Abstract
Background
Marine omega-3 fatty acids have beneficial effects on cardiovascular risk factors. Consumption of fatty fish and marine omega-3 has been associated with lower rates of cardiovascular diseases.
Objective
We examined the association of fatty fish and marine omega-3 with heart failure (HF) in a population of middle-age and older women.
Methods
Participants in the Swedish Mammography Cohort aged 48–83 years completed 96-item food-frequency questionnaires. Women without history of HF, myocardial infarction, or diabetes at baseline (n= 36 234) were followed from January 1, 1998 until December 31, 2006 for HF hospitalization or mortality through Swedish inpatient and cause-of-death registers; 651 women experienced HF events. Cox proportional hazards models accounting for age and other confounders were used to calculate incidence rate ratios (RR) and 95% confidence intervals (CI).
Results
Compared to women who did not eat fatty fish, RR were 0.86 (95% CI: 0.67, 1.10) for <1 serving/week, 0.80 (95% CI: 0.63, 1.01) for 1 serving/week, 0.70 (95% CI: 0.53, 0.94) for 2 servings/week, and 0.91 (95% CI: 0.59, 1.40) for ≥3 servings/week (Ptrend = 0.049). RR across quintiles of marine omega-3 fatty acids were 1 (reference), 0.85 (95% CI: 0.67, 1.07), 0.79 (95% CI: 0.61, 1.02), 0.83 (95% CI 0.65, 1.06), and 0.75 (95% CI: 0.58, 0.96) (Ptrend = 0.04).
Conclusion
Moderate consumption of fatty fish (one to two servings per week) and marine omega-3 fatty acids were associated with a lower rate of first HF hospitalization or death in this population.
Keywords: Heart Failure, Fatty Acids, Omega-3, Diet
INTRODUCTION
Fatty fish and marine omega-3 fatty acids have been shown to have beneficial effects on the cardiovascular system which could slow or stop the development of heart failure (HF), a downstream consequence of many types of injury to the heart (Hunt et al., 2005). HF is the most common reason for hospitalization in the US Medicare population (Kozak et al., 2006). It causes substantial mortality and morbidity, and although treatment is improving, prognosis is still poor, which makes prevention of HF a public health priority (Schocken et al., 2008; Jhund et al., 2009). Cardiovascular effects of marine omega-3 fatty acids including reductions in blood pressure, heart rate, triacylglycerol concentration, and platelet aggregation and increases in heart rate variability (Bays, 2006; Mozaffarian et al., 2006; Mozaffarian et al., 2008; Harris et al., 2008). High intake seems to reduce propensity to arrhythmia (Leaf et al., 2005; Brouwer et al., 2006; Den Ruijter et al., 2008; Chrysohoou et al., 2007; Metcalf et al., 2008). In post-myocardial infarction (MI) and HF patients, supplementation with marine omega-3 fatty acids improved survival (GISSI-Prevenzione, 1999; GISSI-HF Investigators, 2008). In addition, omega-3 fatty acids improve endothelial function (Morgan et al., 2006) and may reduce inflammation (Lennie et al., 2005) in patients with HF.
Several previous studies in a variety of populations with different fish consumption habits have examined the association of fish and omega-3 consumption with incidence of HF events with mixed results. In a study of older US men and women, increasing tuna or other baked or broiled fish intake and marine omega-3 intake was associated with a lower rate of HF, but fried fish was not (Mozaffarian et al., 2005). Total fish consumption, including fried fish, was not associated with HF incidence in a cohort of white and African-American men and women (Nettleton et al., 2008). However, total fish consumption, included tempura fried fish, and total omega-3 fatty acid intake were associated with reduced mortality from HF in a cohort of Japanese adults (Yamagishi et al., 2008). In a population of middle-aged and elderly men from central Sweden, moderate consumption of fatty fish and marine omega-3 fatty acids was associated with a lower rate of HF events, but rates of HF events were similar among the highest and lowest consumers (Levitan et al., 2009).
In Sweden, intake of fish high in omega-3 fatty acids including salmon, herring, and mackerel is common compared to other regions where the association has been evaluated (Becker et al., 2007). In addition, studies to date have examined populations of men or populations including both men and women. We therefore examined the association of fatty fish and omega-3 fatty acids with rate of HF hospitalization or mortality in a large population of Swedish women.
PARTICIPANTS AND METHODS
Participants
This study included participants in the Swedish Mammography Study. The recruitment process, characteristics of the cohort, and study methods have been previously described (Wolk et al., 2006). Briefly, all women in the Swedish population register born between 1914 and 1948 and living in Västmanland and Uppsala counties in central Sweden received a questionnaire between March 1, 1987, and December 14, 1990. Of the 90 303 women identified in the population register, 66 651(74%) returned a completed questionnaire. In September 1997 a second questionnaire was sent to 56,030 participants who were still alive and residing in the study area; 39 227 (70%) returned a questionnaire. Because of the additional information collected on the second questionnaire, such as cigarette smoking, only women who completed the 1997 questionnaire are included in this study.
Participants who did not provide or provided incorrect national identification numbers, who reported implausible energy intakes (>3 standard deviations from the natural logarithm-transformed mean), who had a previous diagnosis of cancer (other than nonmelanoma skin cancer) or HF were excluded (n = 1 126). Because patients with diabetes or MI are often counseled to alter their diets and diabetes and MI are risk factors for HF, only women with no baseline history of MI or diabetes were included (n = 36 234). History of HF and MI was determined through record linkage to the Swedish inpatient register, and diabetes was determined using self-report and record linkage. The study complies with the Declaration of Helsinki and was approved by the Regional Ethical Review Board at Karolinska Institute, Stockholm, Sweden. Completion and return of the self-administered questionnaire was assumed to imply consent.
Diet assessment
Self-administered food-frequency items on the questionnaires asked participants to report usual frequency of consumption of 96 items over the previous year. For foods and beverages such as milk, coffee, cheese, and bread that are commonly eaten in Sweden, participants reported their consumption in servings per day or per week. For other foods and beverages there were 8 predefined responses ranging from never to ≥3 times/day. The questionnaires queried 5 types of fish intake: herring/mackerel, salmon/whitefish/char, cod/saithe/fishfingers, caviar, and shellfish including shrimp. The reported frequency of consumption of herring/mackerel and salmon/whitefish/char was summed to calculate frequency of fatty fish consumption.
For each food and beverage, total consumption was calculated by multiplying the frequency of consumption by age-specific portion sizes which were determined using weighed diet records. Nutrient values were calculated by multiplying the food or beverage intake by the nutrient composition obtained from the Swedish National Food Administration (Bergström et al., 1991) and summing over foods and beverages. Nutrient intakes were adjusted for energy using the residuals method (Willett, 1998). As a secondary exposure, we also adjusted fatty fish intake as for energy using the residuals method. Participants also reported consumption of fish oil supplements in capsules/week. Marine omega-3 fatty acids were calculated as the sum of eicosapentaenoic acid (C20:5n-3) and docosahexaenoic acid (C22:6n-3) from food sources. In a validation study of the food-frequency questionnaire among 129 middle-aged and older women from central Sweden, the correlation between the food frequency questionnaire and 4 1-week weighted diet records was 0.5 for measurement of fatty fish (Wolk et al., 2006). Correlations between food-frequency questionnaires and weighed diet records were 0.58 for eicosapentaenoic acid and 0.56 for docosahexaenoic acid, and correlations between food-frequency questionnaires and adipose tissue content were 0.46 for eicosapentaenoic acid and 0.44 for docosahexaenoic acid (Wolk et al., 1998).
We collapsed responses and categorized study participants into categories of no consumption of fatty fish, <1 serving/week, 1 serving/week, 2 servings/week, ≥3 servings/week for both reported frequency and energy-adjusted frequency. We categorized participants into quintiles of marine omega-3 intake.
HF follow-up
Participants were followed from January 1, 1998 until December 31, 2006 through record linkage to the Swedish inpatient and cause-of-death registers. The inpatient register captures more than 99% of inpatient care (National Board of Health and Welfare, 2005). HF events were defined as hospitalizations for or deaths from HF identified by codes 428 (International Classification of Disease-9), I50, or I11.0 (International Classification of Disease-10) listed as the primary diagnosis. A secondary definition of a HF event included the codes in any diagnosis position. Ingelsson and colleagues found that 95% of people with these codes as primary diagnosis and 82% of people with the codes in any diagnosis position had confirmed HF on medical record review using European Society of Cardiology criteria (Ingelsson et al., 2005). We included only the first HF event recorded in the registers for each individual.
Statistical analysis
Because some participants were missing data on weight or height to calculate body mass index (BMI) (1.7%) and physical activity (22.4%), we used Markov chain Monte Carlo multiple imputation to simulate 5 complete datasets. The percentage of participants missing data on the composite physical activity measure was relatively high because this measure could only be calculated for participants who completed all 6 questions related to physical activity. Statistical analyses were performed in each of datasets separately, and the results were then averaged and the confidence intervals and P values calculated accounting for the uncertainty in the imputed estimates (Schafer, 1997). Results from complete-case analyses were similar, so only the multiple imputation estimates are shown.
We computed means and percentages of demographic, behavioral, and health covariates by intake of fatty fish. We tested for differences using linear regression for continuous variables and χ2 tests for categorical variables. To estimate the incidence rate ratios (RR) associated with fish consumption, we used Cox proportional hazards models that accounted for age by allowing the baseline hazard to vary (Collett, 2003). We adjusted for BMI (linear), physical activity (linear), energy intake (linear), alcohol consumption (linear), fiber consumption (linear), sodium consumption (linear), daily servings of red or processed meat (linear), education (less than high school, high school, university), family history of myocardial infarction at <60 years (yes, no), cigarette smoking (current, past, never), living alone (yes, no), postmenopausal hormone use (yes, no), self-reported history of hypertension (yes, no) and self-reported history of high cholesterol (yes, no). The covariates were selected based on the associations between the variables and fish intake and the variables and incident HF in the literature and in our data. For example, in our data women who lived alone were more likely to be at the extremes of fatty fish intake and being unmarried has been associated with a higher rate of HF (Ingelsson et al., 2006).
We calculated the RR associated with quintiles of marine omega-3 fatty acids and explored the potentially nonlinear shape of the association between marine omega-3 fatty acids and incidence of HF using a restricted cubic spline with 3 knots (Durrleman and Simon, 1989) using models adjusted as described above. For the spline, we excluded individuals with values below the 2.5th and above the 97.5th percentiles to avoid modeling were data were sparse. We created an additional model which adjusted the RR associated with quintiles of marine omega-3 fatty acids for protein (linear), saturated fat (linear), monounsaturated fat (linear), non-marine omega-3 fatty acids (linear), and omega-6 fatty acids (linear). We examined the effect of excluding women with self-reported prevalent hypertension at baseline using models as described above. We additionally examined HF events defined as HF listed in any diagnosis position. Because symptoms of HF occurring prior to hospitalization or death may influence dietary behavior, we performed a sensitivity analysis excluding cases occurring during the first 2 years of follow-up. We tested for violations of the proportional hazards assumption by entering the product of fish intake or marine omega-3 intake and the natural logarithm of time in the model. The proportional hazards assumption did not appear to be violated for any of the models.
Statistical analyses were performed using SAS version 9.1 (Cary, NC) and Stata version 10.0 (College Station, TX). A 2-sided P value < 0.05 was considered statistically significant.
RESULTS
Over 9 years of follow-up, 651 of 36 234 women were hospitalized for HF (n = 596) or died of HF (n = 55), corresponding to a rate of 20 cases per 10 000 person-years. Twelve percent of the women did not consume fatty fish, 25% ate less than one serving per week, 44% ate one serving per week, 17% ate two servings per week and 3% ate 3 or more servings per week. The women who ate the most fatty fish were, on average, older and heavier, less likely to be current smokers, more likely to have a history of hypertension and high cholesterol, and consumed more sodium and more red and processed meat (Table 1).
Table 1.
Baseline characteristics of 36 234 Swedish Mammography Cohort participants by intake of fatty fish1
| Fatty fish intake |
||||||
|---|---|---|---|---|---|---|
| Never (N = 4 344) | <1 servings/wk (N = 8 988) | 1 serving/wk (N = 15 932) | 2 servings/wk (N = 6 026) | ≥3 servings/wk (N = 944) | P2 | |
| Age (y) | 61.9 ± 9.83 | 61.6 ± 9.4 | 60.8 ± 8.9 | 62.9 ± 8.9 | 66.1 ± 9.2 | <0.001 |
| Physical activity (MET hr/d) | 42.4 ± 5.0 | 42.4 ± 4.8 | 42.3 ± 4.6 | 42.8 ± 4.8 | 42.8 ± 5.1 | <0.001 |
| Body mass index (kg/m2) | 24.9 ± 4.0 | 25.0 ± 4.0 | 24.8 ± 3.7 | 25.2 ± 4.0 | 25.5 ± 4.4 | 0.009 |
| Cigarette smoking | <0.001 | |||||
| Current | 1 129 (26.0)4 | 2 119 (23.6) | 3 617 (22.7) | 1 221 (20.3) | 184 (19.5) | |
| Past | 899 (20.7) | 1 912 (21.3) | 3 791 (23.8) | 1 392 (23.1) | 180 (19.1) | |
| Never | 2 316 (53.3) | 4 957 (55.1) | 8 524 (53.5) | 3 413 (56.6) | 580 (61.4) | |
| Living alone | 1 391 (32.0) | 2 324 (25.9) | 3 220 (20.2) | 1 271 (21.1) | 297 (31.5) | <0.001 |
| Postmenopausal hormone use | 1 994 (45.9) | 4 159 (46.3) | 8 113 (50.9) | 3 197 (53.1) | 450 (47.7) | <0.001 |
| Education | <0.001 | |||||
| Less than high school | 3 405 (78.4) | 6 887 (76.6) | 11 214 (70.4) | 4 419 (73.3) | 772 (81.8) | |
| High school | 337 (7.8) | 659 (7.3) | 1 374 (8.6) | 447 (7.4) | 54 (5.7) | |
| University | 602 (13.9) | 1 442 (16.0) | 3 344 (21.0) | 1 160 (19.3) | 118 (12.5) | |
| Family history of myocardial infarction | 550 (12.7) | 1 144 (12.7) | 2 204 (13.8) | 863 (14.3) | 141 (14.9) | 0.008 |
| History of hypertension | 796 (18.3) | 1 820 (20.3) | 3 002 (18.8) | 1 362 (22.6) | 253 (26.8) | <0.001 |
| History of high cholesterol | 287 (6.6) | 626 (7.0) | 1 240 (7.8) | 607 (10.1) | 96 (10.2) | <0.001 |
| Energy intake (kcal/d) | 1 562 ± 541 | 1 651 ± 493 | 1 748 ± 483 | 1 898 ± 519 | 2 234 ±796 | <0.001 |
| Alcohol (g/d) | 2.9 ± 4.5 | 3.5 ± 5.0 | 4.7 ± 5.3 | 4.8 ± 5.5 | 3.6 ± 5.8 | <0.001 |
| Sodium (g/d)5 | 2 443 ± 460 | 2 451 ± 353 | 2 519 ± 347 | 2 620 ± 345 | 3 087 ± 655 | <0.001 |
| Fiber (g/d)5 | 21.6 ± 6.6 | 21.6 ± 5.5 | 22.1 ± 5.2 | 23.0 ± 5.2 | 22.6 ± 5.8 | <0.001 |
| Red/processed meat (servings/d) | 0.9 ± 0.8 | 1.0 ± 0.6 | 1.1 ± 0.7 | 1.2 ± 0.7 | 1.7 ± 1.7 | <0.001 |
| Marine omega-3 (g/d)5 | 0.13 ± 0.11 | 0.22 ± 0.08 | 0.34 ± 0.10 | 0.55 ± 0.14 | 1.26 ± 0.73 | <0.001 |
MET, metabolic equivalent task; SD, standard deviation
Derived from tests of linear trend for continuous variables or χ2 tests for categorical variables
Mean ± standard deviation (all such values)
N (percent) (all such values)
Adjusted for energy using the residuals method
Compared to women who did not consume fatty fish, women who consumed <1 serving/week had a 14% lower rate of HF events, women who consumed 1 serving/week had a 20% lower rate, women who consumed 2 servings/week had a 30% lower rate, and women who consumed ≥3 servings/week had a 9% lower rate after accounting for age and adjusting for dietary, demographic, and lifestyle factors (Table 2). A test for linear trend was significant (P = 0.049) and a test of quadratic trend was not statistically significant (P = 0.21). Results were similar when examining energy-adjusted fatty fish intake. Compared to women who never consumed fatty fish, women who consumed <1 serving/week had a multivariable-adjusted RR of 0.81 (95% CI: 0.62, 1.06), women who consumed 1 serving/week has a RR of 0.83 (95% CI: 0.66, 1.05), women who consumed 2 servings/week had a RR of 0.70 (95% CI: 0.52, 0.94), and women who consumed ≥3 servings/week had a RR of 1.00 (95% CI: 0.66,1.52).
Table 2.
Fatty fish intake and incidence of heart failure hospitalization or mortality1
| Model 12 |
Model 23 |
|||||
|---|---|---|---|---|---|---|
| Fatty fish intake | Cases | Person-years | RR | 95% CI | RR | 95% CI |
| Never | 104 | 37 086 | 1.00 | Reference | 1.00 | Reference |
| <1 serving/week | 177 | 77 451 | 0.88 | 0.69, 1.13 | 0.86 | 0.67, 1.10 |
| 1 serving/week | 237 | 138 614 | 0.79 | 0.62, 0.99 | 0.80 | 0.63, 1.01 |
| 2 servings/week | 101 | 52 189 | 0.69 | 0.53, 0.91 | 0.70 | 0.53, 0.94 |
| ≥3 servings/week | 32 | 7 879 | 0.96 | 0.64, 1.42 | 0.91 | 0.59, 1.40 |
| P for linear trend | 0.03 | 0.049 | ||||
RR, incidence rate ratio; CI, confidence interval
Cox proportional hazards model accounting for age.
Cox proportional hazards model accounting for age and additionally adjusted for education (less than high school, high school, university), body mass index (linear term), physical activity (linear term), cigarette smoking (current, past, never), living alone (yes, no), postmenopausal hormone use (yes, no), total energy intake (linear term), alcohol intake (linear term), fiber intake (linear term), sodium intake (linear term), intake of red or processed meat (linear term), family history of myocardial infarction before 60 years (yes, no), self-reported history of hypertension (yes, no), self-reported history of high cholesterol (yes, no)
The highest quintile of marine omega-3 fatty acid intake was associated with a 25% lower rate of HF events compared to the lowest quintile in multivariable-adjusted models (Table 3). The dose-response analysis using a restricted cubic spline suggested that the rate of HF declined with increasing marine omega-3 fatty acids up to approximate 0.4 g/day; above 0.4 g/day, marine omega-3 fatty acids did not appear to be strongly correlated with rate of HF events (Figure). However, the test for deviation from linearity was not statistically significant (P = 0.32). Further adjusting for macronutrients, including protein, saturated fat, monounsaturated fat, non-marine omega-3 fatty acids, and omega-6 fatty acids, slightly attenuated the difference in rates (compared to the lowest quintile, RR for quintile 2 = 0.87, 95% CI: 0.69, 1.11, RR for quintile 3 = 0.83, 95% CI: 0.64, 1.07, RR for quintile 4 = 0.88, 95% CI: 0.69, 1.12, RR for quintile 5 = 0.81, 95% CI: 0.63, 1.05).
Table 3.
Marine omega-3 intake and incidence of heart failure hospitalization or mortality1
| Model 12 |
Model 23 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Marine omega-3 fatty acids | Median, g/day | Range, g/day | Cases | Person-years | RR | 95% CI | RR | 95% CI |
| Quintile 1 | 0.14 | 0.01–0.19 | 168 | 61 959 | 1.00 | Reference | 1.00 | Reference |
| Quintile 2 | 0.23 | 0.20–0.27 | 123 | 62 897 | 0.87 | 0.69, 1.10 | 0.85 | 0.67, 1.07 |
| Quintile 3 | 0.30 | 0.28–0.33 | 99 | 63 153 | 0.80 | 0.62, 1.02 | 0.79 | 0.61, 1.02 |
| Quintile 4 | 0.38 | 0.34–0.45 | 120 | 63 064 | 0.84 | 0.67, 1.07 | 0.83 | 0.65, 1.06 |
| Quintile 5 | 0.57 | 0.46–7.15 | 141 | 62 146 | 0.77 | 0.61, 0.96 | 0.75 | 0.58, 0.96 |
| P for linear trend | 0.03 | 0.04 | ||||||
RR, incidence rate ratio; CI, confidence interval
Cox proportional hazards model accounting for age.
Cox proportional hazards model accounting for age and additionally adjusted for education (less than high school, high school, university), body mass index (linear term), physical activity (linear term), cigarette smoking (current, past, never), living alone (yes, no), postmenopausal hormone use (yes, no), total energy intake (linear term), alcohol intake (linear term), fiber intake (linear term), sodium intake (linear term), intake of red or processed meat (linear term), family history of myocardial infarction before 60 years (yes, no), self-reported history of hypertension (yes, no), self-reported history of high cholesterol (yes, no)
Figure 1.
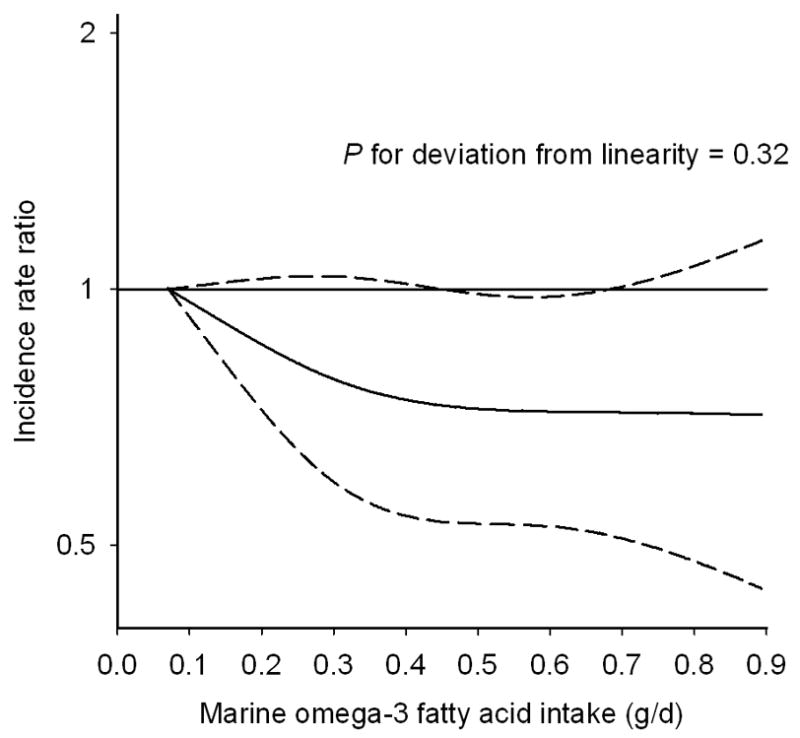
Marine omega-3 intake and incidence rate ratio of heart failure hospitalization or mortality. The solid line represent incidence rate ratio and the dashed line represents the 95% confidence intervals. The curve was produced from a Cox proportional hazards model where marine omega-3 intake was modeled as a restricted cubic spline with 3 knots, accounting for age, and adjusted for education (less than high school, high school, university), body mass index (linear term), physical activity (linear term), cigarette smoking (current, past, never), living alone (yes, no), postmenopausal hormone use (yes, no), total energy intake (linear term), alcohol intake (linear term), fiber intake (linear term), sodium intake (linear term), intake of red or processed meat (linear term), family history of myocardial infarction before 60 years (yes, no), self-reported history of hypertension (yes, no), self-reported history of high cholesterol (yes, no).
Among women without self-reported hypertension at baseline, 401 HF events occurred during follow-up. The results for fatty fish were similar to the total population. Compared to women who did not consume fatty fish, RR were 0.94 (95% CI: 0.69, 1.29) for <1 serving/week, 0.92 (95% CI: 0.68, 1.24) for 1 serving/week, 0.72 (95% CI: 0.50, 1.05) for 2 servings/week, and 1.25 (95% CI: 0.73, 2.14) for ≥3 servings/week (Ptrend = 0.37) in a multivariable-adjusted model. In contrast, there was no apparent association between marine omega-3 intake and HF. RR across quintiles of marine omega-3 fatty acids were 1 (reference), 1.04 (95% CI: 0.77, 1.40), 0.94 (95% CI: 0.68, 1.29), 1.00 (95% CI: 0.73, 1.37), and 0.83 (95% CI: 0.60, 1.15). There were 1 489 events when HF in any diagnosis position was used to define events. Compared to women who did not consume fatty fish, RR were 0.87 (95% CI: 0.73, 1.02) for <1 serving/week, 0.85 (95% CI: 0.73, 1.00) for 1 serving/week, 0.77 (95% CI: 0.64, 0.93) for 2 servings/week, and 0.97 (95% CI: 0.73, 1.29) for ≥3 servings/week (Ptrend = 0.06) in a multivariable-adjusted model. RR across quintiles of marine omega-3 intake were 1 (reference), 0.90 (95% CI: 0.76, 1.05), 0.86 (95% CI: 0.73, 1.02), 0.86 (95% CI: 0.73, 1.01), and 0.88 (95% CI: 0.74, 1.03) (Ptrend = 0.17). Results were not materially different when the first two years of follow-up were excluded or when fatty acids from fish oil supplements were included in the calculation of marine omega-3 consumption.
DISCUSSION
In this population, women who consumed fatty fish 2 times per week had a rate of HF events that was 30% lower than that of women who did not consume fatty fish regularly. Women in the highest fifth of marine omega-3 consumption had a rate of HF that was 25% lower than those in the lowest fifth. A restricted cubic spline analysis suggested a potential threshold in the association with no additional decrease in risk above 0.4 g/day of marine omega-3 consumption. This is similar to the relationship observed for coronary heart disease death (Mozaffarian and Rimm, 2006), but a test for deviation from linearity was not significant.
Previous studies have examined the association of fish intake and omega-3 fatty acids with HF incidence. Mozaffarian and colleagues found that tuna and other broiled or baked fish was associated with lower rates of HF in elderly US men and women, as was marine omega-3 intake, but fried fish was associated with higher rates of HF (Mozaffarian et al., 2005). In contrast, Nettleton and colleagues found no association between total fish intake and HF incidence in US adults, but they were not able to differentiate between fried fish and baked or broiled fish (Nettleton et al., 2008). In a study of Japanese men and women, total fish and total omega-3 intake were associated with lower HF rates (Yamagishi et al., 2008). We previously reported a U-shaped relationship of fatty fish and marine omega-3 intake and HF in Swedish men (Levitan et al., 2009). These studies varied in the way fish intake was assessed and the types of fish commonly consumed; the method of assessing HF also differed across studies.
Fatty fish and marine omega-3 intake may reduce the propensity to develop HF through several pathways. Elevated blood pressure is a major risk factor for HF, (Hunt et al., 2005) and marine omega-3 fatty acids reduce blood pressure in a dose-dependent manner, though the magnitude of the effect is small (Kris-Etherton et al., 2002). Fatty fish and marine omega-3 fatty acid intake can reduce resting heart rate and improve diastolic filling (Mozaffarian, 2007). In rats with pressure overload, marine omega-3 fatty acids prevented left ventricular hypertrophy and dysfunction (Duda et al., 2009). Marine omega-3 fatty acids have also been shown to improve endothelial function and improve arterial compliance and to reduce inflammatory processes (Kris-Etherton et al., 2002).
In this population, the women who consumed 2 servings of fatty fish per week appeared to have the lowest rates of HF, although the test for quadratic trend between fatty fish intake and incidence of HF events was not statistically significant. The appearance of a potentially U-shaped relationship could be due to residual or unmeasured confounding by health status or other factors. If women in worse health consumed more fatty fish than those in better health, the rates of HF events in the highest fatty fish consumers could be biased upward. In fact, women with the highest fatty fish intake were more likely to have a history of high cholesterol or hypertension. Although we controlled for self-reported presence of these cardiovascular risk factors, we did not have information on severity or treatment which could lead to residual confounding. We had limited information on baseline health status and could not control for potential confounding by other indicators of health. Chance could also explain the results, particularly given the small number of women who ate ≥3 servings of fatty fish per week and the wide confidence intervals around the estimates.
HF is a heterogeneous syndrome, and risk factors may not be identical for all subtypes (Hunt et al., 2005); we did not have information on HF etiology or subtype. If the relationship between fish and HF varied by subtype, we would expect that the observed results would be intermediate between the subtype specific relationships. We did not have information regarding the duration of HF symptoms prior to hospitalization or mortality; however, 44% of patients in a Swedish HF registry had new onset HF, defined as HF of less than 6 months duration (Jonsson et al., 2010). Although Swedish inpatient and cause-of-death registers are almost complete and the accuracy of HF diagnosis has been shown to be high (Ingelsson et al., 2005), the registers only captured cases that result in hospitalization or death. Our results may be only generalizable to the subset of HF cases that result in hospitalization or mortality. We expect that these are the most severe cases. As other investigators have suggested (Ingelsson et al., 2005), we included only cases in which HF was listed as the primary diagnosis for our main analysis. This results in greater specificity of diagnosis at the expense of under-diagnosis of HF which would be expected to reduce the power of the analysis. It also reduces the likelihood counting hospitalizations for problems other than HF when HF is mentioned in the medical record.
Power was low for some analyses, particularly those limited to women without a history of hypertension at baseline. Fatty fish consumption and marine omega-3 fatty acids intake were measured using food-frequency questionnaires; we expect some misclassification of intake. However, questionnaires have been validated against weighed diet records and adipose tissue biopsies. As with all observational studies, we were not able to rule out bias due to residual or unmeasured confounding.
In conclusion, moderate consumption of fatty fish (one to two servings per week) and marine omega-3 fatty acids were associated with lower rates of HF in this population of middle-aged and older Swedish women.
Acknowledgments
Supported by the Swedish Research Council/Committee for Infrastructure, the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), and a grant (F32 HL091683) from the US National Heart, Lung, and Blood Institute (to EBL)
The authors’ responsibilities were as follows—EBL: designed the study, performed statistical analysis, interpreted the data, and wrote the manuscript; AW: designed the study, collected data, interpreted the data, and revised the manuscript; MAM: designed the study, interpreted the data, and revised the manuscript. No conflict ofinterest was declared by any of the authors. This study was supported by the Swedish Research Council/Committee for Infrastructure, the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), and a grant (F32 HL091683) from the US National Heart, Lung, and Blood Institute (to EBL).
References
- 1.Bays H. Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. Am J Cardiol. 2006;98:71i–76i. doi: 10.1016/j.amjcard.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Becker W, Darnerud PO, Petersson-Grawé K. Risks and benefits of fish consumption. Livsmedelsverket/National Food Administration; Sweden: 2007. [accessed 27 May 2008]. Internet: http://www.slv.se/default.aspx?id=231&epslanguage=EN-GB. [Google Scholar]
- 3.Bergström L, Kylberg E, Hagman U, Erikson H, Bruce Å. The food composition database KOST: the National Administration’s information system for nutritive values of food. Vår Föda. 1991;43:439–447. [Google Scholar]
- 4.Brouwer IA, Zock PL, Camm AJ, Bocker D, Hauer RN, Wever EF, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 5.Chrysohoou C, Panagiotakos DB, Pitsavos C, Skoumas J, Krinos X, Chloptsios Y, et al. Long-term fish consumption is associated with protection against arrhythmia in healthy persons in a Mediterranean region--the ATTICA study. Am J Clin Nutr. 2007;85:1385–1391. doi: 10.1093/ajcn/85.5.1385. [DOI] [PubMed] [Google Scholar]
- 6.Collett D. Modelling survival data in medical research. 2. Boca Raton: Chapman & Hall/CRC; 2003. [Google Scholar]
- 7.Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, et al. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol. 2001;1:285–298. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- 8.Den Ruijter HM, Berecki G, Verkerk AO, Bakker D, Baartscheer A, Schumacher CA, et al. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008;117:536–544. doi: 10.1161/CIRCULATIONAHA.107.733329. [DOI] [PubMed] [Google Scholar]
- 9.Duda MK, O’Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 11.GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 12.GISSI-Prevenzione. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 13.Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–1754. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 14.Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Ind Med. 1997;32:234–239. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult--Summary article. Circulation. 2005;112:1825–1852. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 17.Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. doi: 10.1016/j.ejheart.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Ingelsson E, Lind L, Arnlov J, Sundstrom J. Socioeconomic factors as predictors of incident heart failure. J Card Fail. 2006;12:540–545. doi: 10.1016/j.cardfail.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Jhund PS, Macintyre K, Simpson CR, Lewsey JD, Stewart S, Redpath A, et al. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: a population study of 5.1 million people. Circulation. 2009;119:515–523. doi: 10.1161/CIRCULATIONAHA.108.812172. [DOI] [PubMed] [Google Scholar]
- 20.Jonsson Å, Edner M, Alehagen U, Dahlström Heart failure registry: a valuable tool for improving the management of patients with heart failure. Eur J Heart Fail. 2010;12:25–31. doi: 10.1093/eurjhf/hfp175. [DOI] [PubMed] [Google Scholar]
- 21.Kozak LJ, DeFrances CJ, Hall MJ. Nation Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat. 2006;13:1–209. [PubMed] [Google Scholar]
- 22.Kris-Etherton PM, Harris WS, Appel LJ for the Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 23.Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, Kang JX, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation. 2005;112:2762–2768. doi: 10.1161/CIRCULATIONAHA.105.549527. [DOI] [PubMed] [Google Scholar]
- 24.Lennie TA, Chung ML, Habash DL, Moser DK. Dietary fat intake and proinflammatory cytokine levels in patients with heart failure. J Card Fail. 2005;11:613–618. doi: 10.1016/j.cardfail.2005.06.434. [DOI] [PubMed] [Google Scholar]
- 25.Levitan EB, Wolk A, Mittleman MA. Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: a population-based prospective study of middle-aged and elderly men. Eur Heart Journal. 2009;30:1495–1500. doi: 10.1093/eurheartj/ehp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metcalf RG, Sanders P, James MJ, Cleland LG, Young GD. Effect of dietary n-3 polyunsaturated fatty acids on the inducibility of ventricular tachycardia in patients with ischemic cardiomyopathy. Am J Cardiol. 2008;101:758–761. doi: 10.1016/j.amjcard.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Morgan DR, Dixon LJ, Hanratty CG, El-Sherbeeny N, Hamilton PB, McGrath LT, et al. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am J Cardiol. 2006;97:547–551. doi: 10.1016/j.amjcard.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 28.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Prineas RJ, Stein PK, Siscovick DS. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol. 2006;48:478–484. doi: 10.1016/j.jacc.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 31.Mozaffarian D. Fish, n-3 fatty acids, and cardiovascular haemodynamics. J Cardiovasc Med (Hagerstown) 2007;8:S23–S26. doi: 10.2459/01.JCM.0000289279.95427.e2. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation. 2008;117:1130–1137. doi: 10.1161/CIRCULATIONAHA.107.732826. [DOI] [PubMed] [Google Scholar]
- 33.The Nation Board of Health and Welfare. The Swedish Hospital Discharge Registry 1964–2003. Stockholm: 2005. [Google Scholar]
- 34.Nettleton JA, Steffen LM, Loehr LR, Rosamond WD, Folsom AR. Incident heart failure is associated with lower whole-grain intake and greater high-fat dairy and egg intake in the Atherosclerosis Risk in Communities (ARIC) study. J Am Diet Assoc. 2008;108:1881–1887. doi: 10.1016/j.jada.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raitt MH, Connor WE, Morris C, Kron J, Halperin B, Chugh SS, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA. 2005;293:2884–2891. doi: 10.1001/jama.293.23.2884. [DOI] [PubMed] [Google Scholar]
- 36.Schafer JL. Analysis of Incomplete Multivariate Data. Boca Raton: CRC Press; 1997. [Google Scholar]
- 37.Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 38.Vena J, Boffetta P, Becher H, Benn T, Bueno-de-Mesquita HB, Coggon D, et al. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ Health Perspect. 1998;106:645–653. doi: 10.1289/ehp.98106645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virtanen JK, Voutilainen S, Rissanen TH, Mursu J, Tuomainen TP, Korhonen MJ, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005;25:228–233. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- 40.Willett WC. Nutritional Epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 41.Wolk A, Ljung H, Vessby B, Hunter D, Willett WC. Effect of additional questions about fat on the validity of fat estimates from a food frequency questionnaire. Study Group of MRS SWEA. Eur J Clin Nutr. 1998;52:186–192. doi: 10.1038/sj.ejcn.1600538. [DOI] [PubMed] [Google Scholar]
- 42.Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296:1371–1376. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- 43.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]


