Abstract
A combination of Cu L-edge and S K-edge X-ray absorption data and density functional theory (DFT) calculations have been correlated with 33S EPR superhyperfine results to obtain the dipole integral (Is) for the S 1s→3p transition for the dithiolene ligand MNT (maleonitriledithiolate) in (TBA)2[Cu(MNT)2]. The results have been combined with the Is of sulfide derived from XPS studies to experimentally obtain a relation between the S 1s→4p transition energy (which reflects the charge on the S atom ()) and the dipole integral over a large range of . The results show that for high charges on S, Is can vary from the previously reported Is calculated using data over a limited range of . A combination of S K-edge and Cu K and L-edge X-ray absorption data and DFT calculations have been used to investigate the one-electron oxidation of [Cu(MNT)2]2- and [Ni(MNT)2]2-. The conversion of [Cu(MNT)2]2- to [Cu(MNT)2]- results in a large change in the charge on the Cu atom in the molecule () and is consistent with a metal-based oxidation. This is accompanied by extensive charge donation from the ligands to compensate the high charge on the Cu in [Cu(MNT)2]- based on the increased S K-edge and decreased Cu L-edge intensity, respectively. In contrast, the oxidation of [Ni(MNT)2]2- to [Ni(MNT)2]- results in a small change in indicating a ligand-based oxidation consistent with oxidation of a molecular orbital, (singly occupied molecular orbital), with predominant ligand character.
Keywords: XAS, S K-edge, Cu, Ni, Dithiolene, Dipole Integral, Covalency
1 Introduction
Ligand K-edge X-ray absorption spectra of transition metal complexes often display intense pre-edge features.1,2 In the case of S K-edge X-ray absorption spectra, these features occur due to electric dipole allowed transitions from the S 1s to the formally filled S 3p orbital which mix into the metal d-orbitals due to bonding. Since the transitions under consideration are localized on the S atom, the pre-edge intensity reflects the amount of S character in these partially occupied or unoccupied metal d orbitals and thus the metal-S covalency.3,4 This methodology has been applied to various systems with metal-S bonds and the S characters in the ground state wave functions have been successfully determined making S K-edge XAS a powerful spectroscopic technique.5-7
The ground state wave function () (i.e the β-spin lowest unoccupied molecular orbital in a spin-unrestricted description) of a metal complex with a S containing ligand with one hole in the 3d orbital (for example, any d9 Cu(II)-S(R) complex) can be written as:
Here β2 corresponds to the amount of S character, α2 the amount of the remaining part of the S containing ligand and [1-β2- α2] the amount of metal 3d character in the . The observed pre-edge transition intensity () is then the intensity of the pure electric dipole-allowed S 1s→3p transition (I[S(1s → 3p)]) weighted by β2.
The value of β2 (percent S(3p) character) can be quantitatively determined from two parameters; the total integrated area under the S-K pre-edge peaks and the dipole integral (Is) (gives the intensity of an electric dipole allowed transition) for the 1s→3p transition for the S species under consideration. The Is has been quantitatively determined for sulfide (S2-) using a valence bond configuration interaction analysis of 2p photoelectron spectra of KFeS2.8 Also, the dipole integral of thiolate (RS-) in plastocyanin has been determined using S K-edge XAS data of a copper thiolate model complex, [Cu(tetb)(o-SC6H4CO2)](H2O).9 Since Is increases with an increase in the charge on sulfur, and increased charge on sulfur leads to an increase in the 1s→4p transition energy (see section 4.1 fordetails), a linear correlation between the Is and S 1s→4p transition energies is expected. Using this relation and the above calibrations as references, the dipole integral term for S in different ligand environments has been determined. However, since both sulfide and thiolate have relatively high negative charges on S, this method needs to be experimentally extended to include S ligands which have high positive charge as part of the calibration of Is. In this study, our S K-edge methodology is extended to include maleonitriledithiolate (MNT), a cyano substituted dithiolene ligand system. [Cu(MNT)2]2- has been extensively studied by EPR and ENDOR methods and the S character in the ground state wavefunction has been determined from 33S EPR superhyperfine data analysis.10-14 These data are used to calibrate the pre-edge intensity obtained for [Cu(MNT)2]2- and to determine the Is for the dithiolene ligand.
Dithiolene ligand systems have been extensively studied over the last ~40 years due to their non-innocent bonding with transition metals.15-20 In particular, the role of the dithiolene ligand in stabilizing the one- and two-electron oxidized forms of various [Ni(dithiolene)2]2- systems has been under considerable debate.21-24 In a recent study, we experimentally found that the ground states of [Ni(S2C2Me2)2]0,1-,2- reflect the strong covalent overlap between Ni and (S2C2Me2)2-. We also showed that there is a strong electrostatic repulsion between the two S atoms in individual (S2C2Me2)2- ligands (which are situated close to each other due to a rigid C=C framework) which leads to inverted bonding with the Ni d-orbitals lower in energy than the dithiolene (highest occupied molecular orbital). These two factors lead to primarily ligand-based (mostly S) one- and two-electron oxidations of [Ni(S2C2Me2)2]2-.25 In the present study, the electronic structure change upon oxidation of [Cu(MNT)2]2- to [Cu(MNT)2]- is investigated using Cu K- and L-edges in addition to S K-edge X-ray absorption spectroscopy see Scheme 1. The data are combined with DFT calculations to gain insight into the role of the ligand in stabilizing the hole created upon oxidation. Additionally, the experimentally derived Is for MNT is used to quantify the covalencies for [Ni(MNT)2]n- systems and to re-evaluate the covalencies for the [Ni(S2C2Me2)2]n- systems. These studies show that while the formal oxidation states of [Cu(MNT)2]- and [Ni(MNT)2]- are Cu(III) and Ni(III), respectively, (shown in Scheme 1) the ligand plays a non-innocent role in bonding and the one-electron oxidation of [Cu(MNT)2]2- and [Ni(MNT)2]2- lead to metal and ligand-based oxidation, respectively. The factors contributing to this difference in redox behavior are discussed and the specific factors involed in a non-innocent ligand system which would lead to ligand-centered vs metal-based oxidation are considered.
Scheme 1.
Ball and stick representation of [Cu(MNT)2]2- (1), [Cu(MNT)2]- (1ox), [Ni(MNT)2]2- (2) and [Ni(MNT)2]- (2ox). The formal oxidation state on the metal in 1ox and 2ox is Cu(III) and Ni(III), respectively.
2. Experimetal Section
2.1 Sample preparation
(TBA)2[Cu(MNT)2] (1), (TBA)[Cu(MNT)2] (1ox) (NEt4)2[Ni(MNT)2] (2) and (NEt4)[Ni(MNT)2] (2ox) [MNT= Maleonitriledithiolate, TBA= tetra n-butyl ammonium, Et= ethyl] were synthesized as previously described.26,27 All complexes are stable at room temperature and were handled on the bench top during sample preparation. For Cu K-edge XAS measurements, the solid samples were finely ground with BN into a homogeneous mixture and pressed into a 1 mm Al spacer between 37 μm Kapton tape. The samples were frozen and stored under liquid N2. During data collection, the samples were maintained at a constant temperature of 10 K using an Oxford Instruments CF 1208 liquid helium cryostat. The Cu L-edge samples were similarly handled and spread thinly over double-sided adhesive conducting graphite tape on an Al sample paddle. The paddles were affixed to a rotary stage and transferred into a vacuum chamber. The samples were positioned at 45° to the incident beam. The S K-edge samples were ground finely and dispersed as thinly as possible on Mylar tape to minimize the possibility of self absorption effects.
2.2 Cu K-edge
The Cu K-edge X-ray absorption spectra of 1 and 1ox were measured at the Stanford Synchrotron Radiation Laboratory (SSRL) on the unfocussed 8-pole 1.8 T wiggler beam line 7-3 under ring conditions of 3 GeV and 60-80 mA. A Si(220) double crystal monochromator was used for energy selection. The monochromator was detuned 50% at 9987 eV to reject components of higher harmonics. Transmission mode was used to measure data to k = 16 Å-1. Data represented here are a three-scan average spectrum. Achievement of internal calibration, background subtraction and data normalization were performed as described in earlier publications.28 The data were normalized using the SPLINE program in the XFIT suite of programs.29 Theoretical EXAFS signals χ(k) were calculated using FEFF (version 7.0)30,31 and fit to the data using EXAFSPAK.32 The distance obtained from the EXAFS analyses of these data (not shown) were in excellent agreement with those obtained from crystal structure parameters and confirmed the sample integrity.
2.3 Cu L-edge
Cu L-edge X-ray absorption spectra of 1 and 2 were recorded at SSRL on the 31-pole wiggler beam line 10-1 under ring operating conditions of 50-100 mA and 3 GeV using a spherical grating monochromator with 1000 lines/mm and set at 30 μm entrance and exit slits. Sample measurements were performed using total electron yield where the sample signal (I1) was measured with a Galileo 4716 channeltron electron multiplier aligned at 45° relative to the copper paddle. The mode of data collection and achievement of external calibration were performed as described in earlier publications.33 Spectra presented here are 3-5 scan averages, which were processed using the SPLINE program in the XFIT suite of programs by fitting a second-order polynomial to the pre-edge region and subtracting it from the entire spectrum as background, resulting in a flat post-edge.29 The data were normalized to an edge jump of 1.0 at 1000 eV. The normalized data were then processes in Microsoft Excel. Two arctangents were subtracted from the data which were separated by 3/2*λL.S (20.25 eV) and fixed with an L3:L2 intensity ratio of 2:1. The total integrated area was obtained between 930-950 eV. Normalization procedures introduce ~5% error in the value of the total integrated area.
2.3 S K-edge
S K-edge data of 1-2ox were measured using the SSRL 54-pole wiggler beamline 6-2 in high magnetic field mode of 10 kG with a Ni-coated harmonic rejection mirror and a fully tuned Si(111) double crystal monochromator. Details of the optimization of this beam line for low energy fluorescence measurements and the experimental setup have been described previously.34 External energy calibration and data normalization were performed as described in earlier publications.5,35 The area under the pre-edge peak was quantified by fitting the data using EDG_FIT.32 The pre-edge and rising edge features were modeled with pseudo-Voigt line-shapes with a fixed 1:1 Lorentzian:Gaussian ratio. The reported intensity and half-width values are based on an average over simultaneous fits that accurately modeled the data and their second derivative. Normalization procedures introduce ~3% error in the value of the integrated area under the pre-edge peak. The error is larger (~6%) in the case of 2ox due to the overlap of pre-edge and edge transitions.
2.4 Electronic Structure Calculations
Gradient-corrected, (GGA) spin-unrestricted, broken symmetry, density functional calculations were carried out using the Gaussian0336 package on a 2-cpu linux computer. Geometry optimizations were performed for each complex. The Becke8837,38 exchange and Perdew8639,40 correlation non-local functionals with Vosko-Wilk-Nusair41 local functionals as implemented in the software package (BP86) were employed in this study to compare the electronic structure differences in 1, 1ox, 2, and 2ox. The triple-ζ 6-311G*42-44 and the double-ζ 6-31G*45-47 basis sets were used on the Cu, Ni and S atoms and the C, H and N atoms, respectively. Population analyses were performed by means of Weinhold's Natural Population Analysis (NPA)48-50 including the Cu 4p orbitals in the valence set. Wave functions were visualized and orbital contour plots were generated in Molden.51 Compositions of molecular orbitals and overlap populations between molecular fragments were calculated using the PyMOlize program.52
2.5 TD-DFT Calculations
TD-DFT calculations were performed with the electronic structure program ORCA.53,54 Single point ground state DFT calculations with the BP86 functional were performed using the geometry optimized coordinates obtained from Gaussian03. The triple-ζ 6-311G* basis set was used for all atoms. The symmetry equivalent S 1s orbitals were localized, and only excitations from the localized S 1s orbitals to the lowest unoccupied orbitals were allowed. Oscillator strengths for the individual S-K pre-edge transitions were obtained.
3. Results
3.1 [Cu(MNT)2]2-/-
3.1.1 Cu K-edge
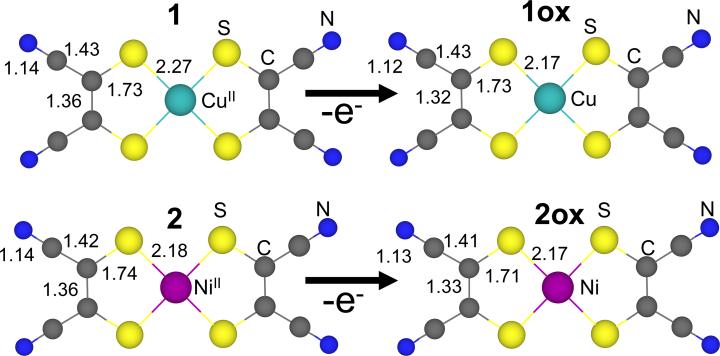
A comparison of the normalized Cu K-edge X-ray absorption spectra of 1 (black) and 1ox (red) is shown in Figure 1. The inset in Figure 1 shows the second derivative spectra of the pre-edge region. The pre-edge feature observed at ~8980 eV is a low-intensity (dipole-forbidden) quadrupole-allowed 1s→3d transition, which gains intensity from 4p mixing into the d-manifold due to deviation from centrosymmetry.55 This pre-edge feature occurs at 8979.3 eV in 1 while it is shifted to higher energy by ~1.4 eV and appears at 8980.7 eV in 1ox. The intense rising edge feature observed at ~8985 eV is a result of a formally two electron 1s→4p+ligand-to-metal charge transfer (LMCT) shakedown transition which becomes allowed due to final-state relaxation.28,56 This feature appears at 8985.0 eV in 1 while it is shifted up in energy by 1.6 eV in 1ox and occurs at 8986.6 eV. In addition to the shift in energy, the intensity ratio between the intense shakedown transition to the rising edge main transition increases on going from 1 to 1ox, (Figure S1, Supporting Information).
Figure 1.
The normalized Cu K-edge XAS spectra of (TBA)2[Cu(MNT)2] 1 ( ) and (TBA)[Cu(MNT)2] 1ox (
) and (TBA)[Cu(MNT)2] 1ox ( ). Inset shows the second derivative of the pre-edge region (1s → 3d transition, ~8978-8982 eV) indicating a ~1.4 eV shift.
). Inset shows the second derivative of the pre-edge region (1s → 3d transition, ~8978-8982 eV) indicating a ~1.4 eV shift.
3.1.2 Cu L-edge
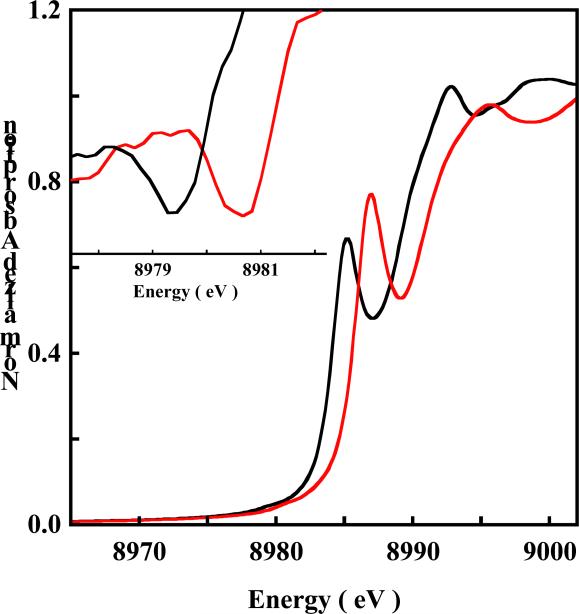
Figure 2 shows a comparison of the normalized Cu L-edge X-ray absorption spectra of 1 (black) and 1ox (red). The spectrum of the well characterized D4h [CuCl4]2- complex (blue) has been included as a reference.57 The L-edge spectrum reflects the metal 2p→3d dipole allowed transition and consists of two sharp spin-orbit split peaks separated by ~20 eV with an intensity ratio of ~2:1. The L2 edge (~950 eV) is broadened due to an additional Auger decay channel of the excited state which is absent for the L3 edge (~930 eV).58 The L3 edge occurs at 931.7 eV in 1 and is shifted up by ~1.6 eV to 933.3 eV in 1ox.
Figure 2.
The normalized Cu L-edge XAS spectra of (TBA)2[Cu(MNT)2] 1 ( ) and (TBA)[Cu(MNT)2] 1ox (
) and (TBA)[Cu(MNT)2] 1ox ( ) and D4h [CuCl4]2- (
) and D4h [CuCl4]2- ( ). The intense peaks at ~ 930 eV and ~ 950 eV represent the L3 edge (2p3/2 → 3d transition) and L2 edge (2p1/2 → 3d transition), respectively.
). The intense peaks at ~ 930 eV and ~ 950 eV represent the L3 edge (2p3/2 → 3d transition) and L2 edge (2p1/2 → 3d transition), respectively.
The L-edge intensity reflects the extent of metal-ligand covalent mixing. Due to the localized nature of the transition, as the unoccupied metal 3d orbitals mix with filled ligand orbitals, the metal character decreases and the intensity of the L-edge transition decreases. A correlation of the total area under the L-edge of 1 and 1ox with the total area of D4h [CuCl4]2- (well studied by various spectroscopic methods with 61 ± 4% Cu character in the ) gives a quantitative estimate of the amount of Cu character in the ground state of 1 and 1ox.57,59,60 The total integrated area under the L-edges of 1 and 1ox are 7.84 and 11.71 (Table 1), respectively which quantitate to a total Cu character of 39% in 1 and 56% in 1ox. Since 1ox is a two-hole system, (in contrast to D4h [CuCl4]2- and 1 which are one-hole systems) the per-hole Cu character is 28%. In both cases the Cu character is lower than that of D4h [CuCl4]2-, indicating that both 1 and 1ox have significant dithiolene character in their ground state wavefunctions.
Table 1.
Cu K- and L-edge X-ray Absorption Edge Energy Positions (eV) and Cu Character in the .
| Cu K-edgea |
Cu L-edgeb |
||||
|---|---|---|---|---|---|
| 1s→4p | 2p→3d |
c |
|||
| Complex | 1s→3d | ||||
| |
|
+ shakedown |
L3 edge |
L2 edge |
|
| 1 | 8979.3 | 8985.0 | 931.7 | 951.7 | 39% |
| 1ox | 8980.7 | 8986.6 | 933.3 | 953.3 | 28% |
Energy resolution ~1 eV.
Energy resolution ~0.1 eV.
Error in total intensity due to data processing and fitting is ± 3%.
3.1.3 S K-edge
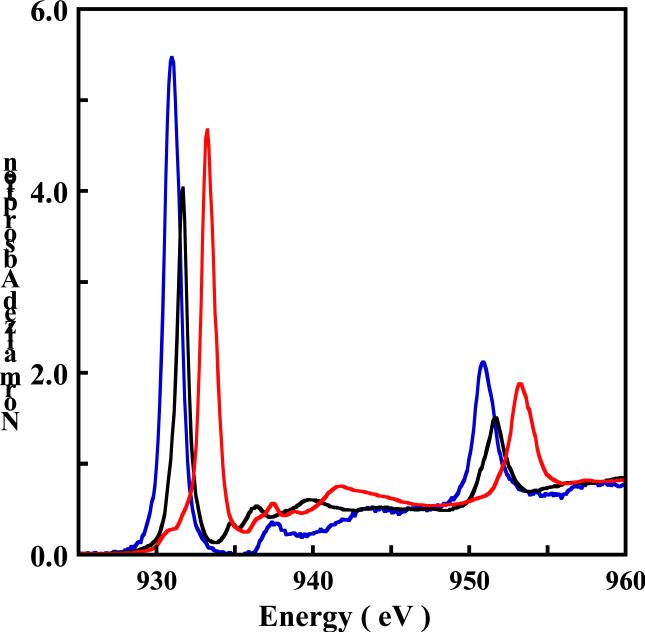
The normalized S K-edge X-ray absorption spectra of 1 (black) and 1ox (red) are shown in Figure 3. A comparison of these spectra with that of the free dithiolene ligand (Na)2[S2C4N2], is shown in Figure S2 (Supporting Information). The spectra of all three compounds consist of multiple transitions between 2469 eV and 2474 eV. However the lowest energy feature in 1 and 1ox is absent in the free ligand and corresponds to a transition from the S 1s to the . This transition occurs at 2470.4 eV for 1, while it is 0.3 eV lower in energy in 1ox and occurs at 2470.1 eV. In contrast, the two edge transitions that occur at 2471.5 eV and 2472.8 eV for 1 are shifted to ~0.5 eV higher energy in 1ox and occur at 2472.1 eV and 2473.2 eV.
Figure 3.
The normalized S K-edge XAS spectra of (TBA)2[Cu(MNT)2] 1 ( ) and (TBA)[Cu(MNT)2] 1ox (
) and (TBA)[Cu(MNT)2] 1ox ( ). The intense peaks between 2471-2474 eV represent the low lying edge transitions (section 3.2).
). The intense peaks between 2471-2474 eV represent the low lying edge transitions (section 3.2).
Due to the localized nature of the S 1s orbital and the fact that an s→p transition is electric-dipole allowed, the transition will gain intensity if the ground state wavefunction has a significant contribution from the S 3p orbital. Thus, the area under the pre-edge peak ( transition) provides a measure of the S(3p) β2 character (see equation 1) in the . The total integrated areas under the S-K pre-edge peaks of 1 and 1ox are 0.63 and 1.41, respectively (Table 2). Since 1ox is a two-hole system, the renormalized per-hole pre-edge intensity is 0.70. The higher per-hole S character in the of 1ox (0.7) compared to 1 (0.63) is consistent with the lower per-hole Cu character in the of 1ox compared to 1 obtained from the Cu L-edge analyses (see section 3.1.2).
Table 2.
S K-X-ray Absorption Edge Energy Positions and Pre-edge Intensities
| Complex | S-K Pre-edgea Energy (eV) | Rising-edge Energy (eV)b | Total Pre-edge Intensitiesc |
|---|---|---|---|
| (TBA)2[Cu(MNT)2] | 2470.4 | 2471.5 | 0.63 |
| (TBA)[Cu(MNT)2] | 2470.1 | 2472.1 | 1.41 |
| (NEt4)2[Ni(MNT)2] | 2471.2 | 2471.8 | 0.99 |
| (NEt4)[Ni(MNT)2] | 2470.2d | 2472.1 | 0.83d |
| 2471.0e | 1.10e |
Energy resolution ~0.2 eV.
Lowest energy edge transition.
Error in total intensity due to data processing and fitting is ± 3%.
The lower and higher energy pre-edge peaks, respectively.
The lower and higher energy pre-edge peaks, respectively.
3.2 [Ni(MNT)2]2-/-
3.2.1 S K-edge
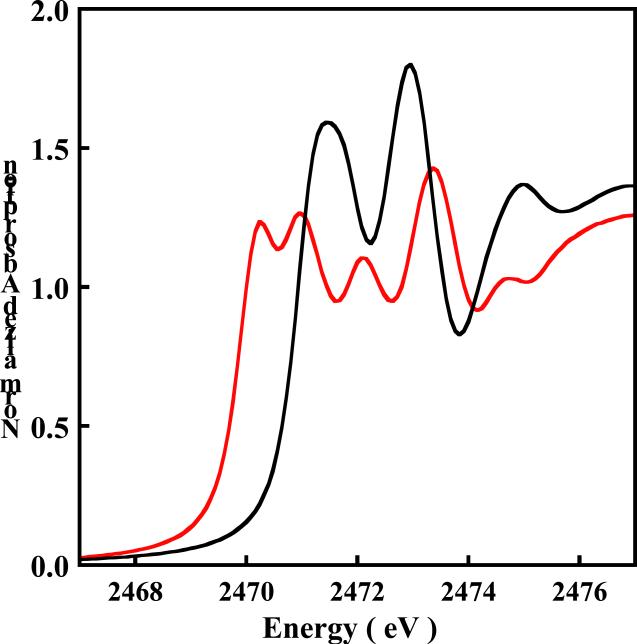
The normalized S K-edge X-ray absorption spectra of 2 (black) and 2ox (red) are shown in Figure 4. In 2 the pre-edge transition overlays with the lowest-energy edge transition which results in a single broad feature. However, the two peaks are better resolved in the second derivative (see Figure S3, Supporting Information). The energy position of the pre-edge transition was determined to be 2471.2 eV. This corresponds to a transition from the S 1s to the , which is unoccupied in 2. Upon oxidation, this peak shifts to lower energy and a second, lower energy peak is observed at ~2470 eV (Figure 4). This peak corresponds to a transition from the S 1s orbital to the of 2 which becomes half-occupied upon oxidation. The intensity of this transition reflects a large amount of S character in this now half occupied orbital (, singly occupied molecular orbital). These two pre-edge features are separated by ~0.8 eV and occur at 2470.2 eV and 2471.0 eV. Similar to the S K-edge shift observed on going from 1 to 1ox, on going from 2 to 2ox, the edge transitions shift to ~ 0.4 eV higher energy and occur at 2471.8 eV and 2472.8 eV for 2 and 2472.1 eV and 2473.3 eV for 2ox. The total integrated area under the single peak in 2 is 0.99 and the two peaks in 2ox is 1.93 (Table 2). Thus, the total integrated area increases by almost a factor of two on going from 2 to 2ox.
Figure 4.
The normalized S K-edge XAS spectra of (NEt4)2[Ni(MNT)2] 2 ( ) and (NEt4)[Ni(MNT)2] 2ox (
) and (NEt4)[Ni(MNT)2] 2ox ( ). The pre-edge feature in 2 is buried in the lowest-energy edge transition.
). The pre-edge feature in 2 is buried in the lowest-energy edge transition.
3.3 Density Functional Theory (DFT) Calculations
3.3.1 Geometry Optimization
Spin-unrestricted density functional calculations were performed on 1, 1ox, 2 and 2ox to correlate with the spectroscopic results and probe their electronic structure differences. The geometry-optimized structural parameters are in good agreement with the experimental data. The relevant bond distances are presented in Table 3. The calculated Cu-S bond distances for 1 and 1ox are 2.32 Å and 2.22 Å compared to 2.27 Å and 2.17 Å, respectively, in the X-ray crystal structure.13,61 The calculated Ni-S bond distances for 2 and 2ox are 2.19 Å and 2.17 Å compared to 2.18 Å and 2.17 Å, from crystallography.27 The Cu-S bond distances become ~0.1 Å shorter upon one-electron oxidation of [Cu(MNT)2]2- while there is a much smaller change in the Ni-S distance upon one-electron oxidation of [Ni(MNT)2]2- (~0.02 Å). The S-C, C-C and C-N distances are in reasonable agreement with the crystal structure data (see Supporting Information for geometry optimized coordinates) and undergo very small changes upon oxidation of both [Cu(MNT)2]2- and [Ni(MNT)2]2-.
Table 3.
Selected DFT Parameters.
| Complex | Cu/Ni-S Bond Distance (Å) | Composition (%)a | ||
|---|---|---|---|---|
| Cu/Ni | S | Lb | ||
| 1 | 2.32 | 37.6 | 52.0 | 10.4 |
| 1ox | 2.22 | 24.7 | 64.8 | 10.5 |
| 2 | 2.19 | 42.9 | 43.8 | 13.3 |
| 39.5 | 46.2 | 14.3 | ||
| 2ox | 2.17 | |||
| 31.2c | 50.5c | 18.2c | ||
Results from Mulliken population analysis.
L represents the C and N atoms of the MNT ligand.
compositions.
3.3.2 Energy Level Diagram
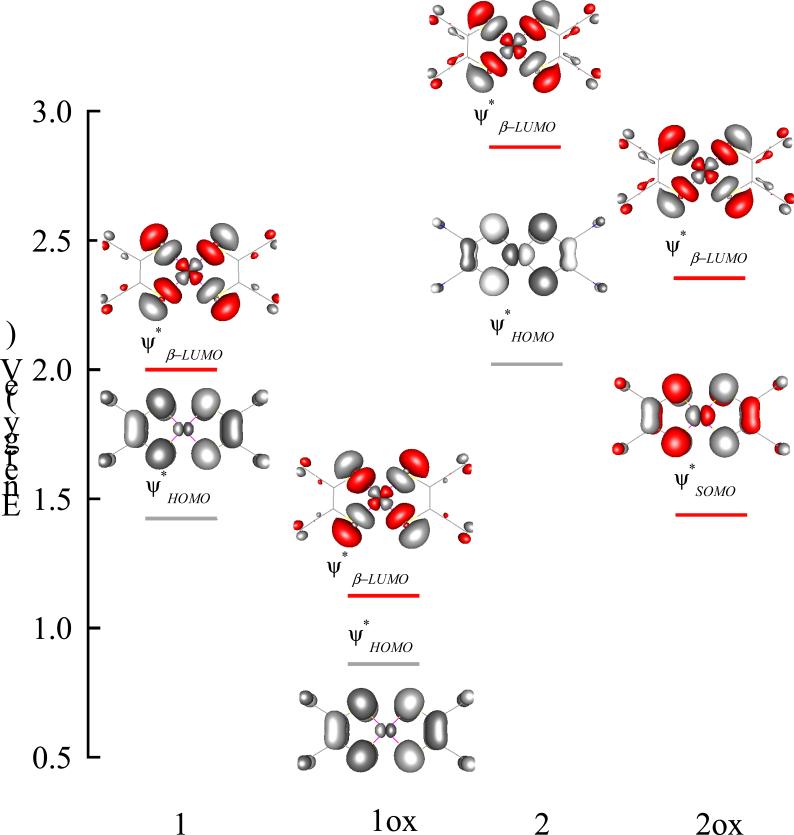
Figure 5 shows a schematic of the energy level diagram for 1, 1ox, 2 and 2ox. The empty β-orbitals, which have been probed by S-K pre-edge spectroscopy, are shown in color while the filled orbitals are in gray. In all four complexes the are qualitatively similar and exhibit an in-plane strong σ-bonding interaction between the metal 3dx2–y2 and the S 3pσ orbitals. The (highest occupied molecular orbital) in 1, 1ox and 2 and the (singly occupied molecular orbital) in 2ox are also qualitatively similar and consist of an out-of-plane π-bonding interaction of the metal 3dxz orbital and the S 3pπ orbital. To compare the relative energies of the , the energies of the N 1s orbitals were aligned. This was possible since the contribution of N atom to bonding is very similar in all the four complexes and hence undergoes similar energy shifts (see section 3.3.3). The of 1ox is 0.85 eV lower in energy compared to 1. The of 2ox is 0.5 eV lower in energy compared to 2. These shifts are in qualitative agreement with the shifts observed in the S transition (see Table 2).62 In addition, the DFT calculated energy difference between the and the of 2ox is ~0.9 eV, which is in good agreement with the energy splitting of the two S-K pre-edge features in 2ox (0.8 eV) (Figure 4 and Table 2).
Figure 5.
Orbital contour plots of the spin-unrestricted broken symmetry wave functions for 1-2ox. The relative energy positions of the orbitals have been obtained by aligning the N 1s energy in each case. Singly and doubly unoccupied orbitals which have been probed by S K-edge XAS are shown in red.
3.3.3 Ground State Wavefunction
3.3.3.1 [Cu(MNT)2]2-/-
DFT calculations using the BP86 functional give ground state descriptions of 1 and 1ox that are in reasonable agreement with experimental ground state properties. Results obtained using the B3LYP38 functional are similar with small quantitative differences in the ground state wave function compositions (Table S1, supporting information). Table 3 summarizes the compositions of selected spin-down molecular orbitals for 1 and 1ox obtained from a Mulliken population analysis. The of the S=1/2 ground state of 1 consists of 37.6% Cu 3dx2–y2 and 52.0% S 3pσ character. The remaining 10.4% is distributed over the non-S atoms of the MNT ligand. The large amount of S character in the ground state is consistent with the in-plane strong σ type interaction between the Cu 3dx2–y2 and S 3pσ orbitals. The one-electron oxidation of 1 to 1ox leads to an S=0 ground state. The resultant orbital is qualitatively similar to that in 1 and involves the same set of atomic orbitals. However, the of 1ox quantitatively differs from that of 1 in that it contains 24.7% Cu 3dx2–y2 and 64.8% S 3pσ character. The remaining 10.5% is distributed over the remaining atoms of the MNT ligand. These results are in excellent agreement with the Cu characters in the ground state wavefunctions obtained from the L-edge spectra (39% in 1 and 28% in 1ox). These results are also consistent with the increased per-hole S character in the ground state of 1ox relative to 1 as observed by an increase in the S-K pre-edge intensity in 1ox (see section 3.1.3).
3.3.3.2 [Ni(MNT)2]2-/-
The compositions of the key orbitals obtained from a Mulliken population analysis of spin unrestricted DFT calculations using the BP86 functional for 2 and 2ox are summarized in Table 3. Results obtained using the B3LYP functional are summarized in Table S1 (Supporting Information). The ground state of 2 is S=0 and contains 42.9% Ni 3dx2–y2 and 43.8% S 3pσ character. The remaining 13.3% is distributed over the remaining atoms of the MNT ligand. The one-electron oxidation of 2 creates a hole in the to generate 2ox (hole in , Figure 5) which has an S=1/2 ground state. The and the of 2ox contain 39.5% Ni 3dx2–y2 and 46.2% S 3pσ and 31.2% Ni 3dxz and 50.5% S 3pπ character, respectively. The remaining 14.3% () and 18.2% () are distributed over the remaining atoms of the MNT ligand.
3.3.4 [Ni(MNT)]2- vs [Ni(S2C2Me2)2]-
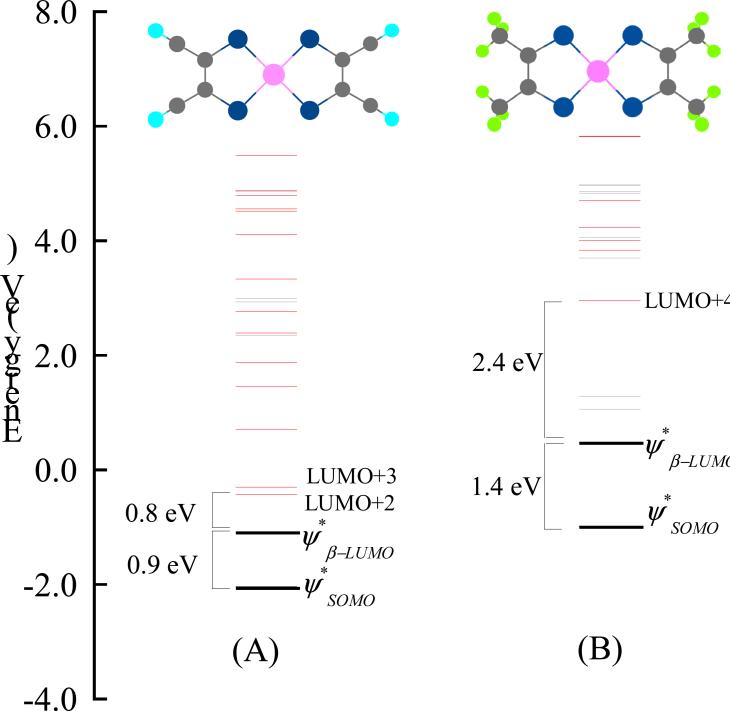
DFT calculations on both 2 and 2ox show very covalent with significant S 3p characters (44% in 2 and 51 % in 3) which are reflected by the intense S-K pre-edge transitions. In an earlier publication DFT studies were performed on [Ni(S2C2Me2)2]n- (n=0,1)25 which showed similar bond distances, ground state wavefunctions and intense S-K pre-edge transitions as in their MNT counterparts in the current study. Despite very similar electronic and geometric structures, the S K-edge spectra of [Ni(MNT)2]-,2- and [Ni(S2C2Me2)2]-,2- are different in the energy range of 2469 eV and 2474 eV (Figure S4, Supporting Information). To understand these differences, a comparison of the key unoccupied β-orbitals of 2ox and [Ni(S2C2Me2)2]- is given in Figure 6. The calculated energy splitting between the and in 2ox and [Ni(S2C2Me2)2]- is 0.9 eV and 1.4 eV, respectively, which is in reasonable agreement with the experimental energy splitting of 0.8 eV (2ox) and 1.0 eV ([Ni(S2C2Me2)2]-). Due to the electron donating nature of the methyl substituents, [S2C2Me2]- is a better donor than MNT which contains electron withdrawing cyano substituents. Thus, the in [Ni(S2C2Me2)2]- is destabilized compared to 2ox, consistent with a larger splitting between and . In [Ni(S2C2Me2)2]-, the lowest lying unoccupied orbital with significant S3p character is LUMO+4 which is ~2.4 eV higher in energy than the . In contrast, in 2ox, significant S3p character is observed in the LUMO+2 and LUMO+3 orbitals, which are ~0.8 eV higher in energy than the orbital. This is consistent with the low lying edge transitions present in the S K-edge data of 2ox (1.1 eV higher than the orbital) which correspond to S 1s→LUMO+3 and 1s→LUMO+4 transitions. Alternatively, the lowest energy edge transition in [Ni(S2C2Me2)2]- is the 1s→LUMO+4 transition which is well separated from the pre-edge features at ~2.5 eV higher energy.
Figure 6.
DFT calculated energy level diagram of 2ox (A) and (NEt4)[Ni(S2C2Me2)2] (B). The orbitals with significant S 3p character are shown in red while those without are shown in gray. 2ox and (NEt4)[Ni(S2C2Me2)2] have been optimized under D2h and C2v symmetries, respectively.
4. Analysis
4.1 Quantitation of Dipole Integral
Single crystal EPR and ESEEM studies and 33S ESR superhyperfine analyses estimated the total S character in the orbital of [Cu(MNT)]- to be 54%.10-14,63 This is in good agreement with the 39% Cu character obtained from the Cu L-edge analysis (considering that the non-S MNT ligands contribute ~11% (see section 3.3.3.1)). These data are also in agreement with the DFT calculated Mulliken populations of the (37.6% Cu(3d), 52.0% S(3p) and 10.4% remaining ligand). The S-K pre-edge intensity, weighted by the transition dipole integral (Is) for the 1s→3p transition, is a measure of the S(3p) character (β2) in the . The relation between Is and β2 is given by D0=(A/3n)β2Is, where D0 is the total area under the pre-edge transition, A is the number of holes (metal 3d or ligand np) and n is the number of absorbing S atoms. Using this relation, the S-K pre-edge intensity, and the S character from 33S EPR results, the resultant S (1s→3p) Is of [Cu(MNT)] is determined to be 14.1. The value of Is depends on the charge on the S in the molecule which modulates the S 1s and 3p radial functions. The S 3p radial function can also depend on the nature of metal-S overlap (σ vs π) (vide infra). In a previous publication, we used a combination of PES and S K-edge pre-edge data for KFeS2 to determine the Is for the S2- ligand to be 5.4.8 Using these two experimentally derived Is's (for [Cu(MNT)]2- and KFeS2), a reasonably accurate estimate of the Is of any S based ligand can be made based on a linear correlation of the 1s→4p energy (2472.3 eV for sulfide and 2476.6 eV for [Cu(MNT)]2-, see Figure S2 in Supporting Information) with Is. It has been shown using DFT calculations that an increase in positive charge on the absorbing S atom leads to a linear increase in the value of Is.64 This is due to the fact that an increase in the charge on the S atom will lead to a larger contraction of the valence 3p orbitals. This leads to better overlap of the 1s and 3p radial functions and hence increase in Is. Again, an increase in charge on the S atom shifts the core 1s orbital to deeper binding energy relative to the valence 4p orbitals and in the process increases the 1s(core)→4p(valence) energy gap. To obtain the relationship between the charge on the S atom and the 1s→4p transition energy, DFT calculations were used to evaluate the correlation between the binding energies of the S 1s and S 4p manifolds without complication from ligand environment and other molecular effects. Using partial charges in a spherically symmetric field, was modulated over a large range, and the theoretical relationship of the S 1s and S 4p binding energies was obtained and is presented in Figure S5. The correlation of and the difference in binding energy of S 1s and S 4p orbitals was found to be close to linear with only an ~3% contribution from a quadratic component. This indicates that a near linear relationship holds between Is and the S 1s→4p transition energy.
In order to assign the S 1s→4p transition energy of (Na)2[Cu(MNT)2], the S K-edge spectra of the free MNT ligand {(Na)2MNT} and (Na)2[Cu(MNT)2] were used as references (shown in Figure S2) and correlated to DFT calculations. In the case of [MNT]2- and [Cu(MNT)]2- the assignment of 1s→S-C(π)* and 1s→S-C(σ)* is complicated by the presence of low lying transitions due to S character mixed into the cyano substituent (see section 3.3.4). Thus, a comparison of the S K-edge XAS data and DFT calculations of (Na)2(S2C2H2) and (NEt)4[Ni(S2C2Me2)] has been made since the spectra of these complexes are not complicated by other low-lying S-based transitions. A detailed analysis is presented in the Supporting Information. In case of the free dithiolene ligand [(C2H2S2)2-], the 1s→S-Cπ* and the 1s→S-Cσ* are separated by only ~0.3 eV and occur under the peak observed at ~2472.8 eV, while the 4p transition occurs ~1.6 eV to higher energy at ~2474.2 eV. In the case of the Ni bound complexes, [Ni(C2S2Me2)]0,-,2-, the S-C bond becomes shorter relative to the free ligand, destabilizing the S-Cσ* orbital and raising the 1s→S-Cσ* transition energy. This is accompanied by an increase in , leading to an increase in the 1s→4p transition energy. Thus, in [Ni(S2C2Me2)]-, the two peaks at ~2472.6 eV and ~2474.2 eV spilt into three peaks at 2473.5 eV, 2474.7 eV and 2475.9 eV corresponding to the 1s→S-Cπ*, 1s→S-Cσ* and 1s→4p transitions, respectively. A similar shortening in the S-C bond is observed in the [Cu(MNT)2]2- complex relative to the free ligand and in analogy to [Ni(S2C2Me2)]-, the 1s→S-Cσ* and the 1s→4p transitions have been assigned to the transitions at 2474.4 eV and 2476.6 eV (see Supporting Information, Section S1).
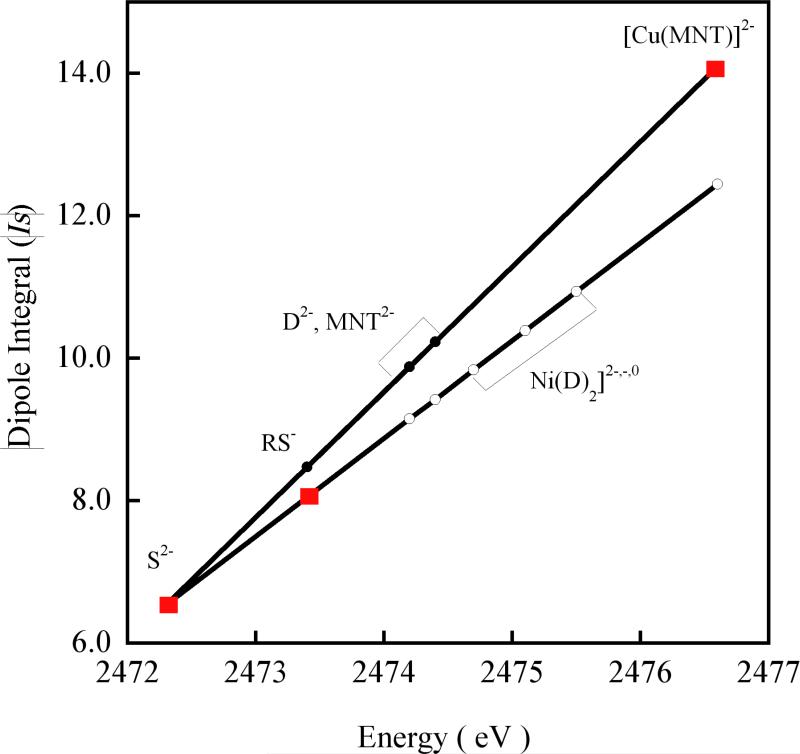
Using the two experimentally derived Is's for [Cu(MNT)]2- (1s→4p at 2472.4 eV) and KFeS2 (1s→4p at 2476.6 eV) and the linear correlation of the 1s→4p energy transition determined above, a reasonably accurate estimate of the Is of any S based ligand can be made. Using this method the Is for the free {(Na)2MNT}, (Na)2(C2H2S2) and Na(C2H5S) have been determined and plotted in Figure 7 which shows the experimentally derived Is (as red squares) and interpolated Is (black circles) based on the 1s→4p energy position of these S(ligand) systems. This is compared to our previous correlation (white circles), which was derived from the experimental data of KFeS2 and a comparison of experimental and theoretical results for plastocyanin and a related thiolate-CuII model complex.9 The spectroscopically derived correlation of Is with 1s→4p is more accurate relative to previously used correlation since sulfide and [Cu(MNT)]- cover a large range of charge on S (in contrast to sulfide and thiolate used for the previous correlation) and the Is value for both has been derived from direct spectroscopic techniques.
Figure 7.
Change in dipole integral (Is) with the S 1s→4p transition energy position. The correlation developed from the Is values for S2- and RS- (red squares) is extrapolated to compounds which have higher positive charge on S (white circles) and compared to the correlation determined from the spectroscopically derived Is values for S2- and [Cu(MNT)]2- (red squares). The Is values for RS-, D2- (D is (S2C2(Me)22-) and MNT2- obtained from interpolation of the two spectroscopically determined Is values are represented by black circles.
It should be noted that the more accurate Is deviates from the previously reported Is by only a small fraction for lower charges on S. The Is for the thiolate ligand system (RS-) changes from 8.05 to 8.47 (Figure 7). This changes the covalency of a metal-thiolate system (such as in the blue copper proteins) by only 2%. However, for high charges on S, Is is seen to deviate significantly. The Is's of dithiolene ligands (MNT2- and (S2C2Me2)2-) are slightly higher than the previously reported Is's. This in effect decreases the Ni-S covalencies for the [Ni(S2C2Me2)]2-,1-,0 complexes,25 and agrees better with the theoretical results in that study (see Table S2, Supporting Information).
Using the Is value from the spectroscopically derived plot in Figure 7, the S character in the ground state wavefunction of 1, 1ox and 2 become 54%, 60% and 42%, respectively (Table 4). These are in good agreement with the ground state wavefunctions obtained from Mulliken population analyses (52.0%, 64.8% and 41% in 1, 1ox and 2, respectively). Since DFT calculations indicate that the contribution of C and N atoms to the ground state in 1 and 1ox is ~10% (Table 3), the remaining contribution of ~36% (1) and 30% (1ox) has to be from Cu, which is in good agreement with Cu L-edge data (39% (per-hole) for 1 and 28% (per-hole) for 1ox). The S characters in the lower () and higher () energy pre-edge features of 2ox are 71% and 47%, respectively. While the S character in the orbital is in good agreement with the calculated value of 46%, the experimental Ni-S covalency in the (70%) is ~20% higher than calculated (~50.5%). TD-DFT calculations indicate that the oscillator strength for the (π orbital) is 17% lower than that for the transition (σ orbital). This may reflect a greater radial distortion in the π-orbitals than in the σ-orbitals.65 Using this ratio of oscillator strength between π- and σ-orbitals of the dithiolene, the experimentally obtained S character in the orbital of 2ox becomes 58%, which is in more reasonable agreement with the calculated value of 50.5%. This is consistent with ENDOR results which indicate that the S character in the ground state of 2ox is 52%.66 It should be noted that although this difference in oscillator strength between π- and σ-orbitals can overestimate the covalency for ligands with high (e.g dithiolene), its effect on ligands with low (e.g sulfide and thiolate) is small.
Table 4.
S Charactera in Obtained from S K-edge XAS
| 1 | 1ox | 2 | 2ox |
|---|---|---|---|
| 54.0% | 60.1% | 42.3% | 46.7% (70.7%)b |
Error in S character is ± 3%.
composition is given in parenthesis
4.2 Metal vs Ligand Centered Redox
The one-electron oxidation of 1 to 1ox leads to a shift in the Cu K- and L- pre-edge transitions to higher energy by ~1.5 eV. It has been shown that these pre-edge shifts, which are transitions to the , are a measure of the ligand field (LF) felt by the absorbing Cu atom in the molecule.67 It is interesting to note that the S-K pre-edge, which is also a transition into the , is shifted to lower energy in 1ox relative to 1 (~0.3 eV). The S-K pre-edge energy position is affected by three factors: LF, and the charges on the S () and the Cu () atoms in the molecule. An increase in ligand field strength shifts the S pre-edge transition to higher energy by destabilizing the , an increase in shifts the transition to lower energy by increasing the binding energy of the 3d-manifold, and an increase in shifts the transition to higher energy (chemical shift induced by a change in charge).5,68 Since Cu K- and L- pre-edge shifts reflect changes in LF and the S K-edge energy shifts (obtained from Figure 3) reflect changes in , these shifts can be used to uncouple the contribution of to the S-K pre-edge shifts. This difference in reflects the difference in the 3d manifold energy between 1 and 1ox due to the different charges on Cu in the two complexes. Using the above relation, the d-manifold of 1ox is calculated to be 2.3 eV lower in energy relative to 1. This difference in the d-manifold energy indicates a large increase in the in 1ox which is indicative of a metal centered oxidation. An oxidation of CuII to CuIII is consistent with the ~ 0.1 Å shorter Cu-S distance in 1ox compared to 1. This decrease in bond distance increases the ligand field and destabilizes the d-manifold, which is manifested in the Cu K- and L- pre-edge shifts to higher energy (~1.5 eV). The total Cu character obtained from Cu L-edge intensities is higher in 1ox (56%) compared to 1 (39%). This is also consistent with a metal-based oxidation since an increase is metal character is reflected by an increase in the total L-edge intensity. However, the per-hole Cu character is smaller in 1ox (28%) compared to 1 (39%). The oxidation of 1 (CuII) to 1ox (CuIII) leads to extensive charge donation from the dithiolene ligands to compensate the increased positive charge on the Cu atom.
The one-electron oxidation of 2 to 2ox leads to a shift in the S-K pre-edge transition to higher energy by ~0.2 eV. Further, S K-edge XAS and DFT calculations show that the one-electron oxidation of 2 to 2ox is very similar to that of [Ni(S2C2Me2)2]2- to [Ni(S2C2Me2)2]- (vide supra). This indicates that the difference in LF between [Ni(S2C2Me2)2]2- and [Ni(S2C2Me2)2]- can be used as a handle on the difference in LF between 2 and 2ox. Ni K-edge XAS on [Ni(S2C2Me2)2]2- and [Ni(S2C2Me2)2]- show that the LF increases by ~0.3 eV upon oxidation of [Ni(S2C2Me2)2]2- (measured by the shift in the Ni-K pre-edge positions).25 Combining this as a measure of the difference in LF between 2 and 2ox with S K-edge energies of 2 and 2ox as a measure of the change in (Figure 4), the d-manifold of 2ox is calculated to be 0.8 eV lower than in 2, due to the change in . This difference is much smaller than the 2.3 eV shift observed on going from 1 to 1ox obtained above. This indicates that the perturbation in is much smaller than in upon oxidation. This is consistent with a smaller change in the Ni-S bond distance on going from [Ni(MNT)2]2- to [Ni(MNT)2]- (~0.02 Å compared to the ~ 0.1 Å decrease in the Cu-S distance on going from 1 to 1ox). Thus, a combination of Cu K-, Ni K- and S K-edge energy shifts indicate that in contrast to 1, the oxidation of 2 is predominantly ligand-based.
S-K pre-edge intensities of 1 and 1ox give a direct comparison of the differences in the Cu-S covalency in the two complexes. The per-hole S character in the of 1 (1-hole) and 1ox (2-holes) is 54% and 60%, respectively. This is in reasonable agreement with DFT calculations which indicate that the S character increases from 52.4% in 1 to 64.8% in 1ox. This increase in the total S character (66%) in the is consistent with the decrease in the per-hole Cu character obtained from Cu L-edge analysis (see section 3.1.2). The per-hole S character in the of 2 (2-holes) and 2ox (2-holes) is 42% and 47%, respectively. This is in reasonable agreement with Mulliken population analyses which indicate that the S character increases from 43.8% in 2 to 46.2% to 2ox. From S-K pre-edge intensities, the increase in the total S character in the is ~10% on going from 2 to 2ox. Upon oxidation of 2 to 2ox a hole is created in the , which contains 58% S character (this incorporates the difference in the oscillator strength between π- and σ-orbitals, see section 4.1). These results are consistent with Mulliken population analyses which indicate that the consists of 50.5% S character and 18.2% C and N character (remaining atoms of the dithiolene ligand). These results are also consistent with 15N and 13C ENDOR results which indicate that the total C and N contribution is ~15% in the ground state of 2ox. ENDOR studies also indicate that the Ni character in the ground state () is 32% which is consistent with the 31.2% obtained from DFT calculated Mulliken population analyses.66
In our earlier publication, DFT calculations were performed on the bonding within the free dithiolene ligands [S2C2(Me)2]2- and [{S2C2(Me2)}2]4- and their interaction with the metal in [Ni(S2C2Me2)2]0,-,2-. These studies showed that the of [{S2C2(Me2)}2]4- is destabilized to higher energy due to strong repulsion between the two negatively charged S atoms in the (S2C2Me2)2- ligand.25 This results in an inverted bonding scheme in [Ni(S2C2Me2)2]n- complexes with the initially occupied ligand orbital at higher energy relative to the metal 3d orbitals. Since the geometric and electronic structures of [Ni(S2C2Me2)2]-,2- and [Ni(MNT)2]-,2- are very similar the relative orbital energies of the metal and ligand orbitals in both these set of complexes are consistent with a ligand-based oxidation. This is also supported by the Ni K-rising edge energies, which are very similar for 2 and 2ox indicating similar in these complexes. In contrast, the one-electron oxidation of 1 to 1ox is shown to be metal-based from Cu K-edge energy shifts. This process creates a hole in the which contains an in-plane strong σ-type interaction between the Cu 3dx2–y2 and S 3pσ orbitals. This strong interaction facilitates extensive charge donation from the dithiolene ligand into the Cu center to neutralize the increased charge on the metal due to oxidation. This is consistent with the increase in the per-hole S-K pre-edge and decrease in the Cu L pre-edge intensity. In contrast, the one electron oxidation of 2 to 2ox creates a hole in the orbital of 2ox which contains an out-of-plane weaker (relative to the σ-type interaction between the Cu 3dx2–y2 and S 3pσ orbital) π-type interaction between the Ni 3dxz and S 3pπ orbitals. This weak interaction leads to predominant ligand character in the leaving the metal relatively unperturbed, which is consistent with the small change in the between 2 and 2ox and a dominantly ligand-based oxidation of 2.25
5. Discussion
S K-edge XAS provides an experimental measure of S 3p character in unoccupied metal d orbitals in complexes with metal-S bonds. A quantitative measure of the S character is possible when both the area under the pre-edge peak(s) and accurate Is values are known. Correlation of S K-edge XAS data with 33S EPR superhyperfine and Cu L-edge results on 1 has enabled the quantitative estimate of the Is for the dithiolene ligand, MNT in [Cu(MNT)2]2-. Using the correlation of to the S 1s→4p edge energy position and the linear dependence of Is on , Is values can now be experimentally established from the interpolation plot shown in Figure 7 (red). This figure also includes our previous correlation (black) which used a limited range of . The different slopes of the two Is versus 1s→4p edge energy plots emphasize the importance of experimentally determining accurate Is values, especially in the case of ligands with high positive charge on the S atom. Thus, this study allows a quantitative extension of our S K-edge XAS methodology to a broader range of S-containing ligand systems.
A combination of Cu K- and L-edge and S K-edge XAS has been used to establish that a metal-based oxidation occurs upon one electron oxidation of 1 to 1ox. The pre-edge and edge energy shifts observed in these XAS spectra have been used to experimentally uncouple the effect of a change in from the effects of change in ligand field and . It is found that the d-manifold of 1ox is shifted to lower energy by ~2.3 eV relative to that in 1 indicating significant change in upon oxidation. In an earlier publication we have shown that the core and valence orbital shifts due to change in are very similar.67 Thus, energy trends in Cu 2p XPS data can be used to investigate trends in 3d manifold energies. XPS data on CuII inorganic complexes and their CuIII analogues are available, which show an ~ 1.5-2.0 eV shift upon one-electron oxidation.69-72 Thus, the large shift of 2.3 eV clearly demonstrates that the conversion of 1 to 1ox involves a dominantly metal-based oxidation. S K-edge XAS studies have been used in combination with previously published Ni K-edge data (Supporting Information of Reference 25) to determine that a predominantly ligand-based oxidation occurs upon conversion of 2 to 2ox. These data show that the shift in Ni 3d-manifold upon oxidation of 2 to 2ox is ~0.8 eV which is much smaller than the shift in the Cu 3d-manifold upon oxidation of 1 to 1ox reflecting a smaller change in . Mulliken population analysis and orbital contour plots obtained from spin-unrestricted DFT calculations show that the one electron oxidation of 1 and 2 lead to very different ground states. In 1ox the ground state wavefunction is , which contains an in-plane strong σ anti-bond between the Cu 3dx2–y2 and S 3pσ orbitals while in 2ox the ground state wavefunction is , which contains an out-of-plane weaker π interaction between the Ni 3dxz and S 3pπ orbitals. These differences in the redox active molecular orbitals strongly affect the redox properties of 1 and 2 and lead to a metal-based oxidation in the case of the [Cu(MNT)]- and a ligand-based oxidation in the case of [Ni(MNT)]-.
Supplementary Material
Acknowledgement
This work was supported by NIH RR-01209 (K.O.H.), NSF CHE-9980549 (E.I.S.), NSF CHE98-76457 and DGI-CTQ2006-06333 (Spain). SSRL operations are funded by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology program is supported by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program and by the Department of Energy, Office of Biological and Environmental Research. XR acknowledges a JdC contract from MEC (Spain).
Footnotes
Supporting Information Available: Fits to the Cu K-edge shakedown and 1s→4p transitions for 1 and 1ox. Comparison of S K-edge data of free (TBA)2[MNT], 1 and 1ox. Comparison of S K-edge data of 2, 2ox and [Ni(S2C2Me2)]-,2-. Pseudo-Voigt fits to all S K- and Cu L-edge data. Correlation plot of and S(1s) and S(4p) orbital energy gap. DFT optimized structures. Results obtained from B3LYP calculations. Comparison of S character in the of [Ni(S2C2Me2)]0,-,2- using the previously reported and spectroscopically determined Is. Details of the S 1s→4p transition energy assignment in 1. Full author list for reference 36. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Shadle SE, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 1995;117:2259–2272. doi: 10.1021/ja002183v. [DOI] [PubMed] [Google Scholar]
- 2.Glaser T, Hedman B, Hodgson KO, Solomon EI. Acc. Chem. Res. 2000;33:859–868. doi: 10.1021/ar990125c. [DOI] [PubMed] [Google Scholar]
- 3.Williams KR, Hedman B, Hodgson KO, Solomon EI. Inorg. Chim. Acta. 1997;263:315–321. [Google Scholar]
- 4.Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 1990;112:1643–1645. [Google Scholar]
- 5.DeBeer George S, Metz M, Szilagyi RK, Wang HX, Cramer SP, Lu Y, Tolman WB, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2001;123:5757–5767. doi: 10.1021/ja004109i. [DOI] [PubMed] [Google Scholar]
- 6.Solomon EI, Hedman B, Hodgson KO, Dey A, Szilagyi RK. Coord. Chem. Rev. 2005;249:97–129. [Google Scholar]
- 7.Solomon EI, Szilagyi RK, DeBeer George S, Basumallick L. Chem. Rev. 2004;104:419–458. doi: 10.1021/cr0206317. [DOI] [PubMed] [Google Scholar]
- 8.Rose K, Shadle SE, Glaser T, de Vries S, Cherepanov A, Canters GW, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 1999;121:2353–2363. [Google Scholar]
- 9.Shadle SE, Penner-Hahn JE, Schugar HJ, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 1993;115:767–776. [Google Scholar]
- 10.Kirmse R, Stach J, Dietzsch W, Hoyer E. Inorg. Chim. Acta. 1978;26:L53–L55. [Google Scholar]
- 11.Stach J, Kirmse R, Dietzsch W, Olk RM, Hoyer E. Inorg. Chem. 1984;23:4779–4780. [Google Scholar]
- 12.Snaathorst D, Doesburg HM, Perenboom JAAJ, Keijzers CP. Inorg. Chem. 1981;20:2526–2532. [Google Scholar]
- 13.Plumlee KW, Hoffman BM, Ibers JA, Soos ZG. J. Chem. Phys. 1975;63:1926–1942. [Google Scholar]
- 14.Maki AH, Davison A, Edelstein N, Holm RH. J. Am. Chem. Soc. 1964;86:4580–4587. [Google Scholar]
- 15.McCleverty JA. Prog. Inorg. Chem. 1968;10:49–221. [Google Scholar]
- 16.Eisenberg R. Prog. Inorg. Chem. 1970;12:295–369. [Google Scholar]
- 17.Olk RM, Olk B, Dietzsch W, Kirmse R, Hoyer E. Coord. Chem. Rev. 1992;117:99–131. [Google Scholar]
- 18.Robertson N, Cronin L. Coord. Chem. Rev. 2002;227:93–127. [Google Scholar]
- 19.White LK, Belford RL. J. Am. Chem. Soc. 1976;98:4428–4438. [Google Scholar]
- 20.Schrauzer GN, Mayweg VP. J. Am. Chem. Soc. 1965;87:1483–1489. [Google Scholar]
- 21.Balch AL, Holm RH. J. Am. Chem. Soc. 1966;88:5201–5209. [Google Scholar]
- 22.Shupack SI, Williams R, Gray HB, Billig E, Clark RJH. J. Am. Chem. Soc. 1964;86:4594–4602. [Google Scholar]
- 23.Stiefel EI, Waters JH, Billig E, Gray HB. J. Am. Chem. Soc. 1965;87:3016–3017. [Google Scholar]
- 24.Herebian D, Wieghardt KE, Neese F. J. Am. Chem. Soc. 2003;125:10997–11005. doi: 10.1021/ja030124m. [DOI] [PubMed] [Google Scholar]
- 25.Szilagyi RK, Lim BS, Glaser T, Holm RH, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2003;125:9158–9169. doi: 10.1021/ja029806k. [DOI] [PubMed] [Google Scholar]
- 26.Davison A, Holm RH, Edelstein N, Maki AH. Inorg. Chem. 1963;2:1227–&. [Google Scholar]
- 27.Billig E, Waters JH, Gray HB, Bernal I, Williams R. Inorg. Chem. 1964;3:663–&. [Google Scholar]
- 28.DuBois JL, Mukherjee P, Stack TDP, Hedman B, Solomon EI, Hodgson KO. J. Am. Chem. Soc. 2000;122:5775–5787. [Google Scholar]
- 29.Ellis PJ, Freeman HC. J. Synchrotron Rad. 1995;2:190–195. doi: 10.1107/S0909049595006789. [DOI] [PubMed] [Google Scholar]
- 30.Mustre de Leon J, Rehr JJ, Zabinsky SI, Albers RC. Phys. Rev. B. 1991;44:4146–4156. doi: 10.1103/physrevb.44.4146. [DOI] [PubMed] [Google Scholar]
- 31.Rehr JJ, Mustre de Leon J, Zabinsky SI, Albers RC. J. Am. Chem. Soc. 1991;113:5135–5140. [Google Scholar]
- 32.George GN. EXAFSPAK & EDG_FIT. Stanford Synchrotron Radiation Laboratory, Stanford Linear Accelerator Center, Stanford University; Stanford, CA 94309: 2000. [Google Scholar]
- 33.Wasinger EC, de Groot FMF, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2003;125:12894–12906. doi: 10.1021/ja034634s. [DOI] [PubMed] [Google Scholar]
- 34.Hedman B, Frank P, Gheller SF, Roe AL, Newton WE, Hodgson KO. J. Am. Chem. Soc. 1988;110:3798–3805. [Google Scholar]
- 35.Gorelsky SI, Basumallick L, Vura-Weis J, Sarangi R, Hodgson KO, Hedman B, Fujisawa K, Solomon EI. Inorg. Chem. 2005;44:4947–4960. doi: 10.1021/ic050371m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pople JA, et al. Gaussian 03, revision C.02. 2004.
- 37.Becke AD. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 38.Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
- 39.Perdew JP, Burke K, Wang Y. Phys. Rev. B. 1998;57:14999. [Google Scholar]
- 40.Perdew JP. Phys. Rev. B. 1986;33:8822–8824. doi: 10.1103/physrevb.33.8822. [DOI] [PubMed] [Google Scholar]
- 41.Vosko SH, Wilk L, Nusair M. Can. J. Phys. 1980;58:1200–1211. [Google Scholar]
- 42.Curtiss LA, McGrath MP, Blaudeau JP, Davis NE, Binning RC, Radom L. J. Chem. Phys. 1995;103:6104–6113. [Google Scholar]
- 43.Krishnan R, Binkley JS, Seeger R, Pople JA. J. Chem. Phys. 1980;72:650–654. [Google Scholar]
- 44.McGrath MP, Radom L. J. Chem. Phys. 1991;94:511–516. [Google Scholar]
- 45.Hariharan PC, Pople JA. Theoret. Chimica Acta. 1973;28:213–222. [Google Scholar]
- 46.Francl MM, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA. J. Chem. Phys. 1982;77:3645–3665. [Google Scholar]
- 47.Rassolov VA, Pople JA, Ratner MA, Windus TL. J. Chem. Phys. 1998;109:1223–1229. [Google Scholar]
- 48.Carpenter JE, Weinhold F. THEOCHEM. 1988;169:41–62. [Google Scholar]
- 49.Foster JP, Weinhold F. J. Am. Chem. Soc. 1980;102:7211–7218. [Google Scholar]
- 50.Reed AE, Curtiss LA, Weinhold F. Chem. Rev. 1988;88:899–926. [Google Scholar]
- 51.Schaftenaar G, Noordik JH. Comput-Aided Mol. Des. 2000;14:123–134. doi: 10.1023/a:1008193805436. [DOI] [PubMed] [Google Scholar]
- 52.Tenderholt A. PyMOlyze. Stanford University; Stanford, CA-94305: 2005. [Google Scholar]
- 53.Neese F. ORCA- An Ab-initio, DFT and Semiempirical Electronic Structure Package, Max-Planck Institut für Bioanorganische Chemie. Mülheim; Germany: Nov, 2004. Version 2.4, Revision 16, 2004. [Google Scholar]
- 54.Neese F, Olbrich G. Chem. Phys. Lett. 2002;362:170–178. [Google Scholar]
- 55.Shulman RG, Yafet Y, Eisenberger P, Blumberg WE. Proc. Natl. Acad. Sci. U.S.A. 1976;73:1384–1388. doi: 10.1073/pnas.73.5.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DuBois JL, Mukherjee P, Collier AM, Mayer JM, Solomon EI, Hedman B, Stack TDP, Hodgson KO. J. Am. Chem. Soc. 1997;119:8578–8579. [Google Scholar]
- 57.George SJ, Lowery MD, Solomon EI, Cramer SP. J. Am. Chem. Soc. 1993;115:2968–2969. [Google Scholar]
- 58.Coster D, Kronig RDL. Physica. 1935;2:13–24. [Google Scholar]
- 59.Solomon EI. Comments Inorg. Chem. 1984;3:227–320. [Google Scholar]
- 60.Shadle SE, Hedman B, Hodgson KO, Solomon EI. Inorg. Chem. 1994;33:4235–4244. [Google Scholar]
- 61.Forrester JD, Zalkin A, Templeton DH. Inorg. Chem. 1964;3:1507–1515. [Google Scholar]
- 62.The ψ*β-LUMO of 2 is 1.0 eV higher in energy compared to 1. The ψ*β-LUMO of 2ox is 1.2 eV higher in energy compared to 1ox. These results are also in good agreement with the experimental shifts observed in the S K- pre-edge transition energies.
- 63.Reijerse EJ, Thiers AH, Kanters R, Gribnau MCM, Keijzers CP. Inorg. Chem. 1987;26:2764–2769. [Google Scholar]
- 64.Neese F, Hedman B, Hodgson KO, Solomon EI. Inorg. Chem. 1999;38:4854–4860. doi: 10.1021/ic990461p. [DOI] [PubMed] [Google Scholar]
- 65.Ray K, DeBeer George S, Solomon EI, Wieghardt K, Neese F. Manuscript Submitted.
- 66.Huyett JE, Choudhury SB, Eichhorn DM, Bryngelson PA, Maroney MJ, Hoffman BM. Inorg. Chem. 1998;37:1361–1367. doi: 10.1021/ic9703639. [DOI] [PubMed] [Google Scholar]
- 67.Sarangi R, Aboelella N, Fujisawa K, Tolman WB, Hedman B, Hodgson KO, Solomon EI. J. Am. Chem. Soc. 2006;128:8286–8296. doi: 10.1021/ja0615223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Randall DW, DeBeer George S, Holland PL, Hedman B, Hodgson KO, Tolman WB, Solomon EI. J. Am. Chem. Soc. 2000;122:11632–11648. [Google Scholar]
- 69.Christ BV. Handbook of Monochromatic XPS Spectra. John Wiley & Sons; 2000. [Google Scholar]
- 70.Bianconi A, Castellano AC, Desantis M, Delogu P, Gargano A, Giorgi R. Solid State Commun. 1987;63:1135–1139. [Google Scholar]
- 71.Sacher E, Klemberg-Sapieha JE, Cambron A, Okoniewski A, Yelon A. J. Electron Spectrosc. Relat. Phenom. 1989;48:C7–C12. [Google Scholar]
- 72.Mizokawa T, Namatame H, Fujimori A, Akeyama K, Kondoh H, Kuroda H, Kosugi N. Phys. Rev. Lett. 1991;67:1638–1641. doi: 10.1103/PhysRevLett.67.1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.