Abstract
The mechanism of the compensatory increase in electromyographic activity (EMG) of a cat ankle extensor during walking shortly after paralysis of its synergists is not fully understood. It is possible that due to greater ankle flexion in stance in this situation, muscle spindles are stretched to a greater extent and, thus, contribute to the EMG enhancement. However, also changes in force feedback and central drive may play a role. The aim of the present study was to investigate the short-term (1- to 2-week post-op) effects of lateral gastrocnemius (LG) and soleus (SO) denervation on muscle fascicle and muscle–tendon unit (MTU) length changes, as well as EMG activity of the intact medial gastrocnemius (MG) muscle in stance during overground walking on level (0%), downslope (−50%, presumably enhancing stretch of ankle extensors in stance) and upslope (+50%, enhancing load on ankle extensors) surfaces. Fascicle length was measured directly using sonomicrometry, and MTU length was calculated from joint kinematics. For each slope condition, LG-SO denervation resulted in an increase in MTU stretch and peak stretch velocity of the intact MG in early stance. MG muscle fascicle stretch and peak stretch velocity were also higher than before denervation in downslope walking. Denervation significantly decreased the magnitude of MG fascicle shortening and peak shortening velocity during early stance in level and upslope walking. MG EMG magnitude in the swing and stance phases was substantially greater after denervation, with a relatively greater increase during stance of level and upslope walking. These results suggest that the fascicle length patterns of MG muscle are significantly altered when two of its synergists are in a state of paralysis. Further, the compensatory increase in MG EMG is likely mediated by enhanced MG length feedback during downslope walking, enhanced feedback from load-sensitive receptors during upslope walking and enhanced central drive in all walking conditions.
Keywords: Locomotion, Proprioceptive feedback, Nerve injury, Muscle length, Muscle spindle, Plasticity, Denervation, EMG
Introduction
An important role of feedback from muscle receptors is to regulate the magnitude and timing of muscle activity (EMG) to satisfy the mechanical demands during locomotion (Pearson et al. 1998; Rossignol et al. 2006). Following denervation or weakening of selected ankle extensors, the EMG magnitude of the intact synergist is markedly increased (e.g., Pearson et al. 1999; Misiaszek and Pearson 2002; Prilutsky et al. 2006b; Frigon and Rossignol 2007). Several mechanisms may be responsible for such adaptive changes in muscle activity: (1) Changes in force-dependent sensory feedback: As fewer muscles are available to contribute to the ankle extensor moment in stance, which does not decrease after denervation (Prilutsky et al. 2006a; Donelan et al. 2009), the increased EMG activity of intact ankle extensors could be mediated by positive force feedback from Golgi tendon organs (Pearson and Collins 1993; Donelan et al. 2009). (2) Changes in length-dependent sensory feedback: Based on a correlation between the amplitude of ankle flexion in early stance during level walking and the mean amplitude of the late EMG component (defined by the authors as a 100 ms period centered on the peak activity following paw contact), but not the initial component (defined as the initial 120 ms of the EMG burst), it has been suggested that the EMG enhancement after denervation of synergists is at least partly mediated by length-dependent feedback from intact ankle extensors (Pearson et al. 1999). This could be due to a larger stretch of the muscle spindles and/or an increased gain of the reflex pathways. Based on the results of a more recent study (Pearson and Misiaszek 2000), the same group suggested that, even though the acute EMG increase may be related to a larger muscle stretch, the persisting higher EMG activity in intact synergists could be explained by an increase in the gain of length-dependent reflex pathways. (3) Changes in the central drive to the motoneurons: In other studies, it has been indicated that the increase in post-contact activity can also be generated by an adaptive increase in central locomotor drive (Bouyer et al. 2001; Gritsenko et al. 2001; Frigon and Rossignol 2007).
Relating mechanical variables to potential feedback from muscle afferents is a valuable approach for studying the neural control of locomotion (see Prochazka 1999; Gregor et al. 2006; Rossignol et al. 2006). Specifically, activity of group Ib afferents originating from Golgi tendon organs is commonly assessed by tendon forces (Prochazka 1999; Donelan and Pearson 2004; Donelan et al. 2009; Ross and Nichols 2009) or joint moments (e.g., Gregor et al. 2006), and was suggested to increase in hindlimb extensors during stance of upslope walking and decrease in downslope walking (Gregor et al. 2006; Donelan et al. 2009). Estimations of activity of group Ia and II afferents originating from muscle spindles are mostly based on changes in single joint angle (e.g., Pearson et al. 1999) or muscle–tendon unit (MTU) length (Bouyer et al. 2001; Gregor et al. 2006), assuming negligibly small influence of tendon and aponeurosis elasticity as well as constant gamma motoneuron activation and presynaptic inhibition. Based on these studies, it was suggested that muscle length-dependent afferent input would be greater during stance of downslope walking compared to level walking and would be small or negligible during upslope walking (Abelew et al. 2000; Gregor et al. 2006). Estimating muscle length changes (and length-dependent feedback) based on a single joint angle neglects the fact that many muscles span more than a single joint (Goslow et al. 1973), which should be accounted for in MTU length calculations. Greater MTU stretch of the two-joint ankle extensors due to an increased ankle flexion (‘yield’) in early stance may be compensated by less MTU stretch due to a greater knee flexion or magnified due to less knee flexion. This is relevant because responses in more proximal joints have been reported after denervation of the ankle flexors (Carrier et al. 1997) as well as after nerve transection and repair of gastrocnemius muscle (Maas et al. 2007).
Neglecting elasticity of tendon and aponeurosis by estimating muscle spindle length changes based on MTU length may also lead to inaccuracies. Muscle spindles lie in parallel with the extrafusal skeletal muscle fibers. Significant discrepancies between MTU and fascicle length change of the cat medial gastrocnemius muscle (MG) have been reported, in particular, in the early stance phase of the walking cycle (Hoffer et al. 1989; Griffiths 1991; Maas et al. 2009). Therefore, muscle fascicle length is considered to be a better measure of strain experienced by the spindles than is MTU length (Prilutsky et al. 1996; Windhorst 2008; Maas and Lichtwark 2009).
The aim of this report was to investigate the short-term (1- to 2-week post-op) effects of paralyzing cat lateral gastrocnemius (LG) and soleus (SO) muscles on muscle fascicle and MTU length changes, as well as muscle activity, in the intact MG during the stance phase of overground level, downslope and upslope walking. The slope conditions were included to facilitate differential length- and force-dependent feedback from MG. We tested the following hypotheses: (1) LG-SO paralysis will increase MTU lengthening and EMG magnitude in MG muscle during early stance for all slope conditions; (2) Locomotor changes in the lengths of muscle fascicles of MG will differ from the MTU length changes, both before and after denervation of the LG and SO muscles; and (3) The expected increase in MG EMG after denervation of LG and SO muscles will correlate with increased MG fascicle stretch in the beginning of stance. The muscle nerves of LG and SO were transected and rejoined to allow for self-reinnervation (Cope et al. 1994). Here, we present the data recorded 1–2 weeks following this procedure when the treated muscles remain essentially in a state of paralysis (Gordon and Stein 1982).
Methods
Five adult female cats (Felis Domesticus, body mass 3.3 kg, SD 0.4) were used. All surgical and experimental procedures were in agreement with the “Principles of laboratory animal care” (NIH No.86-23, 1985) and the Georgia Tech Institutional Animal Care and Use Committee. Prior to the experimental measurements, each cat was trained to walk within a Plexiglas enclosed walkway (2.5 × 0.4 m) on a level surface (0%) as well as on up- and down-sloped surfaces (±50%, i.e. ±26.6°). The surgical and experimental procedures were similar to those described in detail in some of our previous studies (Gregor et al. 2001; Prilutsky et al. 2005; Gregor et al. 2006; Maas et al. 2007, 2009) and will, therefore, only be described briefly below.
Surgical procedures
Under aseptic conditions and using isoflurane anesthesia, the MG muscle of the right hindlimb was surgically instrumented with sonomicrometry crystals and fine-wire electrodes. For EMG measurements, a pair of Teflon-insulated multi-stranded stainless-steel wires (100 μm diameter, Cooner Wire, Chatsworth, CA) was implanted into a compartment of the right MG muscle, located in the mid-region of the muscle and consisting of predominantly fast-twitch muscle fibers (English, personal communication). To verify the absence of muscle activity in the initial weeks following nerve transection and surgical repair, EMG electrodes were also implanted in the midbelly of SO muscle and the medial compartment of LG (English and Letbetter 1982). Procedures for implanting crystals for muscle fascicle length measurements were based on those described by Biewener et al. (1998a). One pair of piezoelectric crystals (2 mm, Sonometrics Corp., Ontario, Canada) was implanted near the origin and insertion of a muscle fascicle in the mid-region of the muscle, taking MG’s architecture into account (see Maas et al. 2009).
After collecting locomotion baseline data, the muscle nerves of LG and SO within the right hindlimb were transected and rejoined, using surgical procedures described previously (Cope et al. 1991). The LG-SO branch of the tibial nerve was identified and transected proximal to where it enters the muscles. The proximal and distal nerve stumps were then immediately aligned to their original position and secured in place using fibrin glue (equal parts of thrombin and a 1:1 mixture of fibrin and fibronectin; Sigma–Aldrich, St. Louis, MO, USA; English et al. 2005).
Data collection
To obtain MTU length changes and determine the stance and swing phase of the walking cycle, joint position data (VICON Inc., UK) and ground reaction forces (Bertec Inc., OH, USA) were collected during walking in each of the three slope conditions according to procedures described previously (Gregor et al. 2006). In short, the right hindlimb was shaved and reflective markers were placed over anatomical landmarks: the iliac crest (IC), greater trochanter, lateral femoral epicondyle, lateral malleolus, 5th metatarsophalangeal (MTP) joint and the distal end of the fifth digit. The coordinates of the greater trochanter and lateral malleolus defined the centers of hip and ankle rotation, respectively. To minimize the influence of skin movement, knee joint position was estimated by triangulation using hip and ankle coordinates as well as thigh and shank segment lengths. Muscle activity (sampling rate 3000 Hz), fascicle length (1059.3 Hz), segment endpoint position (120 Hz) and ground reaction force (360 Hz) data were collected synchronously prior to and 1–2 weeks following nerve transection and repair of LG and SO nerves (i.e., when the animals had fully recovered from the surgery and could walk without pain medication). Note that at this time point, LG and SO muscles are in a state of paralysis (no EMG activity could be recorded) and, thus, cannot actively contribute to movement control.
Data analysis
Walking cycles were accepted only if the cat walked with an uninterrupted gait at a steady pace through the walkway, as assessed by the mean velocity of the IC marker, and the right hindlimb made contact with the force plate independent of the surrounding surface. Also trials in which paw slippage occurred were not used for analysis. For each cat, walking cycles within a narrow range (~ 150 ms) of stance durations (see Table 1) were selected per slope condition to minimize the effects of walking speed. The selection of walking cycles resulted in similar stance durations between conditions before and after LG-SO denervation (Table 1). Therefore, effects of walking speed on MTU, muscle fascicle length and EMG activity can be excluded as a confounding factor. Paw contact (PC) and paw liftoff (PO) were identified using the ground reaction force data. All data were time normalized with respect to stance or swing duration. Linear interpolation between measured points was used to compute a value for each 0.5% of the stance phase and a value for each 1% of the swing phase.
Table 1.
Number of step cycles used in muscle length (top) and EMG (bottom) analyses and corresponding mean stance duration for each cat, walking condition (50% downslope, level, and 50% upslope) and measurement (pre- and post-LG-SO denervation)
| Cat | Walking condition | ||||||
|---|---|---|---|---|---|---|---|
| −50% | 0% | +50% | |||||
| Pre | Post | Pre | Post | Pre | Post | ||
|
Number of step cycles |
#1 |
12 12 |
5 11 |
9 8 |
7 7 |
5 5 |
5 5 |
| #2 |
6 – |
6 – |
7 – |
5 – |
6 – |
5 – |
|
| #3 |
– * |
– 11 |
– 2 |
– 6 |
– 8 |
– 8 |
|
| #4 |
– * |
– 8 |
– 6 |
– 5 |
– 5 |
– 7 |
|
| #5 |
– * |
– 12 |
– 14 |
– 12 |
– 14 |
– 11 |
|
|
Stance Time (ms) |
#1 |
378 (35) 335 (29) |
355 (13) 358 (22) |
424 (34) 421 (21) |
439 (25) 485 (61) |
508 (54) 475 (21) |
445 (37) 463 (39) |
| #2 |
447 (46) – |
411 (13) – |
539 (41) – |
483 (63) – |
485 (23) – |
472 (27) –– |
|
| #3 |
– * |
– 385 (30) |
– 346 (18) |
– 382 (46) |
– 356 (21) |
– 460 (47) |
|
| #4 |
– * |
– 319 (22) |
– 444 (44) |
– 462 (54) |
– 468 (31) |
– 375 (47) |
|
| #5 |
– * |
– 426 (17) |
– 390 (68) |
– 444 (40) |
– 465 (42) |
– 441 (17) |
|
Values are shown as mean (SD). No significant within cat differences in stance duration were found between pre- and post-denervation (separate t-tests for walking condition within each cat). * No measurable EMG was observed in all trials
MTU lengths during the selected walking cycle were calculated using the low-pass filtered kinematic data of ankle and knee joints and the geometric model presented by Goslow et al. (1973). Joint angles were determined from projections of the 3D joint center position data (marker positions) on the sagittal plane using a five-segment 2D model of the cat hindlimb (for more detail see Maas et al. 2007). After MTU lengths were calculated, MTU velocities were computed using the method of finite differences. Positive velocity values indicated lengthening; negative values indicated shortening.
The distance between the sonomicrometry crystals (i.e., muscle fascicle length) was obtained by measuring the elapsed time for a burst of ultrasound that is emitted by one crystal and received by the other one. This transit time was then converted to a distance using the speed of sound in vertebrate skeletal muscle (i.e., 1540 m/s see Biewener et al. 1998b). Fascicle velocities were computed using the method of finite differences. Positive velocity values indicated lengthening; negative values indicated shortening.
Detailed analysis of MTU and fascicle length changes focused on the stance phase, because this is when MG as well as LG and SO are predominantly active during walking (e.g., Gregor et al. 2006) and when motor deficits have been reported following denervation of synergistic muscles (e.g., Pearson et al. 1999; Abelew et al. 2000; Maas et al. 2007). Changes in length and the corresponding peak velocities during early stance (i.e., from paw contact to a change in sign of the length changes, corresponding to the E2 phase Phillipson’s notation, Phillipson 1905) were assessed.
Prior to analysis, the EMG signals were band-pass filtered (30–1000 Hz, 3 dB) and full wave rectified. To assess the onset and offset of MG EMG activity, the mean and standard deviation (SD) of EMG values during muscle silent periods (i.e., most of the swing phase, see Fig. 1) were calculated. The muscle was considered active if the recorded rectified and filtered EMG activity values exceeded by at least two SDs the mean rectified EMG activity recorded during muscle silent periods for at least 50 ms. Correspondingly, the times of muscle activation onset and offset were determined when EMG activity values became greater and smaller, respectively, than the two-SD threshold. The mean magnitude and duration of the total EMG burst in the walking cycle as well as mean EMG magnitudes and burst durations before and after paw contact were determined. As MG activity during swing is not affected by proprioceptive feedback (Gorassini et al. 1994), the mean EMG before paw contact was calculated to reflect the influence of central drive. On the other hand, mean EMG during stance is strongly dependent on length and force feedback (Gorassini et al. 1994; Hiebert and Pearson 1999) and was, thus, calculated to reflect the influence of sensory signals. In addition, low-pass filtered EMG (20 Hz cutoff frequency) was calculated. EMG magnitudes were normalized to their maximum values observed across all conditions within each cat (usually upslope walking, post-LG-SO denervation).
Fig. 1.
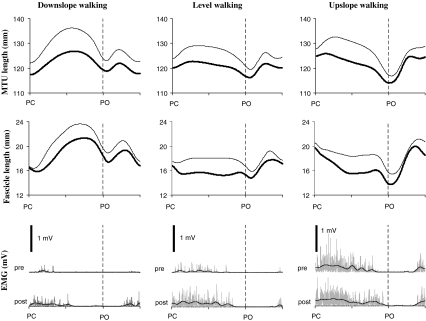
Muscle-tendon unit (MTU) length (top panel), muscle fascicle length (middle panel) and EMG activity (bottom panel) of medial gastrocnemius muscle during typical walking cycles for all three walking conditions prior to (thick lines) and 2 weeks after (thin lines) denervation of lateral gastrocnemius and soleus muscles in Cat #1. Rectified (gray) and low-pass filtered (black) EMG are shown. Note that the pre-post denervation change in EMG amplitude during upslope walking was smaller than the changes during level and downslope walking. This change in EMG was also distinct from the other three cats (see mean data in Fig. 6). Cycle time is normalized to cycle duration; see Table 1 for the mean stance time of Cat #1. PC: paw contact, PO: paw lift-off
Statistics
As length data were available for only two of the five cats (see Table 1), data were analyzed for each cat separately. For EMG data (data were available in four of the five cats), means and standard deviations were calculated on the grouped trials of the four cats (see Table 1). To evaluate the effects of denervation, dependent variable values of pre- and post-measurements were compared using t-tests. A two-way ANOVA (factors: slope and denervation) was used to analyze the effects of denervation (pre and post) and walking slope (downslope, level, upslope) on the stance-swing EMG amplitude difference as well as interaction effects. If significant interaction effects were found, Bonferroni post hoc tests were performed to locate significant differences between slopes. P values < 0.05 were considered statistically significant.
Results
Effects of LG-SO denervation on MG muscle length changes
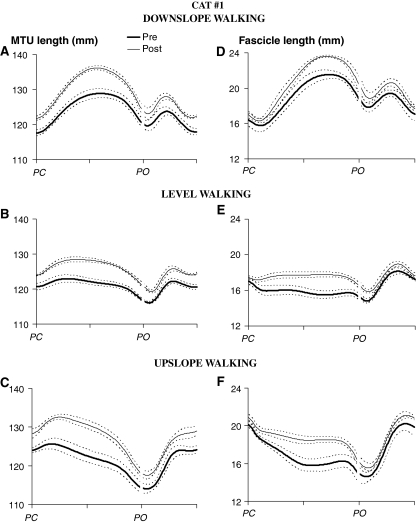
For all three walking conditions and in the two cats analyzed, MTU and fascicle length changes of MG during a walking cycle 1–2 weeks following LG-SO denervation were substantially different from the same measures obtained before denervation (for exemplar data, see Fig. 1). In Cat #1 (Fig. 2), the MTU length pattern after denervation was shifted up, indicating longer absolute MTU lengths throughout the walking cycle. Such a shift was not found in Cat #2 (Fig. 3). The magnitude of MTU stretch in the beginning of stance (i.e., the change in MTU lengthening from paw contact to peak MTU length) in all slope conditions, however, was increased significantly (P < 0.05) following the paralysis of two main ankle extensors in both cats (Fig. 4a, c). Also, peak MTU stretch velocity (Fig. 5a, c) was increased. This is in agreement with the increased range of ankle flexion and the decreased range of knee flexion after paw contact as found in a recent study on LG-MG self-reinnervation (Maas et al. 2007).
Fig. 2.
Mean (±SD) muscle–tendon unit (MTU) and muscle fascicle length of medial gastrocnemius muscle as a function of normalized walking cycle time (in arbitrary units) for all three walking conditions prior to (thick line) and 2 weeks after denervation of lateral gastrocnemius and soleus muscles (thin line) in Cat #1. See Table 1 for the number of analyzed walking cycles. PC: paw contact, PO: paw lift-off
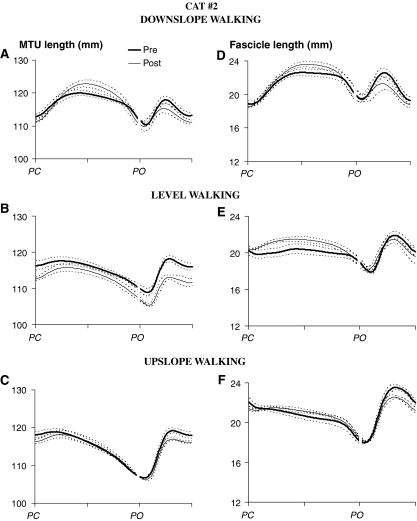
Fig. 3.
Mean (±SD) muscle–tendon unit (MTU) and muscle fascicle length of medial gastrocnemius muscle as a function of normalized walking cycle time (in arbitrary units) for all three walking conditions prior to (thick line) and 1 week after denervation of lateral gastrocnemius and soleus muscles (thin line) in Cat #2. See Table 1 for the number of analyzed walking cycles. PC: paw contact, PO: paw lift-off
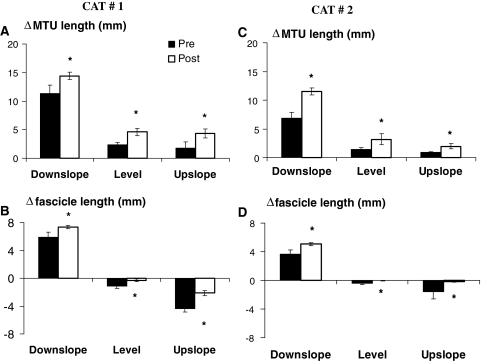
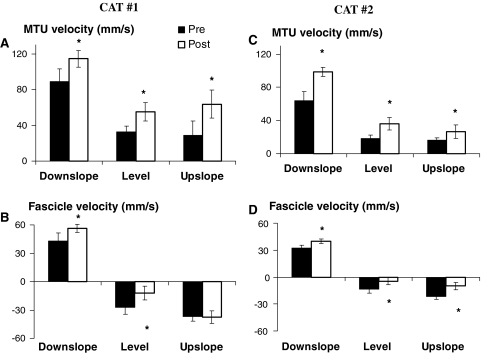
Fig. 4.
Muscle-tendon unit (MTU) and muscle fascicle length changes (Δ) of medial gastrocnemius measured from the beginning of the stance phase to the first maximum or minimum length. For each cat, mean (SD) values prior to and 1–2 weeks after denervation of lateral gastrocnemius and soleus muscles are shown for downslope, level and upslope walking. During level walking, fascicle lengthening was found also in early stance post-denervation (see Figs. 2, 3). For comparison with the pre-denervation condition, shortening is shown only. See Table 1 for the number of analyzed walking cycles. Positive values correspond to MTU and fascicle lengthening, negative values to shortening. Asterisk denotes a value significantly different (P < 0.05) from the pre-denervation value
Fig. 5.
Peak magnitudes of medial gastrocnemius muscle–tendon unit (MTU) and fascicle velocities measured in the beginning of the stance phase. For each cat, mean (SD) values prior to and 1–2 weeks after denervation of lateral gastrocnemius and soleus muscles are shown for downslope, level and upslope walking. During level walking, fascicle lengthening was found also in early stance post-denervation (see Figs. 2, 3). For comparison with the pre-denervation condition, the shortening velocity is shown only. See Table 1 for the number of analyzed walking cycles. Positive velocity values correspond to MTU and fascicle lengthening, negative to shortening. Asterisk denotes a value significantly different (P < 0.05) from the pre-denervation value
Similar to MTU length change patterns, changes in fascicle length (Fig. 4b, d) and peak velocities (Fig. 5b, d) during early stance were affected profoundly by LG-SO denervation in both cats. For downslope walking, the magnitude of fascicle lengthening and peak stretch velocity during early stance increased significantly (P < 0.05). For level and upslope walking, LG-SO denervation significantly (P < 0.05) decreased the magnitude of fascicle shortening just following paw contact (Fig. 4b, d). Peak shortening velocity during this part of the stance phase (Fig. 5b, d) decreased significantly (P < 0.05) except for the upslope condition in Cat #1. In Cat #2 (thin lines in Fig. 3e, f), fascicle shortening after paw contact became negligible (Fig. 4d) after LG-SO denervation. MG fascicles in this cat were stretched in early stance during level walking (Fig. 3e) and remained more or less constant in early stance during upslope walking (Fig. 3f).
Similar to the changes at the MTU level, the fascicle length pattern after denervation was shifted toward longer lengths in Cat #1 (Fig. 2), but not in Cat #2 (Fig. 3). Thus, the observed effects of LG-SO denervation on MTU length of MG muscle are conveyed also at the fascicle level and, thus, may be detected by muscle spindles.
Effects of LG-SO denervation on EMG activity of MG muscle
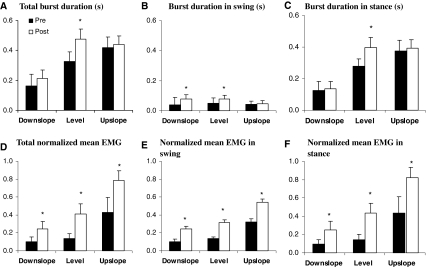
Changes in the timing and magnitude of MG activity during walking at the different slope conditions following LG-SO denervation are shown in Figs. 1 and 6. Significant (P < 0.05) changes in the timing of the EMG bursts were found only for total burst duration (Fig. 6a), burst duration in swing (Fig. 6b) and burst duration in stance (Fig. 6c) during level walking as well as for burst duration in swing during downslope walking. The total MG burst duration and burst duration in stance were statistically higher during level and upslope walking than during downslope walking both before and after LG-SO denervation (P < 0.05, Fig. 6a, c). While the duration of the EMG burst in swing was not different between walking conditions before denervation, the swing burst was significantly longer in downslope and level walking compared to upslope walking after denervation (Fig. 6b). When expressed as a proportion of the maximum value observed across all conditions (i.e., normalized EMG), the magnitude of EMG activity in MG muscle was increased dramatically (2–3 times) for all walking conditions when LG and SO muscles were denervated. No EMG activity was observed in LG and SO muscles. Normalized mean EMG intensity during the whole burst (Fig. 6d) as well as during swing (Fig. 6e) and stance (Fig. 6f) were all significantly (P < 0.05) higher than those found before nerve transection.
Fig. 6.
EMG duration and normalized mean magnitude of medial gastrocnemius muscle in three walking conditions (downslope, level and upslope), pre- and post-denervation of soleus and lateral gastrocnemius. Mean and SD data of four cats (see Table 1). a Duration of EMG burst in whole walking cycle. b Duration of EMG burst before paw contact. c Duration of EMG burst after paw contact. d Normalized mean magnitude of EMG burst in the whole walking cycle. e Normalized mean magnitude of EMG burst before paw contact. f Normalized mean magnitude of EMG burst after paw contact. EMG magnitudes of each cat were normalized to their maximum mean EMG of the cat among all walking trails and conditions. Asterisk denotes statistical significant differences (P < 0.05) between pre- and post-denervation conditions
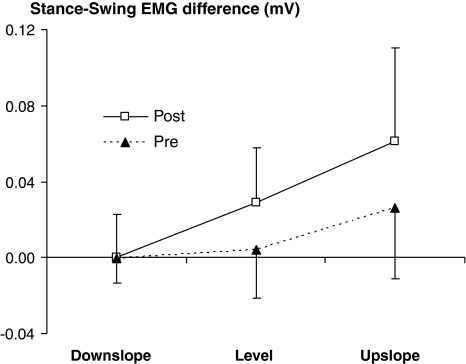
For level and upslope walking, we found a significant (P < 0.05) increase in the difference between the EMG amplitude during stance (contact) and the EMG amplitude during swing (pre-contact) following LG-SO denervation (Fig. 7). In addition, post hoc analysis indicated that the increase in the stance-swing EMG difference during upslope walking was significantly higher than the increase during level walking. In contrast, the stance-swing difference in EMG amplitude during downslope walking was not changed by denervation (Fig. 7).
Fig. 7.
The difference between the EMG amplitude during stance and the EMG amplitude during swing (i.e., stance minus swing). Mean (SD) values prior to and 1–2 weeks after denervation of lateral gastrocnemius and soleus muscles are shown for downslope, level and upslope walking. ANOVA indicated a significant effect of denervation and slope, as well as significant interaction. Subsequent post hoc analysis indicated that the increase in upslope walking was significantly higher than the increase in level walking
Comparison of changes in fascicle length and EMG activity
The amplitude of MG EMG activity did not necessarily follow elongation of MG fascicles (Fig. 1). In intact cats, the greatest MG EMG activity occurred during stance of upslope walking, during which no fascicle elongation took place (Fig. 1). On the other hand, before denervation, very small (in Cat #1, Fig. 1) or no (in Cats #3, #4 and #5) EMG activity was observed during downslope walking, when MG fascicles experienced greater magnitudes of stretch. The limited activity of MG during downslope walking, also reported previously (Gregor et al. 2006; Maas et al. 2009), might be due to implantation of EMG electrodes into an MG compartment with a predominantly fast-twitch muscle fiber composition (see Sect. “Methods”). Fast-twitch motor units may not be recruited during tasks involving low external force demands (English 1984).
In contrast, some correlated changes in EMG activity pattern of MG and changes in fascicle length and velocity were found following LG-SO denervation. For example, both EMG activity and fascicle stretch increased in stance during downslope walking (Fig. 1). During level and upslope walking, EMG activity increased and MG fascicle shortening decreased. Thus, EMG activity of the MG muscle increased as a response to the denervation of two main synergistic ankle extensors. Because of the observed changes in fascicle length under these conditions, we suggest that this modulation of muscle activity may at least partially be brought about by changes in length feedback from the MG muscle.
Discussion
This is the first study in which changes in muscle fascicle length were assessed in MG muscle following denervation of synergistic muscles in the cat. Denervation of LG and SO muscles led either to an increase of fascicle lengthening (both cats, during downslope walking), a switch from shortening to lengthening (Cat #2 during level walking), or a decrease of fascicle shorting (Cat #1 and #2, during upslope walking) in MG during early stance. It should be noted that at the MTU level, early stance is characterized by lengthening in all slope conditions both before and after LG-SO denervation.
As described in the introduction, several mechanisms may be responsible for the modulation of MG muscle activity following synergist denervation that we observed in the present study. For level and upslope walking, it was found that the stance-swing difference in EMG amplitude of MG increased following LG-SO denervation. This is in agreement with the results reported by Pearson et al. (1999) and suggests that changes in length- and force-dependent sensory feedback have at least partly contributed to these neural adaptations. It should be noted, however, that the activity of muscle spindles and Golgi tendon organs was not directly recorded.
Based on our observation of increased fascicle lengthening and stretch velocity during downslope walking and the switch from fascicle shortening to lengthening during level walking, we suggest that enhanced feedback from muscle spindles could be present in the MG muscle following denervation of its primary synergists. Note that a large contribution of length feedback to muscle output is found during active fascicle lengthening, whereas only a limited contribution of length feedback is expected during shortening contractions (Nichols and Houk 1976). Therefore, the fascicle data suggest that changes in length feedback had a significant influence during downslope walking only. It is important to realize that this conclusion would have been different if MTU length changes were considered alone.
Because there were fewer synergistic muscles to generate a similar ankle moment in stance after LG-SO denervation (Prilutsky et al. 2006a; Donelan et al. 2009), higher forces exerted at the MG tendon would be expected, suggesting stronger force-dependent afferent signals from MG. Several studies have shown that positive force feedback arising from Golgi tendon organs contribute substantially to the level of MG muscle activity and burst duration during stance of walking in the cat (Pearson and Collins 1993; Donelan and Pearson 2004; Gregor et al. 2006; Donelan et al. 2009). The present study also demonstrated greater EMG amplitudes and longer EMG burst durations in MG during upslope walking (where greater loads are applied to hindlimbs and greater ankle extensor moments are developed) than during downslope walking (characterized by smaller loads on ankle extensors and MG greater stretch, Figs. 4 and 5, see also Gregor et al. 2006; Donelan et al. 2009; Maas et al. 2009). It is therefore likely that changes in force feedback provided an important source for the modulation of MG activity in stance following LG-SO denervation. This is certainly the case for upslope walking, because the stance-swing difference in EMG amplitude increased most during this condition in which the regulation of MG activity seems to be dominated by force feedback (e.g., Gregor et al. 2006).
Finally, central drive to MG was likely increased following denervation of LG and SO muscles since not only the level of MG activity in the stance phase, but also the level of activity in swing (i.e., just prior to paw contact) increased (Fig. 6e). MG activity levels during swing (pre-contact) are not affected by stance-related proprioceptive feedback but generated by central mechanisms (Gorassini et al. 1994). This finding is in agreement with previous reports describing the effects of synergist denervation on the amplitude of EMG in MG muscle (e.g., Pearson et al. 1999; Gritsenko et al. 2001).
Besides effects of LG-SO denervation on EMG amplitudes during walking, we found also some significant changes in the timing of EMG bursts. It has been suggested that length-dependent and force-dependent signals from ankle extensors regulate the duration of their EMG activity and, hence, stance time in walking cats by reducing the input to the extensor half-center of spinal central pattern generator at the end of stance (Conway et al. 1987; Guertin et al. 1995; Pearson et al. 1998). Since the total burst duration and the burst duration in stance for MG muscle were shortest during downslope walking and longer during level and upslope walking (Fig. 6a, c), our data suggest that force-dependent signals from MG muscle may be responsible. Such a modulation of burst duration on ankle extensors by changing the load as a function of increased slope has been shown as well in the decerebrate cat (Hiebert and Pearson 1999). LG-SO denervation affected the burst duration of MG EMG during level (total, swing and stance burst durations) and downslope walking (swing burst duration) and not during upslope walking. The absence of changes during upslope walking may be explained by the fact that the MG muscle was already active for most of the stance phase in the intact condition (see Fig. 1; also compare values Fig. 6c and stance time in Table 1). Because we selected trials of equal stance duration before and after denervation, the absolute duration of MG activity in stance could hardly increase. Note that during downslope walking, the EMG burst in stance took up a much smaller portion of the stance time and, thus, there was more room for increasing the duration within the timing criteria of the selected trials. Therefore, our data confirm previous findings (Whelan and Pearson 1997; Pearson et al. 1998) that the burst duration of MG muscle may be regulated by force-dependent feedback.
Based on the results of the present study, there is not a single mechanism that explains the neural adaptations following synergist denervation. We conclude that the compensatory changes in MG EMG activity were mediated by enhanced MG length feedback during downslope walking, enhanced feedback from load-sensitive receptors during upslope walking and enhanced central drive in all walking conditions. The possibility of task-specific feedback regulation has previously been hypothesized to occur in intact cats (Pratt et al. 1991; Gregor et al. 2006; Maas et al. 2009) and our results provide further support for this suggestion. The fact that the task-dependent nature of the feedback persisted in MG following denervation of synergistic muscles indicates that differential feedback is an important factor in enabling the neuromuscular system to adapt to injury and maintain locomotor ability over varied terrain. Such a finding has implications for the development of tailored rehabilitation protocols, as it would appear that different sensory pathways could be exploited to facilitate activation of a given muscle or group of muscles and that differential activation of such pathways could be induced with the simple manipulation of a task i.e. the use of slope versus locomotion on level terrain.
Acknowledgments
The authors thank Dr. Alan Sokoloff for allowing us to use his Sonometrics system, Dr. Guayhaur Shue for technical support, Dr. Margarita Bulgakova for data collection in one animal, and Samira Kamran and Alanna Oliver for data analysis. National Institutes of Health Grants HD032571 and NS048844 as well as the Center for Human Movement Studies at the Georgia Institute of Technology provided financial support for this work. HM is currently supported by EU Marie Curie International Reintegration Grant MIRG-CT-2007-203846. EHT is currently funded by a Sir Henry Wellcome Postdoctoral Fellowship.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol. 2000;84:2709–2714. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Corning WR, Tobalske BW. In vivo pectoralis muscle force-length behavior during level flight in pigeons (Columba livia) J Exp Biol. 1998;201:3293–3307. doi: 10.1242/jeb.201.24.3293. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Konieczynski DD, Baudinette RV. In vivo muscle force-length behavior during steady-speed hopping in tammar wallabies. J Exp Biol. 1998;201:1681–1694. doi: 10.1242/jeb.201.11.1681. [DOI] [PubMed] [Google Scholar]
- Bouyer LJG, Whelan PJ, Pearson KG, Rossignol S. Adaptive locomotor plasticity in chronic spinal cats after ankle extensors neurectomy. J Neurosci. 2001;21:3531–3541. doi: 10.1523/JNEUROSCI.21-10-03531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier L, Brustein E, Rossignol S. Locomotion of the hindlimbs after neurectomy of ankle flexors in intact and spinal cats: model for the study of locomotor plasticity. J Neurophysiol. 1997;77:1979–1993. doi: 10.1152/jn.1997.77.4.1979. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Cope TC, Webb CB, Botterman BR. Control of motor-unit tension by rate modulation during sustained contractions in reinnervated cat muscle. J Neurophysiol. 1991;65:648–656. doi: 10.1152/jn.1991.65.3.648. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol. 1994;71:817–820. doi: 10.1152/jn.1994.71.2.817. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol. 2004;92:2093–2104. doi: 10.1152/jn.00325.2004. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Mcvea DA, Pearson KG. Force regulation of ankle extensor muscle activity in freely walking cats. J Neurophysiol. 2009;101:360–371. doi: 10.1152/jn.90918.2008. [DOI] [PubMed] [Google Scholar]
- English AW. An electromyographic analysis of compartments in cat lateral gastrocnemius muscle during unrestrained locomotion. J Neurophysiol. 1984;52:114–125. doi: 10.1152/jn.1984.52.1.114. [DOI] [PubMed] [Google Scholar]
- English AW, Letbetter WD. Anatomy and innervation patterns of cat lateral gastrocnemius and plantaris muscle. Am J Anat. 1982;164:66–77. doi: 10.1002/aja.1001640107. [DOI] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin-4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- Frigon A, Rossignol S. Plasticity of reflexes from the foot during locomotion after denervating ankle extensors in intact cats. J Neurophysiol. 2007;98:2122–2132. doi: 10.1152/jn.00490.2007. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJ. Corrective responses to loss of ground support during walking. I. Intact cats. J Neurophysiol. 1994;71:603–610. doi: 10.1152/jn.1994.71.2.603. [DOI] [PubMed] [Google Scholar]
- Gordon T, Stein RB. Time course and extent of recovery in re-innervated motor units of cat triceps surae muscles. J Physiol-Lond. 1982;323:307–323. doi: 10.1113/jphysiol.1982.sp014074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow GE, Jr, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973;141:1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Smith JL, Smith DW, Oliver A, Prilutsky BI. Hindlimb kinetics and neural control during slope walking in the cat: unexpected findings. J Appl Biomech. 2001;17:277–286. [Google Scholar]
- Gregor RJ, Smith DW, Prilutsky BI. Mechanics of slope walking in the cat: quantification of muscle load, length change and ankle extensor EMG patterns. J Neurophysiol. 2006;95:1397–1409. doi: 10.1152/jn.01300.2004. [DOI] [PubMed] [Google Scholar]
- Griffiths RI. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991;436:219–236. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsenko V, Mushahwar V, Prochazka A. Adaptive changes in locomotor control after partial denervation of triceps surae muscles in the cat. J Physiol-Lond. 2001;533:299–311. doi: 10.1111/j.1469-7793.2001.0299b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault MC, Mccrea DA. Ankle extensor group-I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol-Lond. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. J Neurophysiol. 1999;81:758–770. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- Hoffer JA, Caputi AA, Pose IE, Griffiths RI (1989) Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Progress in Brain Research 80: 75–85; discussion 57–60 [DOI] [PubMed]
- Maas H, Lichtwark GA. Is muscle-tendon unit length a valid indicator for muscle spindle output? J Physiology (Lond) 2009;587:13–14. doi: 10.1113/jphysiol.2008.165555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Prilutsky BI, Nichols TR, Gregor RJ. The effects of self-reinnervation of cat medial and lateral gastrocnemius muscles on hindlimb kinematics in slope walking. Exp Brain Res. 2007;181:377–393. doi: 10.1007/s00221-007-0938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas H, Gregor RJ, Hodson-Tole EF, Farrell BJ, Prilutsky BI. Distinct muscle fascicle length changes in feline medial gastrocnemius and soleus muscles during slope walking. J Appl Physiol. 2009;106:1169–1180. doi: 10.1152/japplphysiol.01306.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiaszek JE, Pearson KG. Adaptive changes in locomotor activity following botulinum toxin injection in ankle extensor muscles of cats. J Neurophysiol. 2002;87:229–239. doi: 10.1152/jn.00410.2001. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976;39:119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius-muscle during locomotor-activity. J Neurophysiol. 1993;70:1009–1017. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE. Use-dependent gain change in the reflex contribution to extensor activity in walking cats. Brain Res. 2000;883:131–134. doi: 10.1016/S0006-8993(00)02880-8. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. Ann N Y Acad Sci. 1998;860:203–215. doi: 10.1111/j.1749-6632.1998.tb09050.x. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Fouad K, Misiaszek JE. Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol. 1999;82:370–381. doi: 10.1152/jn.1999.82.1.370. [DOI] [PubMed] [Google Scholar]
- Phillipson M. L’autonomie et la centralization dans le systeme nerveux des animaux. Travails du Laboratoire de la Physiologie, Institut Solvey. 1905;7:1–208. [Google Scholar]
- Pratt CA, Chanaud CM, Loeb GE. Functionally complex muscles of the cat hindlimb.4. Intramuscular distribution of movement command signals and cutaneous reflexes in broad, bifunctional thigh muscles. Exp Brain Res. 1991;85:281–299. doi: 10.1007/BF00229407. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Herzog W, Leonard TR, Allinger TL. Role of the muscle belly and tendon of soleus, gastrocnemius, and plantaris in mechanical energy absorption and generation during cat locomotion. J Biomech. 1996;29:417–434. doi: 10.1016/0021-9290(95)00085-2. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Sirota MG, Gregor RJ, Beloozerova IN. Quantification of motor cortex activity and full-body biomechanics during unconstrained locomotion. J Neurophysiol. 2005;94:2959–2969. doi: 10.1152/jn.00704.2004. [DOI] [PubMed] [Google Scholar]
- Prilutsky BI, Maas H, Gregor RJ (2006a) Ankle joint moment during walking after self-reinntervation of selected ankle extensors in the cat. In: American Society of Biomechanics, Blacksburg
- Prilutsky BI, Maas H, Nichols TR, Gregor RJ (2006b) Effects of self-reinnervation of selected cat ankle extensors on their activity and hindlimb mechanics in slope walking. In: Society for Neuroscience, Atlanta
- Prochazka A. Quantifying proprioception. Prog Brain Res. 1999;123:133–142. doi: 10.1016/S0079-6123(08)62850-2. [DOI] [PubMed] [Google Scholar]
- Ross KT, Nichols TR. Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat. J Neurophysiol. 2009;101:184–197. doi: 10.1152/jn.90338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc RJ, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Whelan PJ, Pearson KG. Comparison of the effects of stimulating extensor group I afferents on cycle period during walking in conscious and decerebrate cats. Exp Brain Res. 1997;117:444–452. doi: 10.1007/s002210050239. [DOI] [PubMed] [Google Scholar]
- Windhorst U. Muscle spindles are multi-functional. Brain Res Bull. 2008;75:507–508. doi: 10.1016/j.brainresbull.2007.11.009. [DOI] [PubMed] [Google Scholar]