and TXL-2 cells incubated as indicated for 16 hours with or without recombinant human BAFF (100 ng/ml) were examined for cell-surface BAFF-R expression.
We next asked, whether the BAFF-R expressed by these cells is functional. We used a specific monoclonal antibody (clone 11c1) in this experiment to detect cell surface BAFF-R on the ALL cells after incubating overnight with 100 ng/ml recombinant BAFF. Due to spatial competition, the 11c1 anti-BAFF-R antibody is unable to bind to B cells in the presence of high amounts of added recombinant BAFF (28). As shown in Figure 3C, more than 90% of the ALL cells that had previously been incubated with recombinant BAFF were negative for detection of BAFF-R with this antibody –thus, the BAFF-R expressed by ALL cells was occupied with BAFF. This indicates that the BAFF-R expressed by ALL cells shows a high affinity towards its ligand, BAFF.
Expression of BAFF and its signaling in human ALL cells
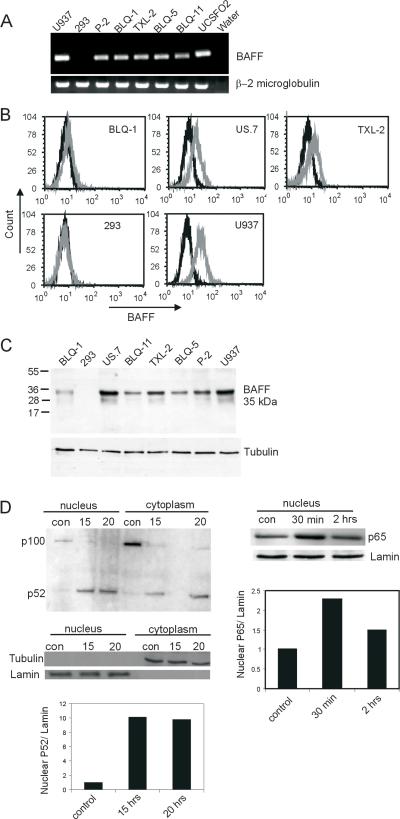
We considered the possibility that the pre-B ALL cells could also express the ligand for the BAFF-R, BAFF. We first evaluated the cells for BAFF mRNA by RT-PCR. As shown in Figure 4A, all four of the ALL cells tested were positive for BAFF mRNA expression. BAFF is member of the broader family of TNF-like cytokines, existing as a membrane-bound and as a secreted form. As shown in Figure 4B, flow cytometry analysis showed that the membrane-associated form of BAFF was present on the surface of BLQ-1, US.7 and TXL-2. The ALL cells were also evaluated for the presence of secreted human BAFF by ELISA, using conditioned medium collected from ALL cell culture. However, we did not detect expression of this form of human BAFF (not shown). We also examined expression of BAFF protein by Western blot. As shown in Figure 4C, BAFF was clearly present in the ALL cells.
Figure 4. BAFF expression and BAFF-induced NF-κB signaling in ALL cells.
(A) BAFF expression using semi-quantitative RT-PCR. U937, positive control; 293 negative control. (B) FACS analysis for cell surface expression of BAFF and (C) Western blot analysis on the indicated cells. Black, isotype control; gray, BAFF antibody. U937 and 293, positive and negative controls. (D, Left panel) Western blot using antibodies for p52 on cytoplasmic and nuclear extracts of BLQ-1 ALL cells incubated for 15 or 20 hours with or without (con) 200 ng/ml of recombinant BAFF. Tubulin and lamin; cytoplasmic and nuclear fraction controls. Densitometric analysis of the nuclear p52 is shown. Ratio of nuclear p52 to lamin of control is taken as 1. (D, Right panel). Nuclear extract of BLQ-1 ALL cells treated for 0.5 or 2 hours with recombinant BAFF (200 ng/ml) blotted with an antibody against of p65. Control, sample not treated with BAFF at 2 hour time point. Densitometric analysis of the nuclear p65 is shown. Ratio of nuclear p65 to lamin of control is taken as 1.
BCMA and TACI signaling activates NF-κB through the canonical pathway, whereas signaling through the BAFF-R was reported to activate both the canonical and the alternative pathways (29-31). We therefore examined intracellular signaling pathways induced by BAFF in the ALL cells. In B cells, the BAFF-mediated activation of the alternative NF-κB pathway involves processing of p100 to p52, with subsequent translocation of p52 to the nucleus (31, 32). When ALL cells incubated with BAFF for 20 hours were separated into a cytoplasmic and a nuclear fraction, there was a substantial (more than 8-fold) increase of processed p52 in the nuclear fraction and a concomitant decrease of p100 in the cytoplasm, as compared to non-BAFF-treated controls (Figure 4D, left panels).
Although most studies suggest that BAFF activates the classical NF-κB pathway through TACI and BCMA (29), Enzler et al (33) reported that BAFF-R also contributes to activation of the classical pathway. We therefore also examined nuclear levels of p65, which migrates to the nucleus upon activation of the classical NF-κB pathway. As compared to control ALL cells without BAFF treatment, nuclear levels of p65 were increased around 2-fold after 30 minutes in the presence of BAFF (Figure 4D, right panels).
Effect of BAFF on growth of ALL cells
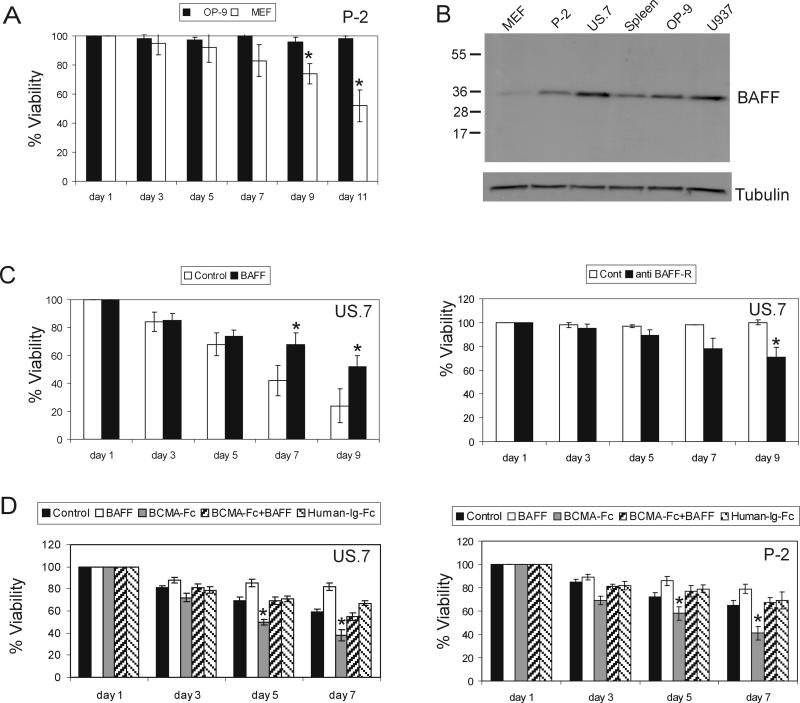
Primary ALL cells do not initially grow in culture without stromal support and are prone to apoptosis in vitro due to lack of proper survival signals. As mentioned above, we found that MEFs did not support ALL growth in long-term culture. However, OP9 bone marrow stromal cells were able to provide the required support to sustain the in vitro growth of ALL cells in long-term culture (Figure 5A). Interestingly, we found that OP9 produced significantly higher amounts of BAFF than MEFs (Figure 5B). This suggested that the human ALL cells require BAFF for long-term growth. To test this, we cultured ALL cells in the absence of stroma and using 5% FBS, with or without recombinant BAFF. Viability of the ALL cells dropped under these stringent conditions, but the presence of BAFF significantly delayed the decrease in viability starting at day 7 of incubation (Figure 5C, left). There are no small molecule inhibitors available to block BAFF-R signaling. However, antibodies have been used to attempt to block BAFF-R function. We therefore incubated the ALL cells with a neutralizing BAFF-R antibody (5 μg/ml) for 9 days. Although there was only a partial inhibition of BAFF induced NF–κB signaling in ALL cells by this antibody (not shown), there was a measurable reduction in cell viability after 5 days of exposure to the neutralizing antibody as shown in Figure 5C (right).
Figure 5. BAFF promotes ALL viability.
(A) Long-term viability of ALL (P-2) cells cultured on OP9 or MEFs. (B) Western blot analysis for BAFF in ALL P-2, US.7 and stromal OP9 and MEF cells. U937, positive human control; mouse spleen, positive murine control. Tubulin, loading control. (C, left) Viability of US.7 in the presence or absence (control) of added recombinant BAFF (1 μg/ml) in the absence of stroma. *p<0.05. (C, right) Viability of US.7 incubated with anti BAFF-R neutralizing antibody (5 μg/ml) as indicated. *p<0.05. (D) Viability of US.7 and P-2 (3×106 cells/ml) cultured in the absence of stroma with BAFF (200 ng/ml), BCMA-Fc (10 μg/ml), human-Ig-Fc (20 μg/ml) or both BAFF (200 ng/ml) and BCMA-Fc (10 μg/ml). *p<0.05 for BCMA-Fc compared to human-Ig-Fc, considered significant.
An autocrine loop could be present on the ALL cells because they express both BAFF and BAFF-R. To investigate this, we incubated a Ph-negative and a Ph-positive ALL with a human BAFF decoy receptor-Fc fusion protein, BCMA-Fc, which can neutralize the effect of human BAFF (19). Interestingly, as shown in Figure 5D, at day 5 in the absence of stroma, there was a significant reduction in cell viability in the presence of BCMA-Fc in comparison with control human IgG-Fc treated wells. Addition of human recombinant BAFF reduced this inhibitory effect, indicating a role of autocrine BAFF signaling in the survival of the ALL cells.
Recombinant BAFF reduces drug-induced ALL cell apoptosis
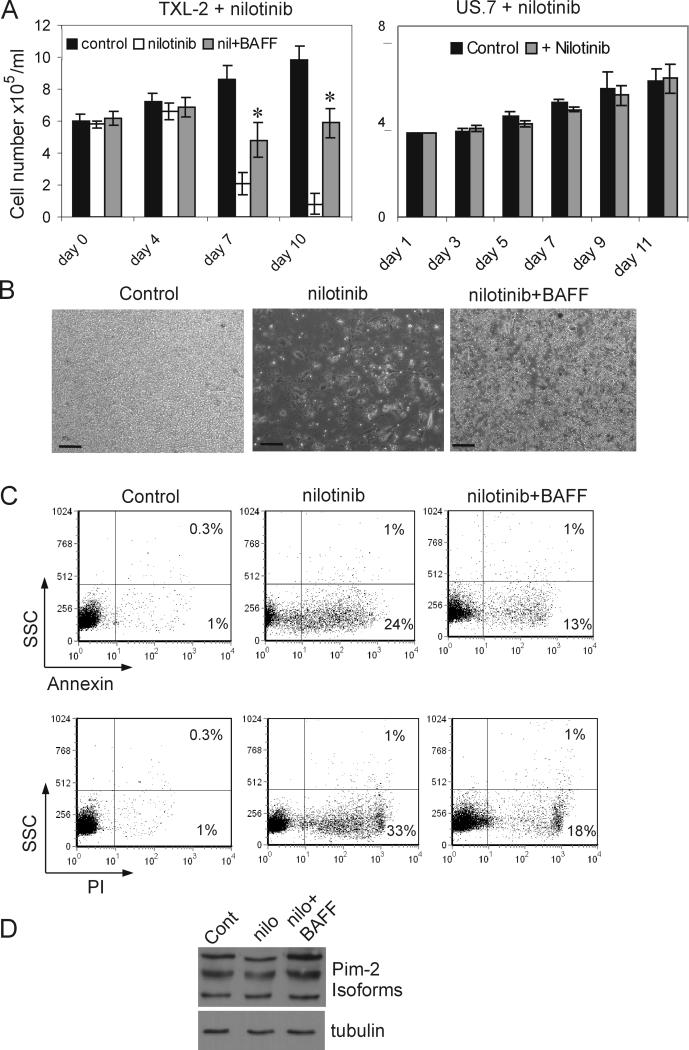
It has been reported that autocrine and paracrine BAFF signaling promote the accumulation of non-Hodgkins lymphoma B cells by attenuating apoptosis (14). BAFF also protected primary myeloma cells cultured with the patient's own bone marrow stroma from dexamethasone–induced apoptosis (13). Therefore we investigated whether BAFF could protect ALL cells from apoptosis induced by a chemotherapeutic drug. Nilotinib is a selective and potent Bcr/Abl tyrosine kinase inhibitor that is currently used to treat Ph-positive leukemias (34). To exclude the possibility that nilotinib might affect the ability of OP-9 stroma to support ALL cell growth, we cultured a Ph-negative ALL (US.7) in the presence of nilotinib. Since nilotinib is a Bcr/Abl tyrosine kinase inhibitor, it has no effect on Ph-negative cells (34). As shown in Figure 6A, right panel, neither viability nor proliferation of US.7 cells growing over OP-9 stroma was affected by the presence of nilotinib, which indicates the that capacity of OP-9 stroma to support ALL growth is not affected by nilotinib. We then cultured Ph-positive TXL-2 ALL cells in the presence of nilotinib with or without recombinant BAFF for 10 days. At day 7, as expected, the total viable cell number in wells treated with nilotinib had decreased (Figure 6A, left). However, there were significantly more viable cells in the nilotinib + BAFF-treated wells, as is exemplified in Figure 6B. To examine the rate of cell death and apoptosis induced by the drug, cells were collected at day 7 and stained with propidium iodide and annexin V. A significant reduction in the number of PI and annexin V positive cells was noticed in the presence of BAFF compared to the drug-alone treated wells (Figure 6C) indicating that BAFF reduced the rate of apoptosis caused by the nilotinib treatment.
Figure 6. Exogenous BAFF reduces effects of drug treatment on ALL cells.
(A, left) Viable cell counts (cell number × 105/ml) of Ph-positive TXL-2 on OP9 stroma incubated with 1 μM nilotinib and human recombinant BAFF (200 ng/ml) for 10 days. Wells without BAFF or nilotinib served as controls. *p<0.05 compared to nilotinib-only treated cells. (A, right) Viable cell counts of Ph-negative US.7 cells treated with 1 μM nilotinib. (B) Phase contrast image of TXL-2 cell cultures at day 10. Scale bar = 100 μm. (C) FACS analysis for annexin V (upper panels) and PI (lower panels) staining of TXL-2 at day 6. (D) Western blot for Pim-2 expression in TXL-2 after 6 days of incubation with nothing (control), nilotinib or nilotinib + BAFF. Tubulin, loading control.
Although the effect of BAFF on in vitro B cell survival is mediated through the classical and alternative NFκB pathways, each pathway makes a distinct contribution to the expression of anti-apoptotic genes. Activation of either the classical or alternative pathway enhances expression of the anti-apoptotic proteins A1/Bfl1 and Bcl-XL, whereas induction of the pro-survival Pim-2 kinase exclusively depends on the alternative pathway (33). Hence, we analyzed Pim-2 expression in the ALL cells treated with nilotinib or nilotinib and BAFF. As shown in Figure 6D, as compared to nilotinib-only treated cells, the addition of BAFF clearly induced the expression of Pim-2, suggesting that the decreased apoptosis of nilotinib-treated ALL cells in the presence BAFF might be mediated at least in part by upregulation of Pim-2.
Discussion
The cytokine BAFF is produced by a number of cell types including monocytes, neutrophils, macrophages, dendritic cells and some subsets of T cells (17). Receptors for BAFF however were initially thought to be restricted to more differentiated B-lineage cells. Therefore, the expression of BAFF receptors on transformed B-lineage lymphocytes in CLL was not entirely unexpected. In contrast, based on BAFF and BAFF-R null mutants as well as other studies, it has been generally accepted that precursor B-lineage cells do not express this receptor. Our studies confirm that there is no expression of this receptor in normal bone marrow pre-B cells. Interestingly, Rodig et al also performed FACS on 2 pre-B ALL samples and reported these were negative for expression of BAFF-R (35). Therefore, the prominent expression of the BAFF-R that we detected on both Ph-positive and Ph-negative ALL samples was unanticipated. In fact, all twelve samples that were tested by us, including original ALL bone marrow and blood samples, were BAFF-R positive, and BAFF-R was detected using both Western blots and FACS analysis. We conclude that this receptor is an excellent tumor-specific marker that distinguishes malignant pre-B cells from their normal counterparts.
We considered the possibility that BAFF-R expression would be downregulated when the ALL cells lost contact with stromal support, but BAFF-R expression was remarkably stable over time and remained even when stromal contact was removed for 5 days. The presence of the BAFF-R on 50-99% of all the cells in the individual samples suggests it has been selected for and has some important function. Since the BAFF-R promotes survival of normal B cells, it could also provide a survival signal to the ALL cells. Indeed, a role for the BAFF-R in the survival of neoplastic B cells, such as CLL, lymphoma, and myeloma has been reported (13, 36-38). We provide evidence that the presence of the BAFF-R on the pre-B ALL cells similarly increases their survival, although viability of ALL cells cultured with rhBAFF alone was not as high as that of cells cultured with OP9 stroma. This was not unexpected, since physical (cell-cell) contact is known to constitute one component of the stromal support to ALL and other leukemia cells (39). In addition, recombinant exogenously added BAFF may not be qualitatively equivalent to that processed by cells, such as that produced in significant amounts by the OP9 cells.
In B cells, BAFF activates the classical and alternative NF-κB pathways. Signals through the BAFF-R mainly activate the alternative pathway, whereas the classical NF-κB pathway is stimulated through BAFF-R, TACI, and BCMA. Both mechanisms of NF-κB activation play important roles in B-cell development and survival (33, 40-42). Interestingly, constitutive NF-κB activation is a molecular signature that has been reported in several malignant B-cell types, including ALL cells (43, 44). We observed BAFF-induced degradation of p100 to p52 and translocation of p65 to the nucleus in ALL cells, suggesting that BAFF induces activation of both classical and alternative NF-κB pathways also in ALL cells.
In vivo and in vitro studies have demonstrated that BAFF signaling increases the expression of anti-apoptotic molecules and promotes cell survival, cell-cycle progression, and proliferation of B cells. Pim-2 is one of the anti-apoptotic genes induced by BAFF signaling in B cells (33, 45). We also measured BAFF-induced Pim-2 expression in ALL cells that were exposed to a therapeutic drug, whereas the levels of Bcl-2 remained unchanged (not shown). Since BAFF-induced long-term survival of B cells with upregulation of Pim-2 depends only on the alternative pathway (33), BAFF may provide protection against drug-induced apoptosis through activation of the alternative NFκB pathway.
The simultaneous expression of the BAFF-R as well as that of BAFF has been reported in both normal murine microglial cells and on malignant human lymphoma cells (14, 36, 46). Remarkably, we observed that the pre-B ALL cells also expressed BAFF mRNA and protein, which points to the possibility of an autocrine BAFF signaling loop in these cells. However, although the pre-B ALL cells expressed amounts of BAFF detectable by Western blots in total cell lysates, we did not detect soluble human BAFF in ALL culture-conditioned medium, with a detection level of below 1 ng/ml. Interestingly, when we blocked human BAFF using a BCMA-Fc decoy receptor, the survival of the ALL cells was reduced, suggesting that the cell-surface BAFF on the ALL cells provides an autocrine or paracrine BAFF survival signal.
Our data show that the BAFF-R is a pre-B leukemia-specific cell surface antigen that could be used for diagnostic purposes. Moreover, inhibitors that block the BAFF/BAFF-R interaction could be valuable specific therapeutic agents in pre-ALL. This receptor-ligand combination is thought to play a role in autoimmune disorders such as rheumathroid arthritis (47), and different approaches to inhibit the interaction have been reported (48). Unfortunately, to date, a potent inhibitor has not been identified. We suggest that specific targeting of pre-B ALL cells with antibody to the BAFF-R conjugated to a toxin, for receptor-mediated delivery of the toxin to ALL cells, could be a promising approach, especially for pre-B ALL cells located in the protective bone marrow environment.
Supplementary Material
Acknowledgements
We would like to thank Dr. Parameswaran Ramakrishnan for providing Ramos and Jurkat cells for the study. This work was supported by R01CA137060 and RO1CA139032 (MM), by funding from a CHLA RCDA, a Jean Perkins Scholar and a StopCancer award (Y-mK) and by funding from the NIH NCI (RO1 CA090321) to NH. ALL samples for Western blots were a kind gift from Ralph Arlinghaus, Viably frozen bone marrow from a normal donor and a patient with ALL were obtained from the National Disease Research Interchange (Philadelphia, PA). We thank Sonia Stoddart for help with growth of the human ALL cells.
Footnotes
Authorship
Contribution: R.P. designed and performed the experiments and wrote the paper; Y-mK passaged the primary human ALL cells in NOD/SCID mice, MM obtained the ALL cells and provided critical input to the study; J.G. contributed to the experimental design, N.H. supported the project, contributed to the experimental design, and wrote the paper.
Conflict of interest disclosure: The authors declare no conflict of interest.
References
- 1.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–43. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 2.Wetzler M, Dodge RK, Mrozek K, et al. Prospective karyotype analysis in adult acute lymphoblastic leukemia: the cancer and leukemia Group B experience. Blood. 1999;93:3983–93. [PubMed] [Google Scholar]
- 3.Heisterkamp N, Groffen J. BCR/ABL Gene Structure and BCR Function. In: Carella AM, Daley GQ, Eaves CM, Goldman JM, Hehlman R, editors. Chronic Myeloid Leukemia: Biology and Treatment. Martin Dunitz Ltd; United Kingdom: 2001. pp. 3–17. [Google Scholar]
- 4.Manabe A, Coustan-Smith E, Behm FG, Raimondi SC, Campana D. Bone marrow-derived stromal cells prevent apoptotic cell death in B-lineage acute lymphoblastic leukemia. Blood. 1992;79:2370–7. [PubMed] [Google Scholar]
- 5.Manabe A, Murti KG, Coustan-Smith E, et al. Adhesion-dependent survival of normal and leukemic human B lymphoblasts on bone marrow stromal cells. Blood. 1994;83:758–66. [PubMed] [Google Scholar]
- 6.Juarez J, Dela Pena A, Baraz R, et al. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia. 2007;21:1249–57. doi: 10.1038/sj.leu.2404684. [DOI] [PubMed] [Google Scholar]
- 7.Khan NI, Bradstock KF, Bendall LJ. Activation of Wnt/beta-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol. 2007;138:338–48. doi: 10.1111/j.1365-2141.2007.06667.x. [DOI] [PubMed] [Google Scholar]
- 8.Bendall LJ, Baraz R, Juarez J, Shen W, Bradstock KF. Defective p38 mitogen-activated protein kinase signaling impairs chemotaxic but not proliferative responses to stromal-derived factor-1alpha in acute lymphoblastic leukemia. Cancer Res. 2005;65:3290–8. doi: 10.1158/0008-5472.CAN-04-3402. [DOI] [PubMed] [Google Scholar]
- 9.Mishra S, Zhang B, Cunnick JM, Heisterkamp N, Groffen J. Resistance to imatinib of bcr/abl p190 lymphoblastic leukemia cells. Cancer Res. 2006;66:5387–93. doi: 10.1158/0008-5472.CAN-05-3058. [DOI] [PubMed] [Google Scholar]
- 10.Bradstock KF, Gottlieb DJ. Interaction of acute leukemia cells with the bone marrow microenvironment: implications for control of minimal residual disease. Leuk Lymphoma. 1995;18:1–16. doi: 10.3109/10428199509064917. [DOI] [PubMed] [Google Scholar]
- 11.Panayiotidis P, Jones D, Ganeshaguru K, Foroni L, Hoffbrand AV. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1996;92:97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 12.Abe M, Kido S, Hiasa M, et al. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia. 2006;20:1313–5. doi: 10.1038/sj.leu.2404228. [DOI] [PubMed] [Google Scholar]
- 13.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–79. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 15.Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–56. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–75. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 17.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–64. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 18.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 19.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 20.Treml JF, Hao Y, Stadanlick JE, Cancro MP. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem Biophys. 2009;53:1–16. doi: 10.1007/s12013-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Endo T, Nishio M, Enzler T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood. 2007;109:703–10. doi: 10.1182/blood-2007-04-081786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng LG, Sutherland AP, Newton R, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;15:807–17. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 23.Baersch G, Mollers T, Hotte A, et al. Good engraftment of B-cell precursor ALL in NOD-SCID mice. Klin Padiatr. 1997;209:178–85. doi: 10.1055/s-2008-1043947. [DOI] [PubMed] [Google Scholar]
- 24.Palacios R, Stuber S, Rolink A. The epigenetic influences of bone marrow and fetal liver stroma cells on the developmental potential of Ly-1+ pro-B lymphocyte clones. Eur J Immunol. 1989;19:347–56. doi: 10.1002/eji.1830190220. [DOI] [PubMed] [Google Scholar]
- 25.Barker J, Verfaillie CM. A novel in vitro model of early human adult B lymphopoiesis that allows proliferation of pro-B cells and differentiation to mature B lymphocytes. Leukemia. 2000;14:1614–20. doi: 10.1038/sj.leu.2401869. [DOI] [PubMed] [Google Scholar]
- 26.Scholzel C, Lowenberg B. Stimulation of proliferation and differentiation of acute myeloid leukemia cells on a bone marrow stroma in culture. Exp Hematol. 1985;13:664–9. [PubMed] [Google Scholar]
- 27.Fu SM, Chiorazzi N, Kunkel HG, et al. Induction of in vitro differentiation and immunoglobulin synthesis of human leukemic B lymphocytes. J Exp Med. 1978;148:1570–8. doi: 10.1084/jem.148.6.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sellam J, Miceli-Richard C, Gottenberg JE, et al. Decreased B cell activating factor receptor expression on peripheral lymphocytes associated with increased disease activity in primary Sjogren's syndrome and systemic lupus erythematosus. Ann Rheum Dis. 2007;66:790–7. doi: 10.1136/ard.2006.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatzoglou A, Roussel J, Bourgeade MF, et al. TNF receptor family member BCMA (B cell maturation) associates with TNF receptor-associated factor (TRAF) 1, TRAF2, and TRAF3 and activates NF-kappa B, elk-1, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. J Immunol. 2000;165:1322–30. doi: 10.4049/jimmunol.165.3.1322. [DOI] [PubMed] [Google Scholar]
- 30.Xia XZ, Treanor J, Senaldi G, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–43. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayagaki N, Yan M, Seshasayee D, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515–24. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 32.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–65. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 33.Enzler T, Bonizzi G, Silverman GJ, et al. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–15. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Deremer DL, Ustun C, Natarajan K. Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Clin Ther. 2008;30:1956–75. doi: 10.1016/j.clinthera.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Rodig SJ, Shahsafaei A, Li B, Mackay CR, Dorfman DM. BAFF-R, the major B cell-activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum Pathol. 2005;36:1113–9. doi: 10.1016/j.humpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Novak AJ, Grote DM, Stenson M, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: correlation with disease activity and patient outcome. Blood. 2004;104:2247–53. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 37.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: a mechanism for survival. Blood. 2002;100:2973–9. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 38.Haiat S, Billard C, Quiney C, Ajchenbaum-Cymbalista F, Kolb JP. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology. 2006;118:281–92. doi: 10.1111/j.1365-2567.2006.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–74. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 40.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Caamano JH, Rizzo CA, Durham SK, et al. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187:185–96. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki Y, Derudder E, Hobeika E, et al. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–39. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Kordes U, Krappmann D, Heissmeyer V, Ludwig WD, Scheidereit C. Transcription factor NF-kappaB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia. 2000;14:399–402. doi: 10.1038/sj.leu.2401705. [DOI] [PubMed] [Google Scholar]
- 44.Munzert G, Kirchner D, Ottmann O, Bergmann L, Schmid RM. Constitutive NF-kappab/Rel activation in philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL). Leuk Lymphoma. 2004;45:1181–4. doi: 10.1080/10428190310001657326. [DOI] [PubMed] [Google Scholar]
- 45.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–53. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 46.Kim KS, Park JY, Jou I, Park SM. Functional implication of BAFF synthesis and release in gangliosides-stimulated microglia. J Leukoc Biol. 2009;86:349–59. doi: 10.1189/jlb.1008659. [DOI] [PubMed] [Google Scholar]
- 47.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 48.Lin WY, Gong Q, Seshasayee D, et al. Anti-BR3 antibodies: a new class of B-cell immunotherapy combining cellular depletion and survival blockade. Blood. 2007;110:3959–67. doi: 10.1182/blood-2007-04-088088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.