Abstract
The attachment of circulating tumor cells to the blood vessels of distant organs is an important step in metastasis. We show here that experimental lung metastasis by two cell lines, B16F1 melanoma and 3LL lung carcinoma, is greatly reduced in transgenic mice that lack plasma fibronectin. This multifunctional adhesive glycoprotein becomes cross-linked to fibrin during clotting. Here we report that eliminating plasma fibronectin from the blood circulation reverses the prometastatic effects of blood clotting and tumor cell integrin αvβ3. In vitro studies showed that fibrin-fibronectin (FibFN) complexes, but not purified fibrin, supported tumor cell attachment and invasion. These functions correlate with the ability of FibFN to induce activation of integrin αvβ3. Our findings reveal an important contribution of plasma fibronectin in lung metastasis. Further, they suggest that the previously noted effects of blood clotting on lung metastasis may be mediated in part by a fibronectin-αvβ3 integrin axis, where plasma fibronectin has to be incorporated into blood clot.
Keywords: plasma fibronectin, fibrin, metastasis, integrin αvβ3, MT1-MMP
INTRODUCTION
Metastasis to distant organs is the most critical complication of malignancies, but our understanding of the molecular mechanisms that govern tumor dissemination is still incomplete. After entering the blood circulation at the primary site, metastatic tumor cells attach within the vasculature of the target organ and invade the surrounding tissue (1, 2). Following initial tumor cell arrest in the vasculature of a distant organ, clotted plasma and platelets cooperatively stabilize circulating tumor cells by generating a thrombus that allows tumor cells to attach and spread at the vessel wall (3). Inhibiting blood clotting by blocking platelet activation and fibrin formation reduces metastasis (4). Metastasis is also reduced in knockout mice that lack fibrinogen as well as in mice that are deficient in coagulation factor XIII, which covalently crosslinks fibrin (5, 6).
Plasma fibronectin (pFN) is one of the most abundant adhesion proteins in the blood and a predominant adhesive component of clotted plasma (7, 8). pFN is incorporated into fibrin clots where FXIII covalently links it to fibrin (7). The recruitment of pFN to clotted plasma represents a major functional modification, because pFN is recognized by a large group of cell adhesion receptors, many of which are members of the integrin family (9). Tumor cells are known to express a variety of fibronectin-binding integrins and some of them, such as integrin αvβ3, have been shown to support tumor growth and metastasis (10, 11). Significantly, tumor cells that express activated αvβ3 metastasize aggressively and inhibiting integrin αvβ3 with antibodies or RGD-containing peptides inhibits metastasis (11–13).
Fibronectin has been found to be up-regulated in several types of malignant tumors and its expression positively correlates with an invasive and metastatic phenotype (14–16). Here, we sought to study the role of pFN for tumor metastasis. While complete loss of fibronectin is embryonal lethal, pFN-deficient mice generated by postnatally deleting the liver fibronectin gene, provide an opportunity to do so (17). Using these mice, we demonstrate that pFN supports tumor cell retention in the lungs. We further show that pFN is required for prometastatic functions of blood clotting and tumor cell integrin αvβ3. Paralleling the in vivo results, we find that tumor cell adhesion to and invasion in fibrin complexed with fibronectin (FibFN) is predominantly mediated by integrin αvβ3, which in turn is activated by FibFN. Collectively, our results establish an important role for pFN in lung metastasis.
MATERIALS AND METHODS
Metastasis Model
Transgenic C57BL/6-Fn(fl/fl) Mx-Cre+ mice become pFN-deficient by postnatally deleting the fibronectin gene in the liver using poly(I):poly(C) (17). To induce metastasis, 5 × 105 B16F1 melanoma or 3LL Lewis lung carcinoma cells (American Type Culture Collection) were injected into the tail vein (i.v.) of transgenic pFN-deficient Fn(fl/fl) Mx-Cre+ mice and their wildtype littermates. Mice were euthanized 10–14 d after tumor cell injection and tumor nodules were counted on the surface of lungs using a stereomicroscope (Leica M50). To inhibit thrombin function, we injected 500 IU hirudin (Calbiochem) with the tumor cell suspension. To inhibit PAR1, we added 10 µg/ml SCH 79797 (Tocris Biosciences) to the tumor cell suspension 30 minutes prior to tail vein injection (18, 19). To inhibit tumor cell integrins, B16F1 cells were incubated with 100 µg/ml of anti-α4 (clones R1–2 and 9C10, BD Biosciences), anti-α5 (clone BMA5, Millipore) or anti-β3 (clone 2C9.G2, BD Biosciences) function blocking antibodies for 30 minutes at 4°C. Tumor cells were washed with PBS prior to i.v. injection in order to remove excess antibody. For histological analysis, tissues were fixed in 4% paraformaldehyde (PFA) and underwent conventional processing for histology. The blocks were cut at 5 µm and stained with H&E or goat anti-mouse CD31 antibody (Santa Cruz Biotechnology Inc.).
Tumor Cell Arrest
To analyze tumor cell arrest, mice were i.v. injected with Cytotracker Green-labeled B16F1 cells (Invitrogen) and perfused through the heart after 1, 4 and 16 hours. Lung tissue was removed, fixed with 4% PFA and analyzed by fluorescence microscopy (Zeiss Axioplan 2). Images were processed with Adobe Photoshop® (Adobe Systems Incorporated). Tumor homing was assessed by counting green-fluorescent B16F1 cells in random optical fields of lung tissue sections from Fn(fl/fl) Mx-Cre+ and wildtype mice. To assess co-localization of green-fluorescent B16F1 cells with fibrin, PFA-fixed lung tissues were stained with biotinylated mouse fibrin(ogen) antiserum (Nordic), followed by streptavidin Alexa Fluor 594 (Invitrogen). Nuclei were stained with DAPI-containing mounting media (Vectashield).
Preparation of Clot Products
Clotted plasma was produced from human citrated plasma (US Biological, Swampscott, MA). pFN-depleted plasma was generated by passing the plasma through sepharose CL4B connected in tandem with a gelatin-agarose column (20). Thawed plasma containing 0.1 M ε-aminocaproic acid (ε-ACA) was added to the column equilibrated with TEC binding buffer (0.05 M Tris-HCl, 0.05M ε-ACA, 0.02 M sodium citrate) and column flow-through was collected as pFN-depleted plasma. Flow-through from the CL4B column was used as pFN-positive control. Collected plasma was clotted with 20 mM CaCl2 overnight at 4°C. To produce fibrin, 2 mg/ml fibrinogen (Enzyme Research Laboratories Inc., South Bend, IN) was mixed with 2.5 mM CaCl2, 2.5 U/ml thrombin and 25 µg/ml Coagulation Factor XIII (Enzyme Research Laboratories). To generate FibFN, 200 µg/ml pFN (EMD Chemicals, Inc., Gibbstown, NJ) was added to the fibrin mixture prior to thrombin. Fibrin and FibFN samples were placed at 4°C and allowed to clot overnight. Then plasma, pFN-deficient plasma, FibFN and fibrin clots were washed in PBS and solubilized in a buffer containing 8 M urea, 2 % SDS, 2 % β-mercaptoethanol and 0.16 M Tris, pH 6.8 (21). To eliminate the solubilization buffer, the solubilized clot material was exhaustively dialyzed against PBS.
Cell Adhesion Assay
Cell adhesion was measured using B16F1, 3LL or THP-1 cells (American Type Culture Collection). THP-1 cells were treated with 160 nM phorbol myristate acetate (PMA; Sigma-Aldrich, St. Louis, MO) to induce macrophage differentiation (22). 48-well plates (Costar, polystyrene, non-tissue culture treated) were coated with 10 µg/ml of fibrinogen or solubilized clot material from plasma (+/− pFN), FibFN or fibrin and incubated at 4°C over night. The plates were then washed with PBS and blocked with 1 % BSA (1 hour, 37°C). Cells were suspended in HEPES-Tyrode's buffer containing 0.1% BSA and 2 mM CaCl2, added to the plate at 2 × 105 in 200 µl/well and allowed to attach for 45–60 minutes (37°C, 5% CO2). Plates were washed to remove floating cells. Attached cells were incubated with para-nitrophenol phosphate (5 mg/ml in 50 mM sodium acetate, 1% Triton X-100, pH 5.2) for 30 minutes and quantified at 405 nm after adding 0.3 M sodium hydroxide. For integrin inhibition, cells were incubated with 100 µg/ml of function blocking anti-α4 (clones R1–2 and 9C10), anti-α5 (clone BMA5), anti-β3 (clone 2C9.G2) or isotype controls on ice for 15 minutes prior to plating.
Flow Cytometry Assay
THP-1 cells were suspended in HEPES-Tyrode's buffer containing 0.1% BSA, 2 mM CaCl2, 1 mM MgCl2, and incubated with antibody specific for αvβ3 (LM609; Millipore), activated αvβ3 (Wow1) (23) or an isotype control (BD Biosciences) in the presence or absence of FibFN (100 µg/ml) ± 10 mM EDTA for 30 minutes at room temperature. Cells were washed with ice cold buffer, and incubated for 30 minutes on ice with Alexa Fluor 488 anti-mouse F(ab’)2 (Invitrogen). Cell viability was monitored by staining cells with 5 µg/ml propidium iodide (Roche Applied Science, Indianapolis, IN). Fluorescence was examined on 15,000 viable cells per sample using a tabletop cytometer (Beckman Coulter).
Invasion Assay
5×104 cells were mixed with 2 mg/ml fibrinogen, 2 mM CaCl2 and 25 µg/ml FXIII with or without 200 µg/ml pFN. Clotting was induced with 2.5 U/ml thrombin. Clot-embedded cells were incubated with DMEM medium supplemented with 10% fetal bovine serum (Mediatech, Inc., Herndon, VA) and Gentamycin (Invitrogen) at 37°C under a humidified, 5% CO2 atmosphere. At designated time points, clots were analyzed for cell invasion at designated areas by phase contrast microscopy (Nikon Eclipse TS100; 20× magnification). Invadopodia formation was classified as complete (i.e. elongated or stellate shape) or incomplete (i.e. round shape with or without rudimentary invadopodia) and calculated for completely spread cells as % of total. Where indicated, cell suspensions were pre-incubated with 100 µg/ml anti-β3 or anti-hamster isotype antibody for 15 minutes on ice prior to thrombin addition and clot formation. Serine protease and matrix metalloproteinase activity was inhibited by adding 1 mg/ml ε-aminocaproic acid (Sigma-Aldrich), 10 µg/ml aprotinin, 25 µM GM6001 (EMD Chemicals, Gibbstown, NJ), 1 µg/ml recombinant TIMP1 or TIMP2 (R&D systems, Minneapolis, MN) to the cell suspension immediately prior to thrombin addition, as well as to the culture medium. Cell invasion in the absence of Factor XIII was assessed by embedding the cells in clots prepared with FXIII-reduced fibrinogen (‘Peak 1’ Fibrinogen, Enzyme Research Laboratories).
siRNA Mediated Gene Silencing
B16F1 cells were plated at 2×103 cells/cm2 and grown for 24 hours prior to transfection with 50 nM integrin β3 (Dharmacon On-TARGETplus SMARTpool L-040746-01; Lafayette, CO), MT1-MMP (Dharmacon On-TARGETplus SMARTpool L-062241-00) or non-targeting control (Dharmacon On-TARGETplus D-001810-10) siRNA. Cells were transfected in Opti-MEM medium (Invitrogen) using LipofectAMINE 2000 reagent (Invitrogen) according to manufacturer instructions. After 6 hours, cells were placed in normal culture medium and grown for an additional 66 hours. Cells were then harvested using 5 mM EDTA, washed twice with PBS and embedded in clot as described above.
Statistical Analysis
All values were analyzed using Student’s t test and are expressed as mean ± S.E.. Treatment differences with a two-sided p value < 0.05 were considered significantly different.
RESULTS
pFN promotes lung metastasis
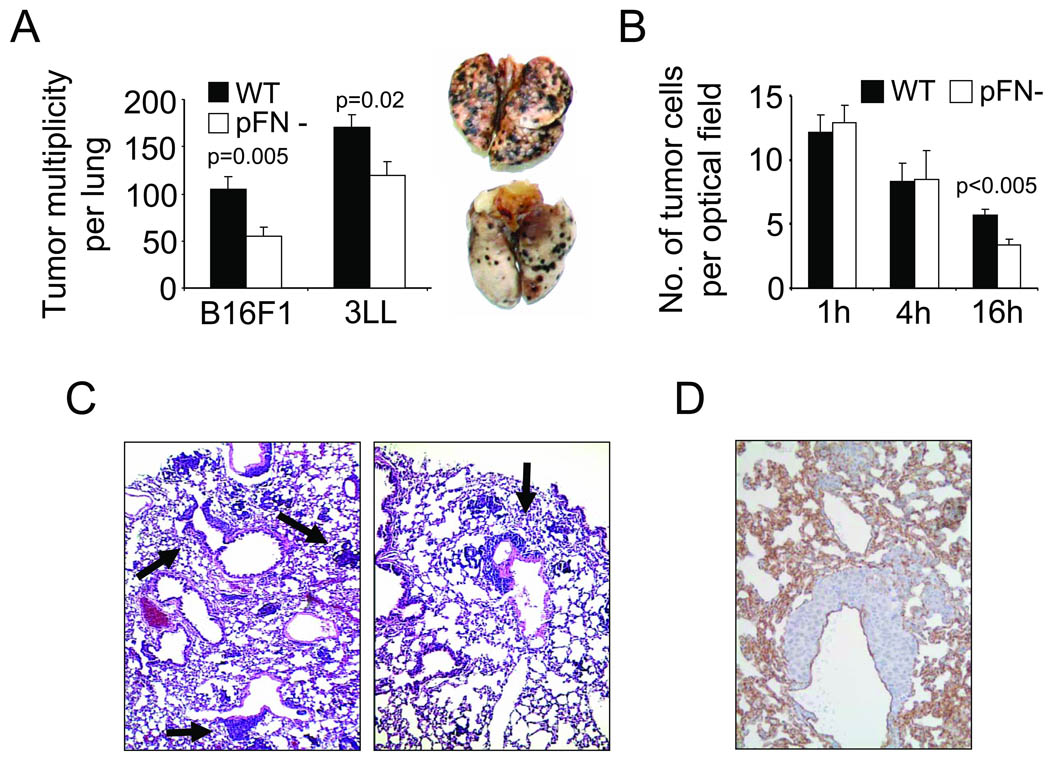
Transgenic C57BL/6-Fn(fl/fl) Mx-Cre mice become pFN-deficient by postnatally deleting the fibronectin gene in the liver (17). We chose B16 melanoma (B16F1) and Lewis Lung carcinoma cells (3LL) as metastasis models because blood clotting promotes lung metastasis from these cell lines (24). We found that lung metastasis from B16F1 melanoma or 3LL lung carcinoma cells injected into the tail vein was significantly reduced in the pFN-deficient mice (Fig. 1A). pFN had no effect on melanoma metastasis to the liver, adrenal gland, kidney and ovaries (not shown). Absence of pFN did not impair initial tumor cell arrest, but 16 hours after the injection, the number of tumor cells was significantly reduced in the lungs of the pFN-deficient mice compared to their normal littermates (Fig. 1B). The same pattern of tumor cell retention was observed in fibrinogen-deficient mice compared to wildtype mice (Supplementary Figure 1). Even though lung metastasis was reduced after two weeks in pFN-deficient mice, there was no obvious qualitative difference between lung histologies from wildtype and pFN-deficient mice as lungs in both groups showed a similar pattern of metastatic lesions at typical locations near distal alveoli and bronchioli (Fig. 1C–D). Metastatic lesions were localized outside of blood vessels indicating prior tumor cell extravasation (Fig. 1D). Together, our results indicate that pFN supports metastasis by promoting the retention of tumor cells in the lungs.
Fig. 1. pFN promotes lung metastasis.
(A), metastatic lesions were counted on lungs of wildtype (WT) and pFN-deficient (pFN-) mice two weeks after i.v. injection of 5 × 105 B16F1 melanoma or 3LL Lewis lung carcinoma cells. (A, inset), representative WT (upper) and pFN- lungs (lower) from B16F1 melanoma. (B), lungs from WT and pFN-deficient mice were isolated 1, 4 and 16 hours after i.v. injection with Cytotracker™-labeled B16F1 cells. Tumor cells were counted by fluorescence microscopy in random optical fields. (C), metastatic lesions (arrows) in H&E-stained lung histologies from WT (left panel) and pFN-deficient mice (right panel) two weeks after i.v. injection with B16F1. (D), representative lung histology depicting metastatic lesions outside of the pulmonary vasculature. Blood vessels were visualized by immunohistochemistry for CD31 (brown).
pFN is required for the prometastatic activity of blood clotting
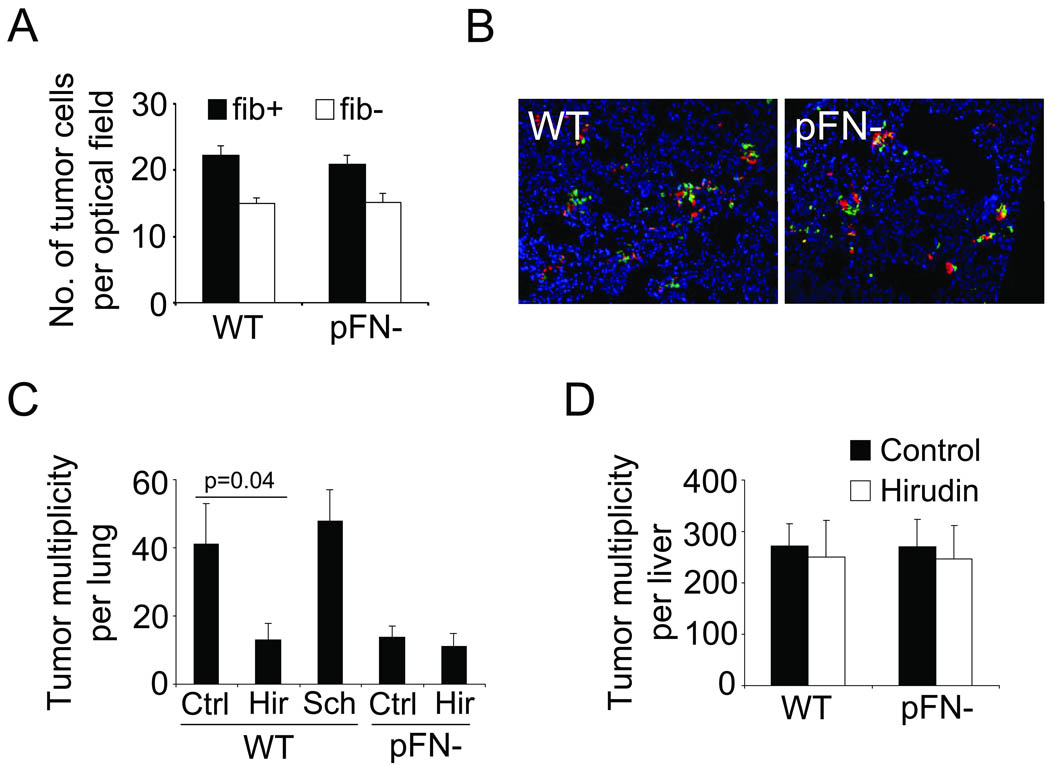
pFN is an important component of plasma clots, which form a provisional extracellular matrix around tumor cells in the lung vasculature. To determine if pFN facilitates clot formation around tumor cells, tissue sections from lungs of wildtype and pFN-deficient mice were probed for co-localization of tumor cells with fibrin(ogen) in fluorescence microscopy. The results revealed no difference in clot formation around circulating tumor cells in the lung vasculature whether pFN was present or not (Fig. 2A–B). To test if pFN requires clotting activity for its prometastatic function, blood clotting was inhibited with the thrombin antagonist hirudin at the time of tumor cell injection. Hirudin reduced lung metastasis in wildtype mice but did not further reduce the already lower rate of metastasis in pFN-deficient mice (Fig. 2C). This effect of hirudin was the result of inhibiting platelet activation and fibrin formation, because blockade of the thrombin receptor PAR1 on tumor and endothelial cells using SCH 79797 did not diminish lung metastasis (Fig. 2C). Interestingly, thrombin inhibition with hirudin had no effect on liver metastasis in wildtype or pFN-deficient mice (Fig. 2D). Together, these results demonstrate that pFN is required for the prometastatic activity of blood clotting in the lung in vivo.
Fig. 2. pFN requires blood clotting for its prometastatic activity.
(A), tumor cells that associate with fibrin (fib +) and those that do not (fib −) were counted in random optical fields of lung tissue sections isolated 1 hour after B16F1 injection. (B), representative micrographs (20× magnification) show the presence of fibrin (red) in lungs of WT and pFN-deficient mice 1 hour after the i.v. injection of Cytotracker™-labeled B16F1 cells (green). Complete co-localization of tumor cells and fibrin appears yellow. Nuclei are stained with DAPI (blue). (C–D), i.v. injection of B16F1 cells into WT or pFN-deficient mice in the presence of 500 IU hirudin (Hir), 10 µg/ml SCH 79797 (Sch) or vehicle (Ctrl). Metastatic lesions were counted on lung (C) and liver (D) isolated two weeks later.
pFN acts through tumor cell integrin αvβ3
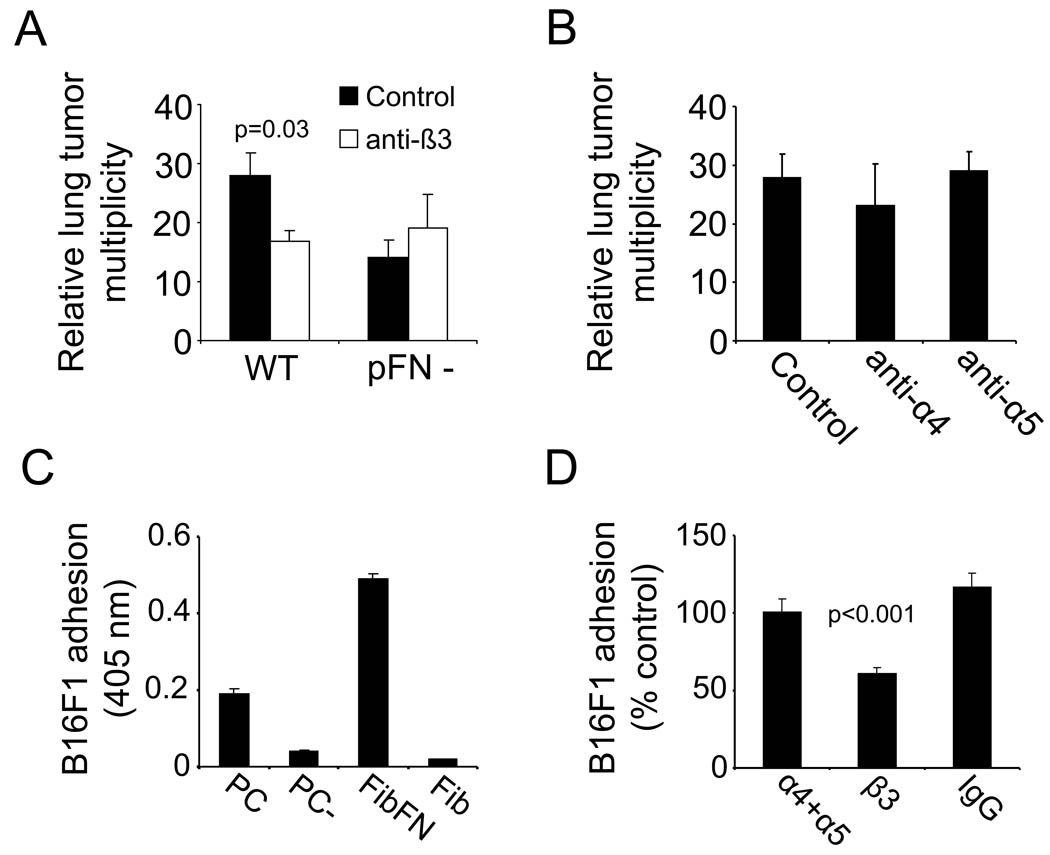
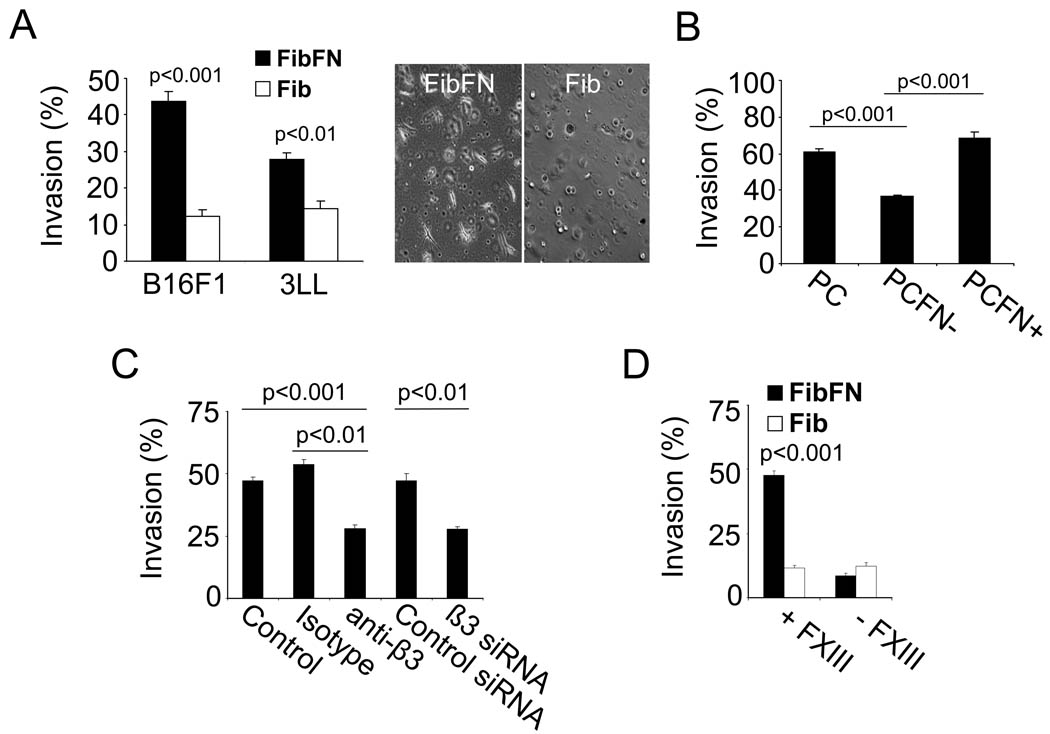
To define the role of fibronectin-binding integrins in the formation of lung metastases, we used antibodies to block α4β1, α5β1 and αvβ3 integrins on B16F1 cells and studied the ability of the cells to form metastases. Washing the antibody-treated cells prior to the injection allowed us to block tumor cell integrins without interfering with the adhesive properties of platelets or other blood cells. Only the anti-β3 integrin antibody (αvβ3 blockade) caused significant inhibition of lung metastasis (Fig. 3A–B). Notably, the β3 blockade had no effect on metastasis in pFN-deficient mice, indicating that a pFN-αvβ3-interaction is important to tumor cell retention in the lungs. Anti-β3-treatment had no effect on clot formation around tumor cells (Supplementary Figure 2). We next employed in vitro assays to analyze the adhesive interactions of tumor cells on surfaces coated with clotted material from complete plasma, plasma depleted of fibronectin, FibFN, or fibrin alone. Paralleling the in vivo results, we found that B16F1 cells bound to clotted plasma and fibrin when fibronectin was included, but not in its absence (Fig. 3C). Antibody inhibition showed that B16F1 adhesion to the FibFN complexes was mediated by integrin αvβ3 (Fig. 3D). In contrast, inhibition of integrins α4 and α5 had no effect on B16F1 adhesion to FibFN. Together, our results underscore the important role of integrin αvβ3 in promoting adhesive interactions of B16F1 cells with FibFN in vitro and for pFN-mediated lung metastasis in vivo.
Fig. 3. pFN acts through tumor cell integrin αvβ3.
(A), number of metastatic lesions on lungs of WT and pFN-deficient mice two weeks after i.v. injection of anti-β3 integrin treated B16F1 cells (100 µg/ml). (B) B16F1 cells were incubated with antibodies against integrin α4 and α5 (100 µg/ml) prior to i.v. injection into WT mice and metastatic lesions were counted two weeks after tumor cell injection. (C), B16F1 cells were allowed to attach to plates coated with solubilized clot materials from plasma (PC), pFN-depleted plasma (PC-), FibFN or fibrin (Fib) (ea. 10 µg/ml). (D), adhesion of B16F1 cells to plates coated with FibFN was tested in the presence of 100 µg/ml function blocking antibody against α4, α5, β3 integrin or control IgG in correlation to adhesion of untreated B16F1 cells.
FibFN activates integrin αvβ3
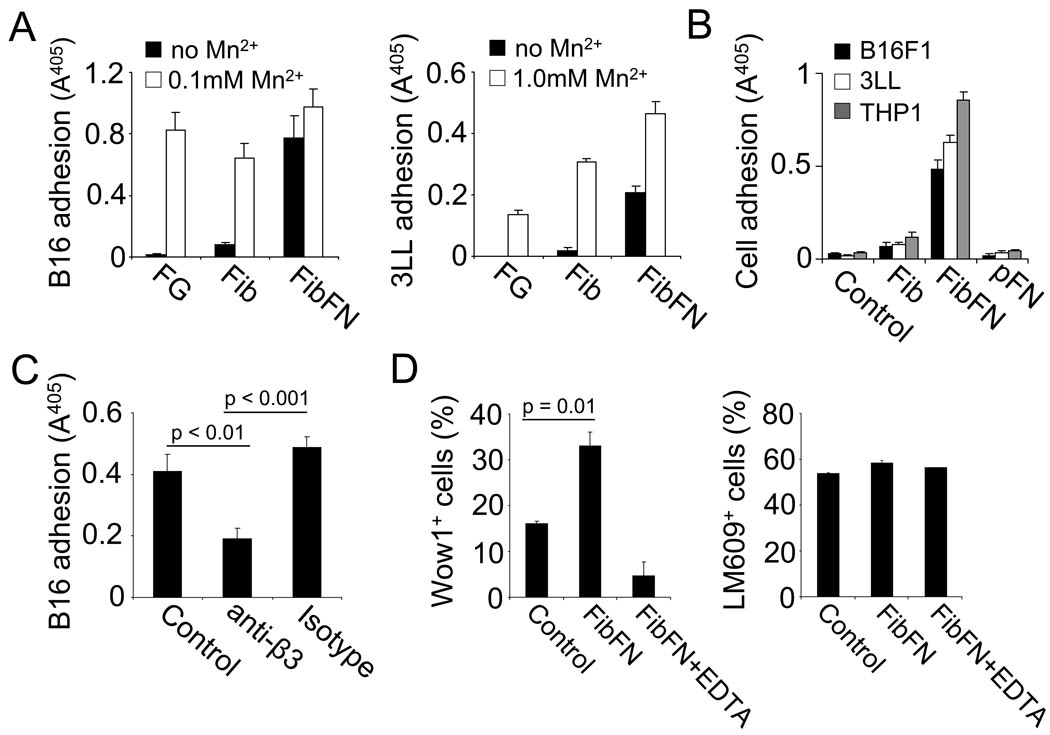
B16F1 and 3LL cells require integrin activation with manganese to adhere on the αvβ3-specific substrates fibrin or fibrinogen, whereas attachment to FibFN complexes occurred spontaneously (Fig. 4A). Notably, addition of FibFN complexes to the binding buffer induced adhesion of B16F1, 3LL and THP-1 cells to immobilized fibrinogen (Fig. 4B). Soluble pFN or fibrin complexes were not sufficient to induce tumor cell attachment. FibFN-induced cell adhesion was significantly inhibited in the presence of a function-blocking antibody against β3 integrin (Fig. 4C). To further explore the effect of FibFN on αvβ3-mediated cell adhesion, we incubated THP-1 cells with Wow1 antibody, which is specific for activated integrin αvβ3 (23). Analyzing THP-1 cells by flow cytometry, we found that Wow1 binding was significantly increased in the presence of FibFN and was reversed when we added FibFN together with EDTA (Fig. 4D). We did not detect any difference in αvβ3 cell surface expression following FibFN treatment. To identify mediators that resemble the effect of FibFN on αvβ3, we screened a number of established integrin agonists for their ability to induce B16F1 cell adhesion to fibrinogen (Supplementary Figure 3). In addition to FibFN, B16F1 cell adhesion to fibrinogen was only induced by PMA and thrombin. Addition of the thrombin inhibitor PPACK reversed thrombin- but not FibFN-induced adhesion. Importantly, out of the group of compounds positive for B16F1 adhesion, only FibFN was sufficient to induce THP-1 cell adhesion as well. Together, our results indicate that FibFN provides a unique stimulus that results in activation of integrin αvβ3.
Fig. 4. FibFN promotes tumor cell adhesion.
(A), B16F1 (left panel) and 3LL cells (right panel) were allowed to attach to plates coated with fibrinogen (FG), fibrin (Fib), or FibFN (ea. 10 µg/ml) in the presence or absence of manganese sulfate (Mn2+). (B), B16F1, 3LL, or THP-1 cells were suspended in HEPES-Tyrode's buffer alone (Control) or in the presence of 100 µg/ml of Fib, FibFN, or pFN and tested for adhesion to fibrinogen-coated plates. (C), FibFN-induced adhesion of B16F1 cells to fibrinogen-coated plates was tested in the presence of 100 µg/ml function blocking antibody against β3 integrin (anti-β3) or control IgG (isotype). (D), THP1 cells suspended in HEPES-Tyrode's buffer (+ 2 mM Ca2+/1 mM Mg2+) were incubated with Wow1 antibody to determine integrin αvβ3 activation (left panel) or with LM609 antibody to determine αvβ3 cell surface expression (right panel) in the presence of 100 µg/ml FibFN, FibFN + 10 mM EDTA or buffer alone (Control). Antibody binding was assessed by flow cytometry.
FibFN promotes tumor cell invasion
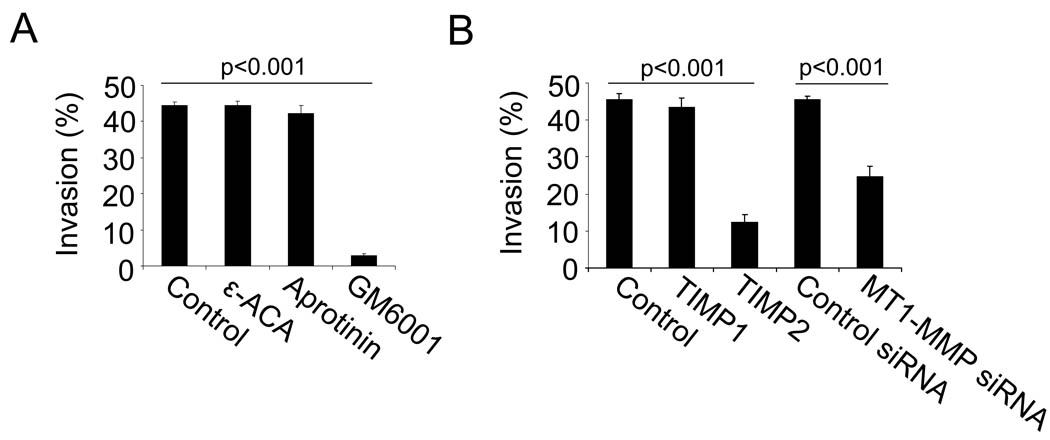
To determine the role of FibFN on tumor cell invasion, we embedded B16F1 and 3LL cells in 3 dimensional gels of fibrin with or without pFN. After 16 hours, 44 % of B16F1 and 28 % of 3LL cells formed invadopodia in FibFN but only 10–15 % in fibrin (Fig. 5A). Invadopodia formation was also significantly reduced when B16F1 cells were embedded in fibronectin-depleted plasma clot (Fig. 5B). FibFN-induced invasion was reduced after β3 integrin inhibition and abolished in absence of FXIII, which cross-links fibronectin to fibrin (Fig. 5C–D). FXIII was not necessary for HUVEC and DU145 cells, which do not require the presence of pFN to invade fibrin (Supplementary Figure 4). We also assessed invadopodia formation in the presence of the matrix metalloproteinase inhibitor GM6001, TIMP2 or MT1-MMP-siRNA, which all caused significant inhibition of FibFN-induced invasion (Fig. 6A–B). TIMP1 as well as the serine protease inhibitors aprotinin and ε-aminocaproic acid had no such effect. Embedding B16F1 cells in FibFN gels had no effect on cell survival or proliferation (not shown). Together, our results indicate that FibFN promotes tumor cell invasion into blood clots via integrin αvβ3 and MT1-MMP.
Fig. 5. FibFN promotes tumor cell invasion.
(A), B16F1 or 3LL cells were embedded in a 3-dimensional matrix of FibFN or fibrin (Fib) and analyzed for invadopodia formation by phase contrast microscopy after 16 hours. (A, inset), representative micrographs (20×) of embedded B16F1 cells after 16 hours. (B), invasion of B16F1 cells after 16 hours in clotted plasma (PC), fibronectin-depleted clotted plasma (PCFN−), and PCFN-containing 100 µg/ml pFN (PCFN+). (C), invasion of FibFN-embedded B16F1 cells after treatment with function blocking antibody and siRNA against integrin αvβ3. (D), invasion of FibFN- and Fib-embedded B16F1 cells in the presence or absence of FXIII.
Fig. 6. FibFN-mediated tumor cell invasion depends on MT1-MMP.
(A–B), B16F1 cells were embedded in a 3-dimensional matrix of FibFN and analyzed for invadopodia formation after 16 hours. (A), invasion of FibFN-embedded B16F1 cells after treatment with ε-aminocaproic acid, aprotinin or the broad spectrum MMP inhibitor GM6001. (B), invasion of FibFN-embedded B16F1 cells after treatment with TIMP1, TIMP2 or MT1-MMP siRNA.
DISCUSSION
We have studied tumor metastasis in pFN-deficient mice and show that pFN supports the retention of tumor cells in the lungs. The pro-metastatic effect of pFN is linked to clotting activity and mediated by tumor cell integrin αvβ3 in vivo. We attributed this novel function of pFN to the capacity of FibFN to activate integrin αvβ3 and, thus, to promote tumor cell adhesion to and invasion into clotted plasma in vitro.
Deposition of fibronectin into the extracellular matrix has been shown to support tumor cell proliferation and angiogenesis, which are crucial steps in the completion of metastasis (25, 26). Our results show that pFN supports lung metastasis at an earlier stage of metastasis when tumor cell fate is determined by the ability of circulating tumor cells to survive in and extravasate from the pulmonary vasculature. Tumor cell survival in the lung vasculature is largely dependent on clot formation, which protects tumor cells from the cytotoxic activity of natural killer cells (4, 6). In addition, clotting has been shown to promote tumor cell adhesion and extravasation (3, 27). The pivotal role of clotting has been demonstrated in several models of spontaneous and experimental lung metastasis (6, 28, 29). Blood clots contain large amounts of pFN, which is cross-linked to fibrin (7). Using the thrombin antagonist hirudin to inhibit clot formation, we found that the prometastatic function of clots depends at least in part on the presence of pFN. In agreement with previous studies, this cooperation between pFN and clotting was important for lung metastasis but was not relevant for metastasis to the liver, where circulating tumor cells have direct access to fibronectin and other extracellular matrix components expressed in the endothelial basement membrane of liver sinusoidal blood vessels (30–32). The initial tumor cell arrest in the lung vasculature, in contrast, is mostly initiated by adhesive interactions with the endothelium suggesting that basement membrane proteins critical for tumor cell survival and extravasation are not immediately accessible (1, 33). In this environment, tumor cells appear to be unable to compensate for the lack of pFN or the inability to form clots.
The association of pFN with fibrin has been shown to promote cell adhesion to clotted plasma (21). Despite this proadhesive function, pFN did not affect the initial tumor cell arrest. This result was not unexpected because soluble pFN is inactive and clots form only after tumor cells adhere to the lung vasculature (3, 34). One of the effects of blood clots is to sustain tumor cell adhesion, which involves integrin activation as tumor cells begin to spread alongside the lung vasculature (3). Our results indicate that adhesive interactions of tumor cells with clotted plasma are uniquely mediated by integrin αvβ3, thus providing a possible explanation for the prometastatic activity of this adhesion receptor. Integrin αvβ3 recognizes a plethora of ligands including fibrin, which is the predominant adhesion protein in clotted plasma (35, 36). Yet, the efficacy of αvβ3 to mediate tumor cell adhesion, invasion and metastasis decreased significantly when pFN was absent from clotted plasma. Based on this finding, we concluded that incorporation of pFN into blood clots promotes metastasis by enhancing the adhesive function of αvβ3 towards clotted plasma. Moreover, we found that FibFN is important for tumor cells as it leads to activation of αvβ3. Activated integrins exhibit a conformational state of increased affinity for their ligands (23). The interaction of integrin αvβ3 with its ligands promotes survival, and can also induce proliferation of metastatic tumor cells (37). However, FibFN had no effect on tumor cell growth suggesting the primary function of clotted plasma is to prepare tumor cells for extravasation. Blood clots have been shown to promote endothelial retraction and, thus, to provide access to the pulmonary basement membrane (27). In order to reach the basement membrane, tumor cells have to invade the surrounding layer of clot. Our results demonstrate that the invasion of blood clots requires FibFN-mediated activation of integrin αvβ3 and the proteolytic activity of MT1-MMP.
In solution, pFN has a compact conformation that limits accessibility to cryptic binding sites for integrins and cell surface proteoglycans buried within the molecule (38). Our results suggest that cross-linking pFN to fibrin via coagulation factor XIIIa gives access to a binding site in pFN that activates integrin αvβ3. It is unlikely that integrin activation is the result of a pFN-binding protein because pFN remained inactive in absence of FXIIIa, which itself had no effect on αvβ3 (Supplementary Figure 3). Moreover, we found that FibFN was unique in its ability to activate αvβ3 when compared to a number of known integrin activators or FN-binding proteins (39–44). FibFN-induced cell adhesion and metastasis did not depend on crosstalk with the fibronectin-binding integrins α4β1 and α5β1. However, it is conceivable that FibFN could activate αvβ3 through ligation of the fibronectin receptors CD26 and CD44 or as a result of cryptic protein disulfide isomerase activity near the C-terminus of fibronectin (45–50). Identifying the molecular mechanism of FibFN binding to tumor cells will expand our understanding of metastasis and may provide a novel target for the development of anti-metastatic treatments.
Supplementary Material
ACKNOWLEGDEMENTS
We thank Dr. Reinhard Faessler (Max Planck Institute for Biochemistry, Martinsreid, Germany) and Dr. Jay Degen (University of Cincinnati, Cincinnati, OH) for knockout mice, Dr. Sanford Shattil (University of California San Diego, San Diego, CA) for Wow1 antibody and Marie Acquafondata and Marianne Notaro for expert help with histologies.
This work was supported by CA119335 (ER), CA134330 (JP), and Cancer Center Support Grant CA30199 from the National Cancer Institute.
REFERENCES
- 1.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 2.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Im JH, Fu W, Wang H, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res. 2004;64:8613–8619. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 4.Gorelik E, Bere WW, Herberman RB. Role of NK cells in the antimetastatic effect of anticoagulant drugs. Int J Cancer. 1984;33:87–94. doi: 10.1002/ijc.2910330115. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo JS, Barney KA, Blevins EA, et al. Factor XIII transglutaminase supports hematogenous tumor cell metastasis through a mechanism dependent on natural killer cell function. J Thromb Haemost. 2008;6:812–819. doi: 10.1111/j.1538-7836.2008.02938.x. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 7.Aeschlimann D, Mosher D, Paulsson M. Tissue transglutaminase and factor XIII in cartilage and bone remodeling. Semin Thromb Hemost. 1996;22:437–443. doi: 10.1055/s-2007-999043. [DOI] [PubMed] [Google Scholar]
- 8.Ruoslahti E. Fibronectin in cell adhesion and invasion. Cancer Metastasis Rev. 1984;3:43–51. doi: 10.1007/BF00047692. [DOI] [PubMed] [Google Scholar]
- 9.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 10.Felding-Habermann B, Mueller BM, Romerdahl CA, Cheresh DA. Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J Clin Invest. 1992;89:2018–2022. doi: 10.1172/JCI115811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felding-Habermann B, O'Toole TE, Smith JW, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries MJ, Olden K, Yamada KM. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986;233:467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- 13.Mitjans F, Sander D, Adan J, et al. An anti-alpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J Cell Sci. 1995;108:2825–2838. doi: 10.1242/jcs.108.8.2825. [DOI] [PubMed] [Google Scholar]
- 14.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 15.Lal A, Lash AE, Altschul SF, et al. A public database for gene expression in human cancers. Cancer Res. 1999;59:5403–5407. [PubMed] [Google Scholar]
- 16.Yao ES, Zhang H, Chen YY, et al. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 17.Sakai T, Johnson KJ, Murozono M, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 18.Ahn HS, Foster C, Boykow G, Stamford A, Manna M, Graziano M. Inhibition of cellular action of thrombin by N3-cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3, 2-f]quinazoline-1,3-diamine (SCH 79797), a nonpeptide thrombin receptor antagonist. Biochem Pharmacol. 2000;60:1425–1434. doi: 10.1016/s0006-2952(00)00460-3. [DOI] [PubMed] [Google Scholar]
- 19.Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. SCH 79797, a selective PAR1 antagonist, limits myocardial ischemia/reperfusion injury in rat hearts. Basic Res Cardiol. 2007;102:350–358. doi: 10.1007/s00395-007-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruoslahti E, Hayman EG, Pierschbacher M, Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82:803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- 21.Corbett SA, Wilson CL, Schwarzbauer JE. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996;88:158–166. [PubMed] [Google Scholar]
- 22.Matikainen S, Hurme M. Comparison of retinoic acid and phorbol myristate acetate as inducers of monocytic differentiation. Int J Cancer. 1994;57:98–103. doi: 10.1002/ijc.2910570118. [DOI] [PubMed] [Google Scholar]
- 23.Pampori N, Hato T, Stupack DG, et al. Mechanisms and consequences of affinity modulation of integrin alpha(V)beta(3) detected with a novel patch-engineered monovalent ligand. J Biol Chem. 1999;274:21609–21616. doi: 10.1074/jbc.274.31.21609. [DOI] [PubMed] [Google Scholar]
- 24.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 25.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkan D, Kleinman H, Simmons JL, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honn KV, Tang DG, Grossi IM, et al. Enhanced endothelial cell retraction mediated by 12(S)-HETE: a proposed mechanism for the role of platelets in tumor cell metastasis. Exp Cell Res. 1994;210:1–9. doi: 10.1006/excr.1994.1001. [DOI] [PubMed] [Google Scholar]
- 28.Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004;104:2746–2751. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 29.Palumbo JS, Potter JM, Kaplan LS, Talmage K, Jackson DG, Degen JL. Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res. 2002;62:6966–6972. [PubMed] [Google Scholar]
- 30.Bruggemann LW, Versteeg HH, Niers TM, Reitsma PH, Spek CA. Experimental melanoma metastasis in lungs of mice with congenital coagulation disorders. J Cell Mol Med. 2008;12:2622–2627. doi: 10.1111/j.1582-4934.2008.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klerk CP, Smorenburg SM, Spek CA, Van Noorden CJ. Colon cancer metastasis in mouse liver is not affected by hypercoagulability due to Factor V Leiden mutation. J Cell Mol Med. 2007;11:561–568. doi: 10.1111/j.1582-4934.2007.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenow F, Ossig R, Thormeyer D, et al. Integrins as antimetastatic targets of RGD-independent snake venom components in liver metastasis [corrected] Neoplasia. 2008;10:168–176. doi: 10.1593/neo.07898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Nakayama J, Ohyama C, et al. Sialyl Lewis X-dependent lung colonization of B16 melanoma cells through a selectin-like endothelial receptor distinct from E- or P-selectin. Cancer Res. 2002;62:4194–4198. [PubMed] [Google Scholar]
- 34.Lai CS, Wolff CE, Novello D, et al. Solution structure of human plasma fibronectin under different solvent conditions. Fluorescence energy transfer, circular dichroism and light-scattering studies. J Mol Biol. 1993;230:625–640. doi: 10.1006/jmbi.1993.1174. [DOI] [PubMed] [Google Scholar]
- 35.Chen YP, O'Toole TE, Leong L, Liu BQ, Diaz-Gonzalez F, Ginsberg MH. Beta 3 integrin-mediated fibrin clot retraction by nucleated cells: differing behavior of alpha IIb beta 3 and alpha v beta 3. Blood. 1995;86:2606–2615. [PubMed] [Google Scholar]
- 36.Pilch J, Habermann R, Felding-Habermann B. Unique ability of integrin alpha(v)beta 3 to support tumor cell arrest under dynamic flow conditions. J Biol Chem. 2002;277:21930–21938. doi: 10.1074/jbc.M201630200. [DOI] [PubMed] [Google Scholar]
- 37.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 38.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- 39.Alon R, Cahalon L, Hershkoviz R, et al. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J Immunol. 1994;152:1304–1313. [PubMed] [Google Scholar]
- 40.Banno A, Ginsberg MH. Integrin activation. Biochem Soc Trans. 2008;36:229–234. doi: 10.1042/BST0360229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byzova TV, Goldman CK, Pampori N, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 42.Dormond O, Bezzi M, Mariotti A, Ruegg C. Prostaglandin E2 promotes integrin alpha Vbeta 3-dependent endothelial cell adhesion, rac-activation, and spreading through cAMP/PKA-dependent signaling. J Biol Chem. 2002;277:45838–45846. doi: 10.1074/jbc.M209213200. [DOI] [PubMed] [Google Scholar]
- 43.Nierodzik ML, Klepfish A, Karpatkin S. Role of platelets, thrombin, integrin IIb–IIIa, fibronectin and von Willebrand factor on tumor adhesion in vitro and metastasis in vivo. Thromb Haemost. 1995;74:282–290. [PubMed] [Google Scholar]
- 44.Pelletier AJ, van der Laan LJ, Hildbrand P, et al. Presentation of chemokine SDF-1 alpha by fibronectin mediates directed migration of T cells. Blood. 2000;96:2682–2690. [PubMed] [Google Scholar]
- 45.Cheng HC, Abdel-Ghany M, Pauli BU. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J Biol Chem. 2003;278:24600–24607. doi: 10.1074/jbc.M303424200. [DOI] [PubMed] [Google Scholar]
- 46.Essex DW, Li M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br J Haematol. 1999;104:448–454. doi: 10.1046/j.1365-2141.1999.01197.x. [DOI] [PubMed] [Google Scholar]
- 47.Inamoto T, Yamochi T, Ohnuma K, et al. Anti-CD26 monoclonal antibody-mediated G1-S arrest of human renal clear cell carcinoma Caki-2 is associated with retinoblastoma substrate dephosphorylation, cyclin-dependent kinase 2 reduction, p27(kip1) enhancement, and disruption of binding to the extracellular matrix. Clin Cancer Res. 2006;12:3470–3477. doi: 10.1158/1078-0432.CCR-06-0361. [DOI] [PubMed] [Google Scholar]
- 48.Jalkanen S, Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992;116:817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langenbach KJ, Sottile J. Identification of protein-disulfide isomerase activity in fibronectin. J Biol Chem. 1999;274:7032–7038. doi: 10.1074/jbc.274.11.7032. [DOI] [PubMed] [Google Scholar]
- 50.Lee JL, Wang MJ, Sudhir PR, Chen GD, Chi CW, Chen JY. Osteopontin promotes integrin activation through outside-in and inside-out mechanisms: OPN-CD44V interaction enhances survival in gastrointestinal cancer cells. Cancer Res. 2007;67:2089–2097. doi: 10.1158/0008-5472.CAN-06-3625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.