Abstract
Functional interactions between drugs acting on either opioid or cholinergic systems have been demonstrated for both neurochemical and behavioral measures. This study used schedule-controlled responding and isobolographic analyses to examine interactions between the μ opioid receptor agonist morphine and the muscarinic acetylcholine receptor antagonist scopolamine as well as the nicotinic acetylcholine receptor agonist nicotine. In 8 rats responding under a fixed ratio 5 schedule of food presentation, morphine (3.2-10 mg/kg), scopolamine (0.032-1.0 mg/kg), and nicotine (0.1-1 mg/kg) each dose dependently decreased responding. Acute injection of scopolamine shifted the morphine dose-response curved leftward and downward and acute injection of morphine shifted the scopolamine and nicotine dose-response curves leftward and downward. The interaction between morphine and nicotine was additive; however, the interaction between morphine and scopolamine was infra-additive or supra-additive, depending on whether scopolamine or morphine was administered first. These results provide quantitative evidence regarding potentially important interactions between drugs acting on either opioid or cholinergic systems, although these interactions are modest and appear to depend on the specific conditions of drug administration.
Keywords: morphine, opioid, acetylcholine, nicotine, scopolamine, rat, isobologram, interaction
Introduction
Opioid receptor agonists remain the drugs of choice for treating moderate to severe pain and they also continue to pose a significant public health problem, particularly with recent increases in the abuse of pharmaceuticals. Some effects of opioid receptor agonists are modified by drugs from other classes, and this is one method by which therapeutic effects of opioids might be enhanced, possibly without enhancing unwanted effects. Thus, by combining small doses of opioids with other drugs it might be possible to provide adequate therapeutic effects and reduced adverse effects (e.g., tolerance and dependence). Interactions between drugs acting on opioid and cholinergic systems have been reported for several effects, including hormonal regulation (e.g., De Marinis et al., 1997). Moreover, morphine induced deficits in water maze performance are attenuated by the muscarinic acetylcholine receptor agonist oxotremorine (Li et al., 2001). Oxotremorine also reverses impairment of memory retention of an inhibitory avoidance task in mice caused by the opioid peptide β-endorphin (Introini et al., 1984) and the muscarinic receptor agonist arecoline decreases i.v. morphine self administration in rats (Buccafusco et al., 2007). On the other hand, the nicotinic acetylcholine receptor agonist nicotine blocks morphine induced state-dependent learning (Zarrindast et al., 2006), inhibits the expression of morphine induced conditioned place preference and locomotor hyperactivity (Shams et al., 2006), and attenuates the development of morphine induced tolerance and dependence (Haghparast et al., 2008).
Qualitatively similar interactions have been reported between opioid receptor agonists and cholinergic receptor antagonists. For example, the muscarinic acetylcholine receptor antagonists scopolamine and atropine attenuate morphine induced conditioned place preference (Zhai et al., 2008; Tan et al., 2007; Lu et al., 2002), locomotor hyperactivity (Li et al., 2007; Oka et al.,1976), and the development of tolerance and dependence (Zhou et al., 1999). Moreover, the nicotinic acetylcholine receptor antagonist mecamylamine reverses morphine-induced conditioned place preference (Zarrindast et al., 2003) and a combination of mecamylamine and dextromethorphan decreases morphine self-administration in rats (Glick et al, 2002). Collectively, these studies suggest potentially important interactions between drugs acting on opioid and cholinergic systems; however, the results fail to identify specific mechanisms (e.g., agonism or antagonism) accounting for these interactions.
Comparisons among studies examining opioid/cholinergic interactions are limited both because often the same drugs are not studied across comparable conditions and because full dose-response curves are seldom reported. The current study used schedule-controlled responding in rats to examine interactions between representative drugs acting on μ opioid (morphine) receptors and either muscarinic acetylcholine (scopolamine [antagonist]) or nicotinic acetylcholine (nicotine [agonist]) receptors.
Materials and Methods
Subjects
Eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighed 250-275 g upon arrival and were housed individually on a 12/12-h light/dark cycle (experiments conducted during the light period) with free access to water in the home cage. Access to food was limited to 10 g/day for several days to facilitate lever press training. Thereafter body weights were allowed to increase at a age-appropriate rate then were maintained at 320 g by providing rodent chow (Rodent sterilizable diet, Harlan Teklad, Madison, WI) in the home cage after daily sessions. All animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
Schedule-controlled responding
Experiments were conducted in commercially available chambers (MED Associates Inc., St. Albans, VT, Model #ENV-008CT) located within sound-attenuating, ventilated enclosures (MED Associates Inc., Model #ENV-022M) that are described in detail elsewhere (Carter et al., 2003). Chambers contained two response levers; responses on the inactive (left) lever were recorded and had no programmed consequence. Data were collected using MED-PC IV software and an interface (MED Associates Inc.).
Rats were trained to press a lever for food under a multiple-cycle procedure. Each cycle began with a 10-min pretreatment period, during which the chamber was dark and responses had no programmed consequence, followed by a 5-min response period, during which a light above the active (right) lever was illuminated and rats could receive a maximum of 10 food pellets (45 mg; Research Diets, New Brunswick, NJ) by responding on the active lever. Initially a single response produced a food pellet; as performance improved the response requirement was progressively increased across days to a maximum of fixed ratio 5. The light was terminated after delivery of 10 food pellets or after 5 min had elapsed, whichever occurred first. Daily sessions consisted of 5 cycles and rats had to satisfy the following criteria for five consecutive sessions before testing began: the daily response rate, averaged across all 5 cycles within a session, did not vary by more than ± 20% of the average daily response rate of the previous 5 training sessions; and the average response rate among the 5 cycles of a daily session did not vary by more than ± 20%. After the first test all tests were preceded by at least two consecutive training sessions that satisfied the same criteria.
Before studying drugs in combination, each drug was studied alone using both an acute (single) dosing procedure and a cumulative dosing procedure. For acute dosing tests, rats received a single injection of drug during the first minute of the first cycle and injections of saline in the first minute of each of 4 subsequent cycles. Cumulative dosing tests were conducted as follows: rats received vehicle in the first cycle followed by drug injections in all subsequent cycles with the cumulative dose increasing by 0.5 log unit per cycle (i.e., 15-min inter-injection intervals throughout). For drug combination studies, a single dose of one drug was administered in the first cycle followed by increasing doses of a second drug in subsequent cycles (i.e., one drug was administered 15 min prior to increasing doses of a second drug, with the inter-injection interval always being 15 min). A single dose of scopolamine was administered before increasing doses of morphine and a single dose of morphine was administered before increasing doses of scopolamine or increasing doses of nicotine. The relatively short duration of action of nicotine (see Results) precluded studying single doses of nicotine prior to increasing doses of other drugs. Drugs were studied up to doses (or dose combinations) that decreased responding so that rats received fewer than 5 pellets in a cycle.
Data Analyses
Rate of responding is expressed as a percentage of the control response rate as follows: control response rates for individual rats were determined by averaging rates across cycles to obtain a mean response rate for a training session during which no drug was administered; the mean rates for 5 training sessions were averaged to obtain a control rate for an individual subject. These percentages were averaged across 8 rats (± SEM) and plotted as a function of dose. Time course data for single doses of drugs were analyzed by a two-way ANOVA followed by Bonferroni's test (time and dose as factors; P<0.05). To determine the potency of drugs to decrease responding, the dose of drug needed to decrease response rate to 50% of the corresponding control rate (ED50) was estimated for individual rats using linear regression.
To determine whether the effects of drug combinations were additive, supra-additive, or infra-additive, isobolograms were constructed (Gessner 1988; Lelas et al., 2001) plotting equieffective doses (e.g. ED50) of one drug in the presence of different doses of a second drug. When the effects of two drugs are additive, the ED50 values for the drug combination will not deviate significantly from a diagonal line connecting ED50 values for the two drugs administered alone (i.e., line of additivity). When a drug combination ED50 value is significantly below the line of additivity the interaction is supra-additive (i.e., in the presence of one drug, smaller than expected [i.e., additivity] doses of a second drug are needed to produce the effect). When a drug combination ED50 value is significantly above the line of additivity the interaction is infra-additive (i.e., in the presence of one drug, larger than expected doses of a second drug are needed to produce the effect). The significance of the deviation of individual points from additivity was determined by connecting the error bars of the ED50 values each drug alone (one plotted on the ordinate and the second on the abscissa). When the error bars of the individual points do not overlap with this variance around the line of additivity, the deviation from additivity is considered to be significant (Lelas et al., 2001).
Drugs
The compounds used in this study were morphine sulfate (Research Technology Branch, National Institute on Drug Abuse, Rockville, Md., USA), nicotine [(−) nicotine hydrogen tartrate], and scopolamine hydrobromide (both purchased from Sigma-Aldrich, St Louis, MO, USA). All drugs were dissolved in saline and administered i.p. in a volume of 1.0 ml/kg body weight.
Results
Control Performance
At the beginning of the study the group average (5 determinations for each of 8 subjects) control response rates (± SEM) for the 5 cycles comprising a session were as follows: 0.71 ± 0.02, 0.73 ± 0.03, 0.70 ± 0.03, 0.71 ± 0.03, and 0.71 ± 0.03 responses/second. At the end of the study the group average control response rates were as follows: 0.71 ± 0.02, 0.76 ± 0.03, 0.84 ± 0.03, 0.89 ± 0.03, and 0.87 ± 0.04 responses per second. Thus, the overall mean response rate for the group of 8 rats increased from 0.71 ± 0.03 to 0.81 ± 0.03 responses per second over the course of these studies.
Single Drug Studies
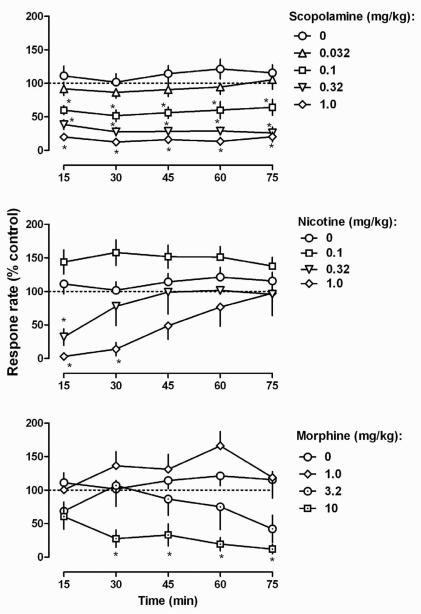
An acute injection of scopolamine dose-dependently decreased responding throughout the 5-cycle, 75-min session (upper panel, Fig. 1). Two-way ANOVA revealed a main effect for time after injection (F [4, 140] =23.60, P<0.0001) and for dose (F [4, 140] =2.66, P<0.05) without a significant interaction (F [16, 140] =0.71, P>0.05). Administration of 0.032 mg/kg scopolamine did not markedly affect rate of responding; however, 1.0 mg/kg scopolamine decreased the rate of responding to less than 20% of control across all 5 cycles.
Fig. 1.
Effects of single injections of scopolamine (upper), nicotine (middle), and morphine (lower) on rate of lever pressing in rats responding under a fixed ratio 5 schedule of food presentation. Abscissa: time in min after i.p. injection. Ordinate: response rate expressed as a percentage of vehicle control rates. Each data point represents the average (± SEM) rate among 8 rats. * = P<.05 compared with saline control rate.
A small dose of nicotine (0.1 mg/kg) increased and larger doses of nicotine decreased responding (middle panel, Fig. 2). For example, a dose of 0.1 mg/kg nicotine increased responding to as much as 150% of the control rate throughout the session, although this change was not statistically significant. In contrast, a still larger dose (1.0 mg/kg) nearly eliminated responding in the first cycle; responding recovered through the session and to control values in the last (fifth) cycle. Two-way ANOVA indicated a main effect for time after injection (F [4, 112] =8.91, P<0.0001), and for dose (F [3, 112] =5.46, P<0.005) as well as a significant interaction (F [12, 112] =3.47, P<0.001).
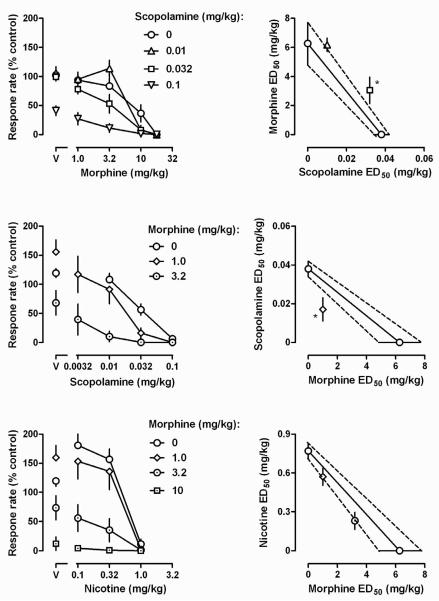
Fig. 2.
Effects of drug combinations on rate of lever pressing (left panels) and corresponding isobolograms of the same data (right panels) as follows: scopolamine and morphine (upper and middle panels]; morphine and nicotine (lower panels). Left panels: abscissa, dose in mg/kg body weight; ordinate, rate expressed as a percentage of vehicle control rates. Right panels: abscissa, ED50 values for scopolamine (upper) and morphine (middle and lower); ordinate, ED50 values for morphine (upper), scopolamine (middle), and nicotine (lower) in mg/kg body weight. Points above “V” represent response rate after vehicle administration. * = outside the estimated line of additivity.
Similarly, a small dose of morphine (1.0 mg/kg) increased responding to more than 160% of the control rate in the fourth cycle, although this increase did not reach statistical significance. A larger dose of morphine (10 mg/kg) significantly decreased responding with a maximum decrease to less than 20% of the control rate occurring in the last (fifth) cycle (lower panel, Fig. 1). Two-way ANOVA revealed a main effect for dose (F [3, 112] =7.57, P<0.001), not for time after injection (F [4, 112] =2.03, P>0.05), although there was a significant interaction (F [12, 112] =2.32, P<0.05).
ED50 (mean ± SEM) values calculated for each drug at the time of peak effect in the single dosing procedure (i.e., 15, 30, and 75 min post injection for nicotine, scopolamine, and morphine, respectively) were as follows: 0.32 ± 0.05 mg/kg for nicotine; 0.15 ± 0.03 mg/kg for scopolamine; and 3.32 ± 1.19 mg/kg for morphine. Each of the drugs also decreased responding in a dose-related manner under the cumulative dosing procedure (left panels, Fig. 2), resulting in the following ED50 (mean ± SEM) values: 0.77 ± 0.06 mg/kg for nicotine; 0.03 ± 0.01 mg/kg for scopolamine; and, 6.27 ± 1.44 mg/kg for morphine. Thus, nicotine and morphine were less potent and scopolamine was more potent under the cumulative dosing procedure, as compared with the acute dosing procedure.
Drug Combination Studies
Scopolamine pretreatment dose-dependently decreased responding and shifted the morphine dose-response curve leftward and downward (upper left panel, Fig. 2). Isobolographic analyses indicated that 0.01 mg/kg scopolamine enhanced the rate-decreasing effects of morphine in an additive fashion and that 0.032 mg/kg enhanced the effects of morphine in infra-additive fashion (square, upper right panel, Fig. 2). A dose of 0.1 mg/kg scopolamine alone decreased responding to less than 50% of the control rate, precluding determination of an ED50 value for morphine in combination with this dose of scopolamine. Morphine alone first increased then, at larger doses, decreased responding (points above “V”, middle and lower left panels, Fig. 2). Morphine also shifted the scopolamine and nicotine dose-response curves leftward and downward. Isobolographic analyses indicated that 1.0 mg/kg of morphine enhanced the rate-decreasing effects of scopolamine in a supra-additive fashion (diamond, middle right panel, Fig. 2). A larger (3.2 mg/kg) dose of morphine in combination with the smallest (0.0032 mg/kg) dose of scopolamine decreased responding to less than 50% of control, precluding determination of an ED50 value for scopolamine in combination with this dose of morphine. Isobolographic analyses indicated that 1.0 and 3.2 mg/kg morphine interacted with increasing doses of nicotine in an additive fashion (lower right panel, Fig. 2). A dose of 10 mg/kg of morphine decreased responding such that an ED50 value could not be determined for nicotine with this dose of morphine.
Discussion
Opioids have a number of important clinical effects, but they also have adverse effects that can limit their use. One method by which the adverse effects of a drug can be reduced and, therefore, its clinical utility potentially enhanced, is by administering that drug in combination with another drug; in some cases, a desired therapeutic effect can be achieved with smaller doses of a drug when it is administered in combination with a second drug. Many studies have investigated opioid receptor agonists in combination with other drugs, to assess the potential clinical utility of those drug combinations and to examine interactions between the neurochemical systems where those drugs are acting (e.g., Li et al., 2008). The current study examined the μ opioid receptor agonist morphine in combination with the muscarinic cholinergic antagonist scopolamine and the nicotinic cholinergic agonist nicotine. Comparison among dose-response curves for these drug combinations indicate that these interactions are modest and are impacted by the particular conditions of drug administration.
Opioid receptor agonists can affect cholinergic systems as well as drugs acting on those systems. For example, acute administration of morphine decreases acetylcholine concentrations in brain regions that are thought to be important in opioid dependence and addiction, including the nucleus accumbens (Rada et al, 1996; Fišerová et al, 1999), prefrontal cortex (Rada et al, 1996; Osman et al, 2005), hippocampus (Ragozzino et al, 1994), and striatum (Tjon et al, 1995). The opposite effect is observed when chronic morphine treatment is discontinued, with acetylcholine concentrations increasing (Tjon et al, 1995; Fišerová et al, 1999). These and many other studies clearly indicate neurochemical interactions between opioid and cholinergic systems, although dopamine systems might also play a role in these interactions (Zhang et al, 2002).
Consistent with neurochemical studies, functional interactions between drugs acting on opioid or cholinergic have also been reported. For example, the muscarinic cholinergic receptor agonist oxotremorine reverses morphine induced memory deficits in a water maze test (Li et al., 2001) and impairment of retention of an inhibitory avoidance task by β-endorphin (Introini et al., 1984). The nicotinic cholinergic receptor agonist nicotine blocks morphine induced state-dependent learning (Zarrindast et al, 2006), inhibits the expression of morphine induced conditioned place preference and locomotor hyperactivity (Shams et al, 2006), and attenuates the development of morphine tolerance and dependence in mice (Haghparast et al, 2008). However, the muscarinic antagonists scopolamine and atropine also attenuate morphine induced conditioned place preference (Zhai et al, 2008; Tan et al, 2007; Lu et al, 2002), locomotor hyperactivity (Li et al , 2007; Oka et al, 1976), and the development of tolerance and dependence (Zhou et al, 1999). Finally, the nicotinic cholinergic receptor antagonist mecamylamine also reverses morphine-induced conditioned place preference (Zarrindast et al, 2003) -- an effect shared by the agonist nicotine. Thus, the interaction between drugs acting on opioid and cholinergic systems appears to vary widely across studies and laboratories.
In many of the procedures that have been used to examine interactions between opioid and cholinergic drugs, the two drugs of interest do not have the same behavioral effect (e.g., opioids, but not cholinergic drugs, are readily self administered by rats and other species). That being the case, possible interactions between drugs can be assessed in only a limited fashion (e.g., drug A affecting drug B, but not vice versa). Moreover, in many studies only single doses of drugs were examined in combination, thereby further limiting interpretation. One potential advantage of using schedule-controlled responding to study drug interactions is that most drugs have effects in this procedure, thereby allowing determination of complete dose-response curves for both (all) drugs, alone and in combination. Thus, morphine, scopolamine, and nicotine decreased responding in a time- and dose-related manner when administered alone. When morphine was administered prior to increasing doses of nicotine, the interaction was additive. The very short duration of action of nicotine precluded the converse experiment with nicotine administered prior to increasing doses of morphine. When morphine was administered prior to scopolamine, the interaction was supra-additive, with smaller than predicted doses of scopolamine deceasing responding. However, when the order of drug administration was reversed (i.e., scopolamine was administered prior to increasing doses of morphine), the interaction between morphine and scopolamine was infra-additive, with larger than predicted doses of morphine needed to decrease responding. These differences are not unlike those reported in previous studies on opioid/cholinergic drug interactions and they could result from pharmacodynamic (e.g., actions at other [non-opioid and non-cholinergic] targets) or pharmacokinetic factors. For example, it has been shown that the interaction between other opioid and cholinergic drugs varies markedly depending on pharmacokinetic factors and, specifically, the order of drug administration (Ishizaki et al, 1998).
In summary, this study used schedule-controlled responding in rats to examine interactions between the μ opioid receptor agonist morphine and drugs acting on cholinergic receptors. Multiple doses of drugs were studied together and the data were assessed by examination of isobolographic analyses. The results show that morphine and nicotine interact in an additive manner. On the other hand, morphine and scopolamine interact in an infra-additive or supra-additive manner, depending on the order of drug administration. It is clear that procedural details are especially important in drug interaction studies and apparent inconsistencies in the literature on opioid/cholinergic interactions might be due to pharmacokinetic factors.
Acknowledgements
CPF is supported by a Senior Scientist Award (DA17918).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buccafusco JJ, Bain JN. A 24-h access I.V. self-administration schedule of morphine reinforcement and the estimation of recidivism: Pharmacological modification by arecoline. Neuroscience. 2007;149:487–98. doi: 10.1016/j.neuroscience.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABAB receptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–74. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- De Marinis L, Mancini A, Valle D, Fiumara C, Conte G, Bianchi A, Perrelli M, Gentilella R, Giustina A. Physiological role of the opioid-cholinergic interaction in growth hormone neuroregulation: effect of sex and food intake. Metabolism. 1997:740–44. doi: 10.1016/s0026-0495(97)90116-5. [DOI] [PubMed] [Google Scholar]
- Fis̆erová M, Consolo S, Krs̆iak M. Chronic morphine induces long-lasting changes in acetylcholine release in rat nucleus accumbens core and shell: an in vivo microdialysis study. Psychopharmacology. 1999;142:85–94. doi: 10.1007/s002130050866. [DOI] [PubMed] [Google Scholar]
- Gessner PK. A straightforward method for the study of drug interactions: an isobolographic analysis primer. J Am Coll Toxicol. 1988;7:987–1012. [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking α3β4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–91. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Haghparast A, Khani A, Naderi N, Alizadeh AM, Motamedi F. Repeated administration of nicotine attenuates the development of morphine tolerance and dependence in mice. Pharmacology, Biochemistry & Behavior. 2008;88:385–92. doi: 10.1016/j.pbb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Harris RA. Interactions between narcotic agonists, partial agonists and antagonists evaluated by schedule-controlled behavior. J Pharmacol Exp Ther. 1980;213:497–503. [PubMed] [Google Scholar]
- Introini IB, Baratti CB. The impairment of retention induced by β-endorphin in mice may be mediated by reduction of central cholinergic activity. Behav Neural Biol. 1984;41:152–63. doi: 10.1016/s0163-1047(84)90527-2. [DOI] [PubMed] [Google Scholar]
- Ishizaki J, Yokogawa K, Nakashima E, Takayasu T, Ohshima T, Ichimura F. Effect of sequence of administration on the pharmacokinetic interaction between the anticholinergic drug biperiden and [3H]quinuclidinyl benzylate or [3H]N-methylscopolamine in rats. J Pharm Pharmacol. 1998;50:189–96. doi: 10.1111/j.2042-7158.1998.tb06175.x. [DOI] [PubMed] [Google Scholar]
- Lelas S, Rowlett JK, Spealman RD. Isobolographic analysis of chlordiazepoxide and triazolam combinations in squirrel monkeys discriminating triazolam. Psychopharmacology. 2001;158:181–89. doi: 10.1007/s002130100868. [DOI] [PubMed] [Google Scholar]
- Li J-X, McMahon LR, Gerak LR, Becker GL, France CP. Interactions between △9-tetrahydrocannabinol and μ opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology. 2008;199:199–208. doi: 10.1007/s00213-008-1157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu A, Yu B, Wang J, Guo C. Effects of scopolamine on behavioral sensitization induced by morphine in rats. Acta Psychologica Sin. 2007;39:299–305. [Google Scholar]
- Li Z, Wu CF, Pei G, Xu NJ. Reversal of morphine-induced memory impairment in mice by withdrawal in Morris water maze possible involvement of cholinergic system. Pharmacol Biochem Behav. 2001;68:507–13. doi: 10.1016/s0091-3057(01)00456-7. [DOI] [PubMed] [Google Scholar]
- Lu L, Nan-Jie Xu XG, Yue W. Reactivation of morphine conditioned place preference by drug priming: role of environmental cues and sensitization. Psychopharmacology. 2002;159:125–32. doi: 10.1007/s002130100885. [DOI] [PubMed] [Google Scholar]
- Oka T, Hosoya E. Effects of humoral modulators and naloxone on morphine-induced changes in the spontaneous locomotor activity of the rat. Psychopharmacology. 1976;47:243–8. doi: 10.1007/BF00427608. [DOI] [PubMed] [Google Scholar]
- Osman NI, Baghdoyan HA, Lydic R. Morphine inhibits acetylcholine release in rat prefrontal cortex when delivered systemically or by microdialysis to basal forebrain. Anesthesiology. 2005;103:779–87. doi: 10.1097/00000542-200510000-00016. [DOI] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. Apparent pA2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther. 1999;290:1222–9. [PubMed] [Google Scholar]
- Rada PV, Mark GP, Taylor KM, et al. Morphine an naloxone, i.p or locally, affect extracellular acetylcholine in the accumbens and Prefrontal Cortex. Pharmacol Biochem Behav. 1996;53:809–16. doi: 10.1016/0091-3057(95)02078-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Wenk GL, Gold PE. Glucose attenuates a morphine-induced decrease in hippocampal acetylcholine output: an in vivo microdialysis study in rats. Brain Res. 1994;655:77–82. doi: 10.1016/0006-8993(94)91599-7. [DOI] [PubMed] [Google Scholar]
- Shams J, Sahraei H, Gholami A, Haeri-Rohani A, Alaf-Javadi M, Sepehri H, Salimi SH, Ghoshooni H. Effects of ultra-low doses of nicotine on the expression of morphine-induced conditioned place preference in mice. Behav Pharmacol. 2006;17:629–35. doi: 10.1097/FBP.0b013e3280102d68. [DOI] [PubMed] [Google Scholar]
- Tan H, Liu N, Wilson FA, Ma Y. Effects of scopolamine on morphine-induced conditioned place preference in mice. Addiction Biology. 2007;12:463–9. doi: 10.1111/j.1369-1600.2007.00062.x. [DOI] [PubMed] [Google Scholar]
- Tjon GH, De Vries TJ, Nestby P, Wardeh G, Mulder AH, Schoffelmeer AN. Intermittent and chronic morphine treatment induces long-lasting changes in delta-opioid receptor-regulated acetylcholine release in rat striatum and nucleus accumbens. Eur J Pharmacol. 1995;283:169–76. doi: 10.1016/0014-2999(95)00319-g. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Faraji N, Rostami P, Sahraei H, Ghoshouni H. Cross-tolerance between morphine- and nicotine-induced conditioned place preference in mice. Pharmacol Biochem Behav. 2003;74:363–9. doi: 10.1016/s0091-3057(02)01002-x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Nouraei N, Khallilzadeh A, Askari E. Influence of acute and sub-chronic nicotine pretreatment on morphine state-dependent learning. Behav Brain Res. 2006;173:268–73. doi: 10.1016/j.bbr.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Zhai H, Wu P, Chen S, Li F, Liu Y, Lu L. Effects of scopolamine and ketamine on reconsolidation of morphine conditioned place preference in rats. Behav Pharmacol. 2008;19:211–6. doi: 10.1097/FBP.0b013e3282fe88a0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–52. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ge X, Wang LZ, Ma L, Pei G. Attenuation of morphine tolerance and dependence in scopolamine-treated rats. Neuroreport. 1999;10:2007–10. doi: 10.1097/00001756-199907130-00003. [DOI] [PubMed] [Google Scholar]