Abstract
To capitalize on the response of tumor cells to ionizing radiation, we developed a controlled-release nanoparticle drug delivery system using a targeting peptide that recognizes a radiation-induced cell surface receptor. Phage display biopanning identified Gly-Ile-Arg-Leu-Arg-Gly (GIRLRG) as a peptide that selectively recognizes tumors responding to ionizing radiation. Membrane protein extracts of irradiated glioma cells identified glucose-regulated protein GRP78 as the receptor target for GIRLRG. Antibodies to GRP78 blocked the binding of GIRLRG in vitro and in vivo. Conjugation of GIRLRG to a sustained-release nanoparticle drug delivery system yielded increased paclitaxel concentration and apoptosis in irradiated breast carcinomas for up to three weeks. Compared to controls, a single administration of the GIRLRG-targeted nanoparticle drug delivery system to irradiated tumors delayed the in vivo tumor tripling time by 55 days (P=0.0001) in MDA-MB-231, and 12 days in GL261 (P<0.005). This targeting agent combines a novel recombinant peptide with a paclitaxel encapsulating nanoparticle that specifically targets irradiated tumors, increasing apoptosis and tumor growth delay in a manner superior to known chemotherapy approaches.
Keywords: Radiation-induced neoantigens, targeted drug delivery, nanoparticle drug delivery system, GRP78, recombinant peptide
Introduction
In vitro, many agents are capable of killing cancer cells effectively. These agents trigger cancer cell death through numerous complex pathways, such as apoptosis or prevention of further cell division. However, when these agents are transferred from use in cell culture to an entire system, the effect on normal tissue limits their use in a clinical setting. With a small therapeutic index between cancer destruction and toxic side effects, drugs are often not used in patients or discontinued far before they achieve a maximal effect.
Thus, targeted therapy provides a means to circumvent the toxicities and lack of treatment response of conventional systemic chemotherapy. With the development of targeted biologics, such as trastuzumab and imatinib, therapies for specific cancer types have been developed. However, these therapeutics are often limited to cancers expressing various mutations and thus are limited in broad use (1, 2). Treatments aimed at universal solid tumor therapy, such as angiogenesis inhibitors, have had limited success thus far (3).
The discovery of receptors expressed at much greater levels on tumors than on normal tissue would provide targets for drug delivery. Therefore, receptor induction in tumors could play a critical role in providing new targets. Ionizing radiation (XRT), while also therapeutic, could potentially provide a cellular stress, localized to cancer cells, capable of causing new receptor translocation. Beyond XRT's cytotoxic effects, it has been shown to induce gene transcription (4), and protein expression on tumor microvasculature (5). Using phage display biopanning, recombinant peptides that bind only to treated cancers have been found (6, 7). However, combining these peptides with chemotherapeutic agents has not been effectively translated to clinical use.
Through targeted therapeutics, nanoparticle delivery systems have the potential to overcome the normal tissue toxicity of traditional chemotherapy. However, even though paclitaxel encapsulation in albumin nanoparticles, nab-paclitaxel, increases the efficacy and safety over paclitaxel formulated in cremophor (8-10), more nausea, diarrhea, and grade-3 sensory neuropathy occurs in patients treated with nab-paclitaxel (8). Another remaining challenge of nanoparticle delivery systems is the lack of control of drug release profiles. This distinct “burst-effect,” in which the majority of drug is released in a rapid and uncontrollable fashion, creates unpredictable pharmacokinetics thereby making effective dose regimens difficult to predict.
Our goal was to identify a novel recombinant peptide and a radiation-inducible receptor pair in XRT-treated cancers and conjugate the peptide to a novel nanoparticle drug delivery system (DDS) for use in XRT-targeted chemotherapy. In this work, we present three new findings: the discovery of a recombinant peptide that recognizes XRT-treated tumors, the discovery of this peptide's radiation-inducible receptor, and the therapeutic benefits of a targeted recombinant peptide/nanoparticle DDS.
Materials and Methods
Animals used
Athymic nude and C57/Bl6 mice were purchased from Harlan Laboratories. All animal protocols were IACUC approved.
Tumor models
GL261 murine glioma and MDA-MB-231 human breast cancer cell lines were purchased from American Type Culture Collection (ATCC). Heterotopic tumor models were developed by subcutaneously inoculating cell suspensions (6×106 cells) into nude or C57/Bl6 mice.
Co-culture assays
Human umbilical vein endothelial cells (HUVECs) in the sixth passage (Lonza) and GL261 murine glioma cells were co-cultured as previously described (7). The cells were allowed to interact for one day before treatment with 3 Gy of XRT and incubated for 3 hours before they were harvested. Coverslips were blocked for 30 minutes with 5% BSA and 1% Streptavidin (ThermoScientific). Cells were incubated for one hour with a Streptavidin-Peptide-AlexaFluor594 complex (AlexaFluor594 carboxylic acid succinimidyl ester was purchased from Invitrogen) (Supplementary Fig. S1). HUVEC nuclei were stained with DAPI and images of nuclei and peptide binding were taken by Vanderbilt Cell Imaging Shared Resource center using a Zeiss Axiophot fluorescent microscope at 40× magnification. Cell co-localization was performed using Metamorph Offline software in all assays.
Similarly, 3×105 HUVECs were layered in co-culture plates alone and treated with 3 Gy or left untreated, incubated with Streptavidin-Peptide-AlexaFluor594 complex and imaged as before. Positive and negative controls of XRT treated and untreated GL261/HUVEC co-cultures with the peptide incubated on HUVECs were used. Assays were performed three times in triplicate.
Near infrared imaging
Tumor bearing mice were treated with three once daily doses of 3 Gy XRT or sham XRT (three per group) and injected with peptide or antibody three hours after the last XRT treatment. In one experiment, labeled complexes of biotinylated peptide-AlexaFluor750 conjugates were injected (AlexaFluor750 carboxylic acid succinimidyl ester was purchased from Invitrogen). In a second experiment, an antibody to GRP78 conjugated with AlexaFluor750 was injected and tumors were removed seven days after labeled antibody injection; polyclonal serum IgG antibody was used as a control. In a third experiment, mice received an unlabeled blocking antibody to GRP78 or unlabeled polyclonal IgG serum and then injected with the labeled GIRLRG peptide. Near infrared images were taken using the IVIS imaging system with an ICG filter setting at various time points after the injection.
Membrane protein extraction
GL261 tumor samples either treated with 3 Gy XRT or sham-XRT were removed from the hind limbs of athymic nude mice 48 hours after treatment and frozen at -80°C. Forty mg samples of treated and untreated frozen tumors were homogenized and the protein was extracted using the Mem-PER Eukaryotic Membrane Protein Extraction Kit (ThermoScientific). The extracted protein was then incubated overnight in the Slide-A-Lyzer Dialysis Cassettes (ThermoScientific). The protein was then incubated overnight with NeutrAvidin-coated agarose beads (ThermoScientific) bound to biotinylated GIRLRG or scrambled peptide (RILGGR). After incubation the beads were washed with 1× PBS, boiled at 100°C and run on an Invitrogen NuPAGE 10% gel. The gel was stained with Invitrogen Simply Blue SafeStain. Bands from the gel were analyzed by the Vanderbilt Proteomics Core via the LC/MS/Mass Spectrometry technique.
Western blotting
Nude mice implanted subcutaneously in the hind limb with either GL261 and MDA-MB-231 tumors were treated with 3 Gy XRT for three consecutive days, and tumors were removed 48 hr post-XRT. Tumor samples were homogenized, and the protein from the samples was extracted. Protein was probed with antibodies for GRP78 and actin (Cell Signaling) on a polyvinylidene difluoride membrane and exposed to film that was later developed using the Western Lightning Chemiluminescence Plus detection system (PerkinElmer) according to the manufacturer's protocol.
Immunohistochemistry
Paraffin embedded tumor samples were stained using an antibody for the von Willebrand Factor (vWF) (DakoCytomation) at a 1:100 dilution from the original stock solution of 3.1 g/L and incubated overnight. Samples were then incubated with a Streptavidin-Peptide-AlexaFluor594 complex and washed three times with PBS.
In a second assay, samples were stained with vWF and incubated with an antibody to GRP78 at dilutions of 1:250 and 1:1000 of original stock solution. These samples were then incubated with Streptavidin-Peptide-AlexaFluor594 complex and imaged.
Images were taken using a fluorescent microscope at 20× magnification. Assays were performed in triplicate.
Nanoparticle synthesis and attachment of GIRLRG peptide to nanoparticles
Polyester nanoparticle DDS was synthesized by the procedure described in van der Ende et. al. (11). To a solution of nanoparticles from poly (valerolactoneepoxyvalerolactone-allylvalerolactone-oxepanedione) containing 11% epoxide and cross-linked with 1 equivalents of 2,2-(ethylenedioxy)bis(ethylamine) per epoxide (11) (105.6 mg, 0.78 μmol) in DMSO (1 mL), KKCGGGGIRLRG peptide (56 mg, 3.35 μmol) in DMSO (2 mL) was added. The reaction mixture was heated for 72 h at 34 °C. Residual peptide was removed by dialyzing with SnakeSkin® Pleated Dialysis Tubing (MWCO = 10,000) against 50/50 THF/CH3CN.
Encapsulation of paclitaxel in GIRLRG conjugated nanoparticles
The paclitaxel was encapsulated by the procedure described in van der Ende et al (12). The weight percent of paclitaxel encapsulated in the nanoparticles was determined by NanoDropTM UV-Vis at 254 nm as mentioned in the literature and was found to be 11.3%.
Paclitaxel antibody staining
Nude mice were implanted in the hind limb with MDA-MB-231 tumors. Once tumors reached 450 mm3 in volume, mice were treated with 3 Gy XRT once daily for three consecutive days, or they were left untreated. On the second day mice were injected with one of either i.) systemic paclitaxel, ii.) paclitaxel/Nanoparticle with RILGGR or iii.) paclitaxel/Nanoparticle with GIRLRG. The paclitaxel concentration used was 10 mg/kg. Tumors were removed one week and three weeks after treatment, imbedded in paraffin and sectioned. Tumor sections were incubated with a monoclonal antibody to Paclitaxel (Santa Cruz) at a concentration of 1:500. Three mice per group were used. All paclitaxel antibody staining was performed in triplicate.
TUNEL staining
Nude mice were treated and tumors were collected as described in “Paclitaxel antibody staining” above. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was done with the DeadEnd Colorimetric TUNEL System (Promega) following the manufacturer's instructions. Positive staining was observed by light microscopy.
Paclitaxel antibody staining and TUNEL staining evaluation
All slides were evaluated and graded based on color intensity of immunoreactions using a 6-tier grading system of 5-6 (strong), 3-4 (moderate), 1-2 (faint), and 0 (none). Assays were performed in triplicate.
Statistical analyses
Student's t-test was used to perform group comparisons. Linear correlations of peptide binding and tumor response to treatment were developed by use of the correlation coefficient of tumor growth and radiance datasets (SigmaPlot).
Results
Discovery of a peptide that recognizes irradiated tumors
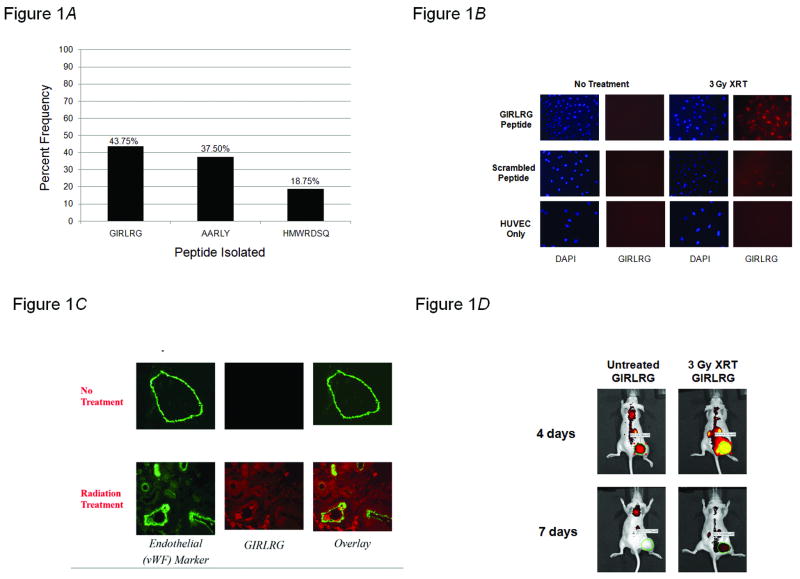
We utilized phage display technology to identify a targeting peptide that would discriminately bind to irradiated tumors. Using a previously characterized in vivo biopanning method to screen the T7 phage-based random peptide library (6, 7), the novel peptide Gly-Ile-Arg-Leu-Arg-Gly (GIRLRG) was identified as the predominant phage-encoded peptide recovered from irradiated GL261 gliomas in mice (Fig. 1A). We then investigated GIRLRG's specificity for irradiated tumors using in vitro co-culture experiments. To model the tumor microenvironment, we used human umbilical vein endothelial cells (HUVECs) co-cultured with GL261 tumor cells. These experiments revealed that the GIRLRG targeting peptide bound to tumor vasculature only when two criteria were met: tumor cells were irradiated, and tumor cells were able to interact with HUVECs (Fig. 1B). To simulate normal tissue, HUVECs were cultured alone. There was no binding of the GIRLRG recombinant peptide to this normal tissue model suggesting an obligate interaction between the tumor and tumor vasculature for the peptide's target receptor to be available for binding. We also found that the GIRLRG peptide colocalizes with an endothelial cell marker in irradiated tumor samples ex vivo (Fig. 1C). We then used an in vivo binding model utilizing near infrared imaging with GL261 tumors implanted in the hindlimbs of mice. The GIRLRG targeting peptide was again shown to preferentially bind to radiation-treated tumors in vivo over untreated tumors (Fig. 1D).
Figure 1.
GIRLRG binds to tumors responding to ionizing radiation. A, Biopanning of the T7 phage-based peptide library followed by sequencing was used in vivo to identify phage peptides binding specifically to glioma (GL261) treated with 3 Gy of ionizing radiation and 40mg/kg of sunitinib. Shown here are the three amino acid sequences isolated most frequently and the percentage of peptide-bearing phages in the sequenced clones. B, GIRLRG preferentially binds to radiation treated co-cultures of GL261 gliomas and HUVECs. GIRLRG (top row) or a scrambled peptide (middle row) were incubated on HUVECs from XRT-treated and untreated GL261/HUVEC co-cultures. GIRLRG was also incubated on XRT-treated and untreated HUVEC cultures alone (bottom row). C and D, GIRLRG binds to tumors responding to ionizing radiation ex vivo and in vivo. C, Nude mice were implanted in the right hindlimb with GL261 glioma cells and were either left untreated or treated daily with radiation (3 Gy) for 5 consecutive days (three mice per treatment group). Tumor sections were incubated with von Willebrand Factor, an endothelial marker, followed by fluorescently-labeled GIRLRG and imaged. Shown are untreated tumor sections and XRT-treated tumor sections 48 hours post-treatment. D, Nude mice were implanted in the hind limb with GL261 glioma cells and were either left untreated or treated daily with radiation (3 Gy) for 3 consecutive days (three mice per treatment group). Fluorescently-labeled GIRLRG peptide preferentially binds to radiation treated tumors in GL261 xenografts compared to untreated tumors.
GRP78 is induced by ionizing radiation in tumors
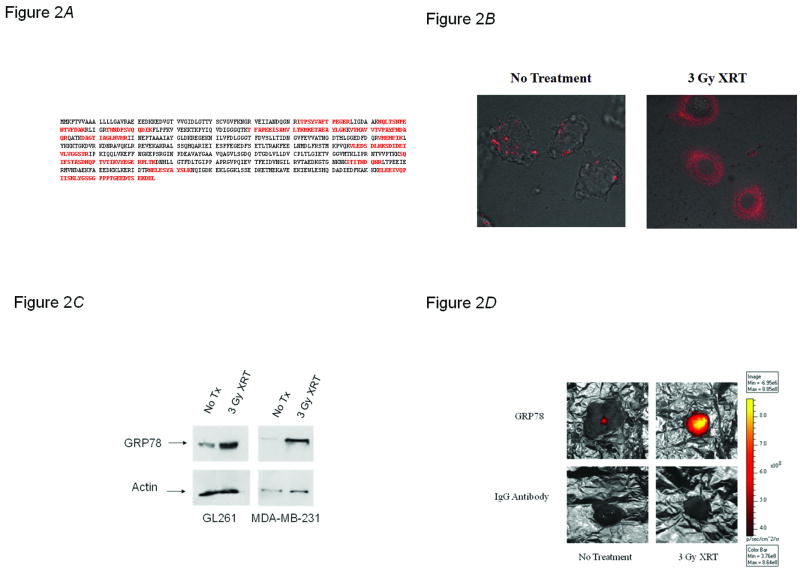
We next sought to discover the target of GIRLRG. Agarose beads coated with GIRLRG were incubated with membrane protein extracts from irradiated GL261 tumors and removed 48 hours post-irradiation. We isolated a 78-kDa band by gel electrophoresis. Mass spectrometry of that band revealed it to be the 78-kDa glucose regulated protein (GRP78) (Fig. 2A). Therefore, we investigated the effects of radiation on GRP78 concentration. In vitro, we found that GRP78 is induced in HUVECs grown in co-culture with GL261 gliomas after XRT treatment (Fig. 2B). Membrane protein extract from GL261 gliomas and MDA-MB-231 breast carcinomas were analyzed for GRP78 expression via Western blot (Fig. 2C). The results revealed increased GRP78 expression after radiation treatment, in both tumor types, as compared to controls. GRP78 upregulation in response to XRT in GL261 gliomas was validated ex vivo (Supplementary Fig. S2) and in vivo (Fig. 2D). Tumors implanted in the hind limbs of nude mice were treated with XRT, then injected with either a fluorescently labeled GRP78 antibody or labeled control polyclonal serum IgG, and imaged using near infrared imaging. Tumors treated with ionizing radiation, showed intense binding of fluorescently-labeled antibody to GRP78 compared to IgG serum controls (Fig. 2D).
Figure 2.
GRP78 is induced by ionizing radiation. A, The highlighted sequences are the sequences identified by mass spectrometry as extracted and precipitated with GIRLRG peptide coated on agarose beads. B, GRP78 is induced in HUVECs grown in co-culture with GL261 gliomas after XRT treatment. GL261 glioma cells (3 × 105) and HUVECs (1 × 104) were co-cultured for 24 hours prior to treatment with 3 Gy of XRT. Three hours post-treatment, AlexaFluor594 labeled GRP78 antibodies were added to the culture plates. C, Western blot of GRP78 expression in XRT-treated and untreated GL261 glioma and MDA-MB-231 breast carcinoma tumor sections showing GRP78 is upregulated to the membrane in response to XRT. D, Antibody to GRP78 binds selectively to XRT-treated tumors. GL261 tumors were implanted in the hind limbs of nude mice and treated with 3 Gy of XRT for three consecutive days. On the third day an antibody to GRP78 or IgG antibody serum conjugated with AlexaFluor 750 was injected through the tail veins of mice. Tumors were removed seven days after peptide injection and imaged using near infrared imaging to assess relative levels of peptide binding.
GRP78 and GIRLRG interaction studies
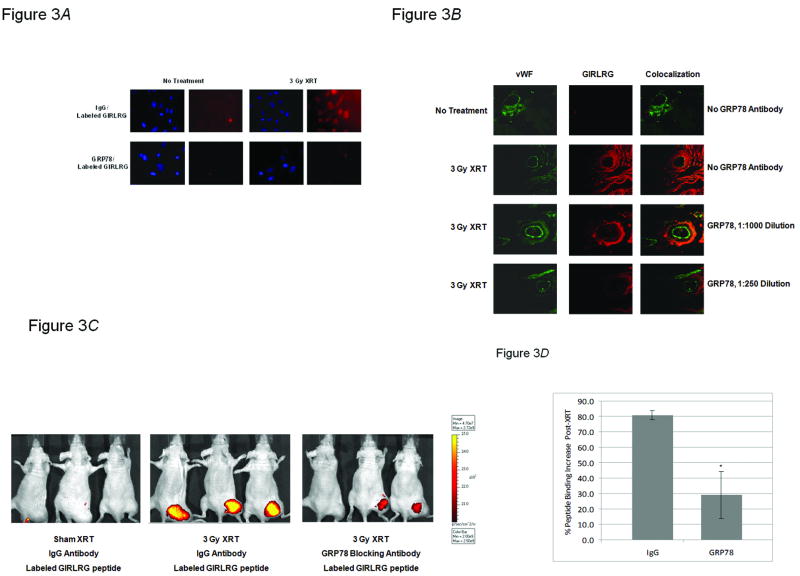
We next studied the putative ligand-receptor interaction between the GIRLRG peptide and GRP78. This was accomplished through in vitro, ex vivo and in vivo experiments using blocking antibodies to GRP78. In vitro co-cultures with GL261/HUVECs showed decreased binding of GIRLRG to HUVECs when blocking antibodies to GRP78 were added to the co-culture (Fig. 3A). Next, we treated implanted GL261 tumors in mice with XRT, removed and sectioned the tumors, and treated with varying concentrations of GRP78 blocking antibody. We found that as the GRP78 blocking antibody concentration increased, binding of fluorescently labeled GIRLRG decreased (Fig. 3B). An in vivo imaging study was performed to assess if fluorescently-labeled GIRLRG peptide could still bind to XRT-treated tumors following the addition of an antibody to GRP78. The GRP78 antibody attenuated GIRLRG signal intensity by > 70% compared to control IgG serum antibody in irradiated GL261 tumors (P<0.05) (Fig. 3C and D).
Figure 3.
GRP78 is the target of GIRLRG. A, GL261 glioma cells and HUVECs were co-cultured for 24 hours prior to treatment with 3 Gy of XRT followed by blocking antibody to GRP78 or a control IgG antibody serum. Cells were then incubated with AlexaFluor594-labeled GIRLRG peptide and imaged for peptide binding. B, GL261 gliomas implanted in the hind limbs of nude mice were treated with 3 Gy of XRT daily for three days, sectioned and incubated with a polyclonal antibody to GRP78 at antibody concentrations of 1:1000 and 1:250. C and D, GL261 gliomas implanted in the hind limbs of nude mice (three mice per treatment group) were treated with 3 Gy of XRT daily for three days. One hour after the final XRT treatment, mice were injected with either a polyclonal antibody to GRP78 or IgG antibody serum. Three hours after antibody administration mice were injected GIRLRG peptide labeled with AlexaFluor 750. C, Mice were imaged 24 hours after the final XRT treatment using near infrared imaging. D, Shown is the graph of relative peptide binding in vivo in mice treated with XRT and injected with either an antibody to GRP78 or an IgG control antibody serum. Differential peptide binding is shown as percentage tumor fluorescence increase relative to an untreated tumor-bearing mouse injected with an IgG control antibody serum.
Creation of a GIRLRG-targeted nanoparticle DDS
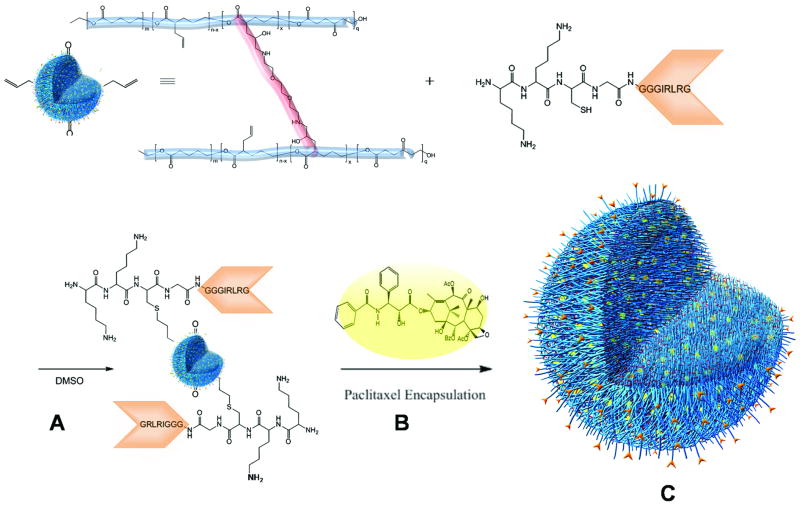
We postulated that conjugating the GIRLRG recombinant peptide with a nanoparticle DDS could target chemotherapeutics specifically to radiated tumors. After nanoparticle formation (11), the peptide was conjugated using a high yielding thiol-ene reaction, reacting the free thiol of the cysteine near the N-terminus of the KKCGGGGIRLRG with allyl functionalities on the nanoparticle (12). NMR Spectroscopy methods could determine the conjugation of 37 peptides (Fig. 4). In the final step, paclitaxel was incorporated resulting in a DDS that is well-dispersed in a cremphor-free_solution. The biocompatibity of peptide targeted particles in concentrations applied for in vivo studies was confirmed in cytotoxcity assays (Supplementary Fig. S3).
Figure 4.
Synthesis of a GIRLRG-targeted nanoparticle drug delivery system (DDS). A, Polyester nanoparticle formation resulting from a via intermolecular cross-linking of diamine (2,2′-(ethylenedioxy)bis(ethylamine)) (shown in red) and linear polymer precursor (poly(vl-avl-evl-opd)) (shown in blue) containing epoxide units as cross-linking partners to form well-defined spherical nanoparticles (33). B, The nanoparticle-GIRLRG conjugate was synthesized through thiol-ene chemistry via the free allyl moieties on the nanoparticle (11) and the free cysteine at the N-terminus of the targeting GIRLRG peptide (shown in orange). Paclitaxel (shown as yellow spheres) was incorporated into the cavities of the particles' three-dimensional nano-network by a Vitamin E TPGS formulation technique (12) to lead to the nanoparticle DDS. C, Illustration of an opened fully synthesized nanoparticle DDS, showing the linear polymers (blue), cross-linking (red), paclitaxel (yellow spheres), and GIRLRG targeting peptide (orange).
GIRLRG-targeted nanoparticle DDS increases paclitaxel concentration and apoptosis in irradiated tumors
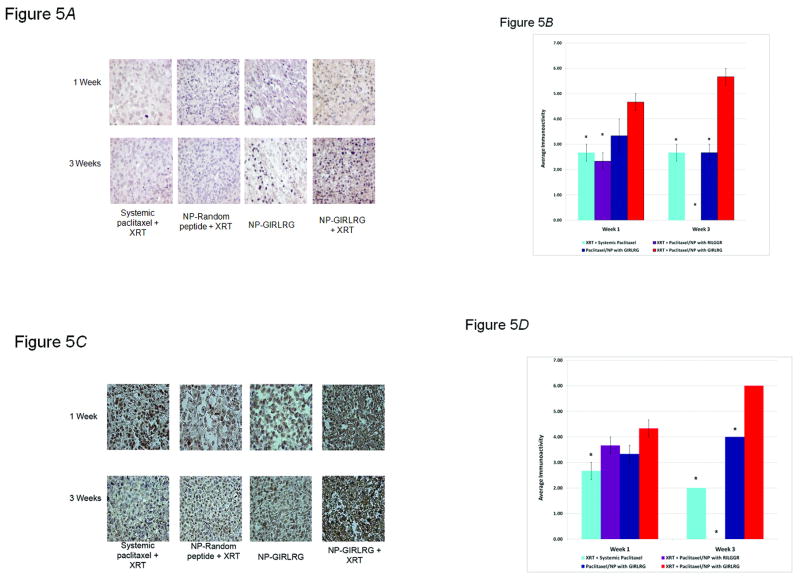
We next investigated the effects of the GIRLRG-targeted nanoparticle DDS on paclitaxel concentration and apoptosis in tumors as compared to controls. MDA-MB-231 breast carcinomas were implanted in the hind limbs of nude mice and treated as described in Fig. 5. Tumors were harvested at one and three weeks post-treatment and the levels of paclitaxel (Fig. 5A and B) and apoptosis (Fig. 5C and D) were determined with the respective cell staining assays and quantified. Paclitaxel was found in significantly greater concentrations in the targeted-nanoparticle group with the use of irradiation over all other treatment groups at one and three weeks (P<0.05) (Fig. 5A and B). Similarly, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of these tumor sections showed that at one and three weeks, the nanoparticle-GIRLRG DDS was superior to radiation and systemic paclitaxel in maintaining persistent cytotoxicity (P<0.05) (Fig. 5C and D). In fact, staining for paclitaxel and apoptosis significantly persisted for three weeks after just a single administration of the nanoparticle over the other control groups (P<0.05), indicating that the nanoparticle-GIRLRG peptide complex provides a prolonged and sustained release of paclitaxel when properly targeted to the tumor with ionizing radiation.
Figure 5.
Nanoparticle DDS increases paclitaxel concentration and apoptosis in irradiated tumors. A-B, Analysis of paclitaxel levels shows that GIRLRG-targeted nanoparticle DDS increases paclitaxel concentration in irradiated breast cancers. MDA-MB-231 breast carcinomas were implanted in the hind limbs of nude mice and treated with 3 Gy of XRT daily for three days, and systemic paclitaxel, targeted nanoparticle (NP) DDS or random peptide DDS on the second day once they reached 450 mm3 (three mice per treatment group). C-D, TUNEL staining was used to assess relative levels of apoptosis between treatment groups in tumor sections removed at one week and three weeks post-treatment. Otherwise mice were treated in the same fashion as in Panel A. In B, D a * indicates P<0.05 when compared to the XRT + Paclitaxel/NP with GIRLRG group.
Treatment with targeted nanoparticle DDS produces in vivo tumor growth delay
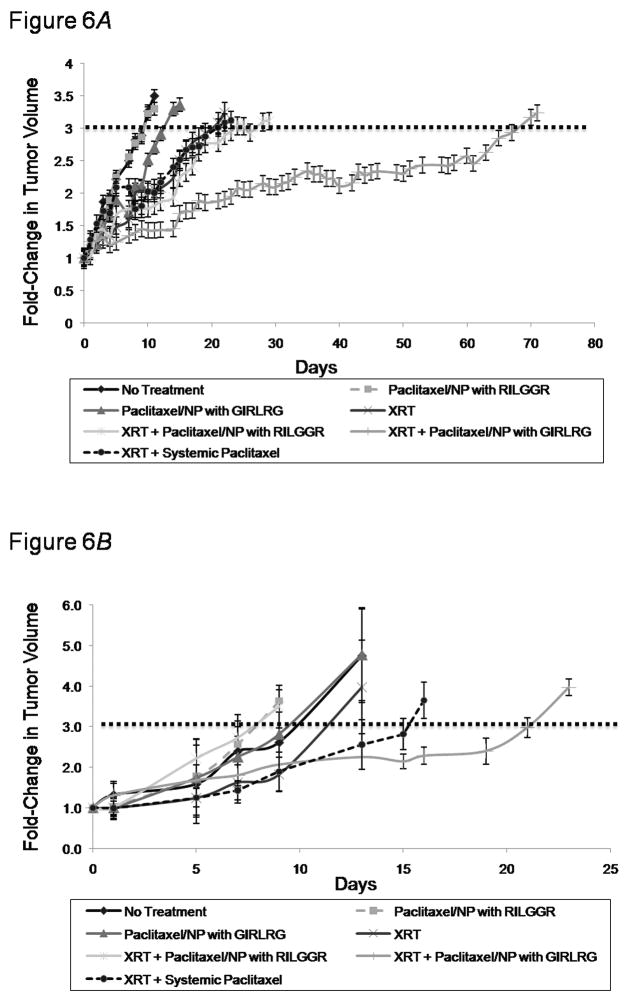
Our primary outcome to determine the overall efficacy of our novel targeting nanoparticle DDS was to assess tumor volume tripling time in human tumor cell lines and in syngeneic mouse tumors. Therefore, we implanted MDA-MB-231 breast carcinomas in nude mice and GL261 gliomas in C57/B16 mice and performed a tumor growth delay study after treating the mice as shown in Fig. 6. Our results showed that MDA-MB-231 tumor tripling time was delayed 55 days with the nanoparticle-targeted peptide with XRT (P=0.0001), compared to 11-14 days by the three other XRT-treatment groups (P<0.05) (Fig. 6A). Both unirradiated nanoparticle groups provided no significant tumor growth delay when compared to the untreated control, suggesting that even the nanoparticle-GIRLRG is not adequately targeted in unirradiated tumors. The administration of radiation with systemic paclitaxel or with untargeted nanoparticle (nanoparticle-RILGGR) provided no significant tumor growth delay when compared to radiation alone (Fig. 6A). Similarly, in the GL261 group, tumor tripling time was significantly delayed by 12 days by nanoparticle-targeted peptide with XRT-treatment (P<0.005); however, all other treatment groups failed to significantly delay tumor tripling time compared to untreated controls (Fig. 6B).
Figure 6.
GIRLRG-targeted nanoparticle DDS causes tumor growth delay in vivo. A, MDA-MB-231 or B, GL261 tumors were implanted in the hind limbs of nude mice or C57/Bl6 respectively. Once tumors reached 300 mm3 in volume, mice were treated with 3 Gy of XRT daily for three days, or were left as untreated controls. On the second day mice were injected with either systemic paclitaxel, nanoparticle-RILGGR scrambled peptide, or nanoparticle-GIRLRG targeted peptide at a concentration of 10 mg/kg (five mice per treatment group) (A) or 20 mg/kg (three mice per treatment group) (B). Tumor volumes were monitored throughout using calipers.
Discussion
We began designing our targeted DDS by seeking peptides capable of recognizing irradiated cancer cells. Using a previously characterized in vivo biopanning method (6, 7, 13, 14), we discovered several candidate peptides (Fig. 1A). One of the candidates, GIRLRG, proved to be specific to irradiated cancer cells capable of interacting with tumor vascular endothelial cells in vitro (Fig. 1B) and tumors ex vivo and in vivo (Fig. 1 C and D). In order for the GIRLRG peptide to be of clinical utility for targeted therapy, its receptor must not be a ubiquitously expressed surface protein. Ionizing radiation (XRT) provides an intense cellular stress, causing activation of DNA damage repair cascades and endoplasmic reticulum stress pathways (15). Therefore, we hypothesized that the receptor for GIRLRG could potentially be involved in one of these stress pathways and be induced by XRT.
We sought to find this receptor using GL261 tumor membrane protein affinity purification with GIRLRG. The receptor identified for GIRLRG is the 78-kDa glucose-regulated protein (GRP78) (Fig. 2A). GRP78 is known as an endoplasmic reticulum chaperone involved in suppression of stress-induced apoptosis (16), but can exist as a cell surface protein to transduce extracellular stimuli to intracellular signals to promote tumorigenesis (17-19). Signaling via cell surface GRP78 increases cytosolic calcium concentration, Akt phosphorylation, IP3, and NF-κB, leading to an increase in DNA and protein synthesis as well as cellular proliferation (19). A breadth of research supports the correlation of GRP78 to higher pathological grade, metastasis, chemotherapeutic response, cancer prognosis, and patient survival in gliomas and breast carcinomas (16, 18, 20-23). Importantly for clinical translation, GRP78 is expressed at much higher levels in a variety of tumors and tumor vasculature compared to much lower in normal tissues and non-tumor bearing vasculature where expression potentially increases during tissue inflammation (17, 18, 20, 21, 23-25). This “natural” gradient of GRP78 expression between tumor vasculature and non-tumor bearing vasculature is consistent with our in vitro data of GIRLRG selectively binding only to irradiated HUVECs incubated with cancer cells, not to HUVECs alone (Fig. 1B).
The discovery that GRP78 is upregulated at the cell surface in XRT-treated tumors and tumor vasculature (Fig. 2) may be a further indicator of its role in the cellular stress response, and in a cancer cell's ability to escape stressors that would lead a normal cell to apoptosis (16, 20-23). The mechanism by which GRP78 translocates to the cell surface is not fully understood, but hypotheses include particular mechanisms adapted by cancer cells, oversaturation of the ER retention system, transmembrane cycling of ER GRP78 to the cell surface, and cotrafficking with cell surface client proteins (21). Since GRP78 is expressed at the cell surface of tumors but not normal organs, cell surface GRP78 has become an attractive strategy for targeted therapy (26). Ligand peptides for GRP78 are rapidly internalized via clathrin-mediated endocytosis (27). Previously, GRP78 targeting peptides linked with paclitaxel (28, 29), doxorubicin (29), or proapoptotic peptides (27), have been shown to induce melanoma cell death in vitro. Once weekly systemic administration over four weeks of proapoptotic chimeric peptides fused to GRP78 binding motifs suppressed tumor growth in xenograft models without affecting normal organs (30). Nonetheless, these observations have not been approved for clinical use.
Having discovered a receptor induced by ionizing radiation, we sought to utilize nanoparticle technology to deliver a paclitaxel drug payload using GIRLRG as a targeting molecule for GRP78. We postulated that conjugating the GIRLRG recombinant peptide with a nanoparticle DDS capable of controlled pharmacokinetics could target chemotherapeutics specifically to radiated tumors. In addition, the control over particle sizes has been recognized to be crucial to predict the interaction with cells and other biological barriers (31) and reduce the risk of undesired clearance from the body via the liver or spleen (32). Therefore, the nanoparticle we used applies an intermolecular cross-linking technique (33) that not only allows for predetermined nanoparticle dimensions with standard deviations of 10%, but also provides adjustable cross-linking densities to control the degradation of the particles and allows for post-modification reactions with bioactive groups such as the targeting peptide. The adjustable cross-linking densities of the nanoparticle-targeting peptide complex can be applied towards the controlled and sustained release of paclitaxel. Consistent with the nanoparticle's biodegradation profile (12, 33), paclitaxel was found in significantly greater concentrations in our MDA-MB-231 breast cancer xenografted mice in the targeted-nanoparticle group with the use of XRT over all other treatment groups at one and three weeks (P<0.05) (Fig. 5A and B). Similarly, TUNEL staining for apoptosis at both one and three weeks were greatly increased indicating that the nanoparticle-GIRLRG DDS was superior to radiation and systemic paclitaxel in maintaining persistent cytotoxicity (P<0.05) (Fig. 5C and D). Our data supports the model that the GIRLRG peptide is able to achieve significant targeting of paclitaxel to the tumor (Fig. 5A and B) when there is high expression of GRP78 at the surface, which can be induced in tumors with XRT (Fig. 2).
Our primary outcome to determine the overall efficacy of our novel targeting nanoparticle DDS was to assess tumor volume tripling time in both human tumor cell lines and in syngeneic mouse tumors. Our results showed that MDA-MB-231 tumor tripling time was delayed 55 days with the nanoparticle-targeted peptide with XRT (P=0.0001), compared to 11-14 days by the three other XRT-treatment groups (P<0.05) (Fig. 6A). Both unirradiated nanoparticle groups provided no significant tumor growth delay when compared to the untreated control, suggesting that even the nanoparticle-GIRLRG complex itself is not adequately targeted in unirradiated tumors. The administration of radiation with systemic paclitaxel or with untargeted nanoparticle (nanoparticle-RILGGR) provided no significant tumor growth delay when compared to radiation alone (Fig. 6A), suggesting that the nanoparticle itself (nanoparticle-RILGGR) does not target irradiated tumors. Similarly, in the GL261 group, tumor tripling time was significantly delayed by 12 days by nanoparticle-targeted peptide with XRT-treatment (P<0.005); however, all other treatment groups failed to significantly delay tumor tripling time compared to untreated controls (Fig. 6B). Thus a single administration of the targeted nanoparticle DDS achieved tumor growth delay in irradiated tumors that was significantly greater than conventional systemic chemotherapy and radiation.
In conclusion, our results indicate that administration of ionizing radiation to tumors and tumor vasculature causes migration of GRP78 to the cell surface where the nanoparticle-GIRLRG DDS specifically delivers paclitaxel to the radiated site. By combining the controllable, sustained drug release of the nanoparticle with the newly identified GIRLRG targeting peptide, we were able to specifically target chemotherapeutics directly to an ionizing radiation-inducible receptor causing significant tumor cell death. The receptor identified for the peptide, GRP78, is an ideal target for our nanoparticle-peptide DDS because it is inducible by XRT (Fig. 2). Even after a single administration of the nanoparticle-GIRLRG complex, paclitaxel can be detected in radiated tumors after three weeks, which translates into significantly increased levels of apoptosis and tumor growth delay (Figs. 5 and 6). Thus, we have utilized novel nanotechnology in vivo to produce a significant increase in the efficacy of cancer treatment over current clinical models. We expect our targeted nanoparticle DDS to have clinical utility, and with further investigation hope to implement our system into clinical trials.
Supplementary Material
Acknowledgments
We thank Samuel Spratt for figure illustrations; Erkki Ruoslahti (Burnham Institute) for the gift of T7 phage-based random peptide library; Vanderbilt University Medical Center's Cell Imaging Shared Resource center, the Vanderbilt Academic Fund Venture Capital for Proteomics, and the Vanderbilt Mass Spectrometry Core for experimental support; and Jessica Huamani and Allie Fu for technical support. Roberto Diaz is a recipient of the Leonard B. Holman Research Pathway fellowship.
Grant Support: This work was supported by Department of Defense Breast Cancer Research Program Grant BC061828 (R. Diaz); National Science Foundation Career Award CHE-0645737 (E. Harth.); startup funds from Vanderbilt University (E. Harth) and Emory University (R. Diaz); Resident Research Seed Grant from the American Society for Radiation Oncology (R. Diaz); National Institutes of Health Grant R01-CA112385 (D. Hallahan); Vanderbilt In Vivo Cellular and Molecular Imaging Center Grant P50CA128323 (D. Hallahan); StarBRITE microgrant from Vanderbilt University (R. Passarella, D. Spratt, and R. Diaz).
References
- 1.Varmus H. The new era in cancer research. Science. 2006;312(5777):1162–5. doi: 10.1126/science.1126758. [DOI] [PubMed] [Google Scholar]
- 2.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006;312(5777):1175–8. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 3.Ebos JM, Lee CR, Kerbel RS. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin Cancer Res. 2009;15(16):5020–5. doi: 10.1158/1078-0432.CCR-09-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallahan DE. Radiation-Mediated Gene Expression in the Pathogenesis of the Clinical Radiation Response. Seminars in Radiation Oncology. 1996;6(4):250–67. doi: 10.1053/SRAO00600250. [DOI] [PubMed] [Google Scholar]
- 5.Hallahan D, Geng L, Qu SM, et al. Integrin-mediated targeting of drug delivery to irradiated tumor blood vessels. Cancer Cell. 2003;3(1):63–74. doi: 10.1016/s1535-6108(02)00238-6. [DOI] [PubMed] [Google Scholar]
- 6.Han Z, Fu A, Wang H, et al. Noninvasive assessment of cancer response to therapy. Nat Med. 2008;14(3):343–9. doi: 10.1038/nm1691. [DOI] [PubMed] [Google Scholar]
- 7.Passarella RJ, Zhou L, Phillips JG, Wu H, Hallahan DE, Diaz R. Recombinant peptides as biomarkers for tumor response to molecular targeted therapy. Clin Cancer Res. 2009;15(20):6421–9. doi: 10.1158/1078-0432.CCR-09-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 9.Henderson IC, Bhatia V. Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert Rev Anticancer Ther. 2007;7(7):919–43. doi: 10.1586/14737140.7.7.919. [DOI] [PubMed] [Google Scholar]
- 10.Morris PG, Fornier MN. Novel anti-tubulin cytotoxic agents for breast cancer. Expert Rev Anticancer Ther. 2009;9(2):175–85. doi: 10.1586/14737140.9.2.175. [DOI] [PubMed] [Google Scholar]
- 11.van der Ende A, Croce T, Hamilton S, Sathiyakumar V, Harth E. Tailored polyester nanoparticles: post-modification with dendritic transporter and targeting units via reductive amination and thiol-ene chemistry. Soft Matter. 2009;5(7):1417–25. [Google Scholar]
- 12.van der Ende AE, Sathiyakumar V, Diaz R, Hallahan DE, Harth E. Linear release nanoparticle devices for advanced targeted cancer therapies with increased efficacy. Polymer Chemistry. 2010;1(1):93–6. [Google Scholar]
- 13.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–80. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 14.Diaz R, Passarella RJ, Hallahan DE. Determining glioma response to radiation therapy using recombinant peptides. Expert Rev Anticancer Ther. 2008;8(11):1787–96. doi: 10.1586/14737140.8.11.1787. [DOI] [PubMed] [Google Scholar]
- 15.He L, Kim SO, Kwon O, et al. ATM blocks tunicamycin-induced endoplasmic reticulum stress. FEBS Lett. 2009;583(5):903–8. doi: 10.1016/j.febslet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Lee HK, Xiang C, Cazacu S, et al. GRP78 is overexpressed in glioblastomas and regulates glioma cell growth and apoptosis. Neuro-Oncology. 2008;10(3):236–43. doi: 10.1215/15228517-2008-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arap MA, Lahdenranta J, Mintz PJ, et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6(3):275–84. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5(7):741–4. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxid Redox Signal. 2009 doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- 20.Dong D, Ni M, Li J, et al. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer Res. 2008;68(2):498–505. doi: 10.1158/0008-5472.CAN-07-2950. [DOI] [PubMed] [Google Scholar]
- 21.Lee AS. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Research. 2007;67(8):3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 22.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Research. 2006;66(16):7849–53. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 23.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67(20):9809–16. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 24.Mintz PJ, Kim J, Do KA, et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21(1):57–63. doi: 10.1038/nbt774. [DOI] [PubMed] [Google Scholar]
- 25.Quinones QJ, de Ridder GG, Pizzo SV. GRP78: a chaperone with diverse roles beyond the endoplasmic reticulum. Histol Histopathol. 2008;23(11):1409–16. doi: 10.14670/HH-23.1409. [DOI] [PubMed] [Google Scholar]
- 26.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67(8):3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Steiniger SC, Kim Y, Kaufmann GF, Felding-Habermann B, Janda KD. Mechanistic studies of a peptidic GRP78 ligand for cancer cell-specific drug delivery. Mol Pharm. 2007;4(3):435–47. doi: 10.1021/mp060122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y, Lillo AM, Steiniger SC, et al. Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45(31):9434–44. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 29.Yoneda Y, Steiniger SC, Capkova K, et al. A cell-penetrating peptidic GRP78 ligand for tumor cell-specific prodrug therapy. Bioorg Med Chem Lett. 2008;18(5):1632–6. doi: 10.1016/j.bmcl.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arap MA, Lahdenranta J, Mintz PJ, et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6(3):275–84. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Pan S, Zhang W, Du Z. Novel thermo-sensitive core-shell nanoparticles for targeted paclitaxel delivery. Nanotechnology. 2009;20(6):65104. doi: 10.1088/0957-4484/20/6/065104. [DOI] [PubMed] [Google Scholar]
- 32.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57(15):2106–29. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 33.van der Ende AE, Kravitz EJ, Harth E. Approach to formation of multifunctional polyester particles in controlled nanoscopic dimensions. Journal of the American Chemical Society. 2008;130(27):8706–13. doi: 10.1021/ja711417h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.