Abstract
Studies investigating the effect of visual illusions on saccadic eye movements have provided a wide variety of results. In this study, we test three factors that might explain this variability: the spatial predictability of the stimulus, the duration of the stimulus and the latency of the saccades. Participants made a saccade from one end of a Müller-Lyer figure to the other end. By changing the spatial predictability of the stimulus, we find that the illusion has a clear effect on saccades (16%) when the stimulus is at a highly predictable location. Even stronger effects of the illusion are found when the stimulus location becomes more unpredictable (19–23%). Conversely, manipulating the duration of the stimulus fails to reveal a clear difference in illusion effect. Finally, by computing the illusion effect for different saccadic latencies, we find a maximum illusion effect (about 30%) for very short latencies, which decreases by 7% with every 100 ms latency increase. We conclude that spatial predictability of the stimulus and saccadic latency influences the effect of the Müller-Lyer illusion on saccades.
Keywords: Saccades, Eye movements, Illusion, Perception, Action
Introduction
Current models of the primate visual system propose a division between two visual systems: vision-for-perception (implemented by the V1-IT cortico-cortical (ventral) stream) and vision-for-action (the V1-PPT (dorsal) stream). This proposal (Milner and Goodale 1995; see also Jacob and Jeannerod 2003; Trevarthen 1968; Ungerleider and Mishkin 1982) has received support from human and monkey studies using diverse methods, including neuropsychology, imaging and psychophysics. However, the degree of functional independence between the two systems remains controversial. According to the original proposal (Goodale and Milner 1992) both visual systems operate independently. Vision-for-perception encodes object properties relative to the environment, on a relatively slow time scale and with conscious control, whereas the vision-for-action system uses spatial representations relative to the body, on a faster time scale than the vision-for-perception system and without the need for conscious control. This characterization predicts that perceptual responses, such as adjustments and verbal reports, should be affected by contextual information, whereas motor responses, such as pointing or grasping, should not. Aglioti et al. (1995) tested this prediction with the Ebbinghaus illusion (a size-contrast illusion). They found that participants perceived the circle surrounded by small circles as being larger than the one surrounded by large circles. Conversely, when picking up a disk that was put on the inner circle, participants opened their hands as a function of the physical size of the disk instead of the perceived size (Aglioti et al. 1995).
Overall, however, the current literature provides mixed evidence for a dissociation between perception and action (for differing opinions on the literature see Franz and Gegenfurtner 2008; Glover 2004; Milner and Goodale 2008; Schenk and McIntosh 2010; Smeets and Brenner 2006). Many studies have shown that actions are substantially affected by illusions. These results have been interpreted as evidence that vision-for-perception and vision-for-action are not (completely) independent. Several studies found similar effects of visual illusions on perception and action, suggesting that the two systems have a common source of information (Franz et al. 2000; Franz 2001; Franz and Gegenfurtner 2008; Pavani et al. 1999). According to others, task demands determine whether an effect of the illusion can be found (Bruno 2001; Smeets and Brenner 1995; Vishton et al. 1999). Some have suggested that illusion effects on actions can be explained by the two visual systems interacting under specific conditions, e.g., delayed actions (Goodale and Westwood 2004; Goodale 2008). Two recent meta-analyses on pointing (Bruno et al. 2008) and grasping (Bruno and Franz 2009) in the Müller-Lyer illusion analyzed a number of factors influencing the effect the illusion has on those actions. The results pinpointed the availability of visual feedback during the response as a major factor.
Illusion effects have not only been investigated in pointing and grasping but also in saccadic eye movements. This is interesting for a number of reasons. The neuroanatomy of saccadic control is known to involve a number of brain areas, including regions of the parietal and the frontal cortices as well as the basal ganglia, thalamus, superior colliculus, cerebellum and brainstem reticular formation (see Munoz 2002). Given the involvement of the parietal cortex, and most notably of the lateral intraparietal area (LIP) that is classically assigned to the dorsal stream, the two visual systems hypothesis predicts no or very small illusion effects on saccades. On the other hand, saccades are ballistic movements that cannot be corrected online based on novel visual information that becomes available during saccade execution (although some form of feedforward control may still be possible; see West et al. 2009). If the availability of online visual feedback is critical, one would predict substantial illusion effects on saccades. Finally, studies on illusion effects on saccades show a large variability in results, ranging from 20–30% (Bernardis et al. 2005; De Grave et al. 2006b; Knox 2006; Lavrysen et al. 2006; McCarley et al. 2003) to effects between 10 and 20% (De Grave et al. 2006b; Ehresman et al. 2008; Festinger et al. 1968; Lavrysen et al. 2006; Thompson and Westwood 2007) or even less than 10% (Binsted and Elliott 1999; McCarley et al. 2003; Tegetmeyer and Wenger 2004, 2006). Finally, Wong and Mack (1981) reported that saccadic eye movements were not affected by an illusion of displacement. This large range of results suggests that additional factors modulate illusion effects on saccades.
In this study, we want to investigate possible explanations for this wide variability in results on saccadic eye movements. All the afore-mentioned saccade studies, except for Wong and Mack (1981), used versions of the Müller-Lyer illusion. Therefore, we will focus on that type of illusion. The studies differed in three main characteristics. The first one is spatial predictability of the stimulus. In most studies that investigated the effect of the Müller-Lyer illusion on saccades, the stimulus was always presented in the same location relative to the starting position. Thus, participants know the saccade direction in advance and only have to determine an end position in each trial. This could be done by computing an egocentric position. Conversely, when the saccade direction varies from trial to trial, saccades need to be computed by a vector (a direction and an amplitude). Assuming that position and vector coding are performed by separate mechanisms (De Grave et al. 2004) one might expect differences in illusion effects. Indeed, studies in which the spatial location of the stimulus was predictable (Binsted and Elliott 1999; Ehresman et al. 2008; Lavrysen et al. 2006; Tegetmeyer and Wenger 2004, 2006; Thompson and Westwood 2007) showed smaller illusion effects than studies in which the stimulus was presented randomly in one of several directions (Bernardis et al. 2005; De Grave et al. 2006a, b; Knox 2006; McCarley et al. 2003). To systematically investigate the effect of spatial predictability of the stimulus, we asked participants to perform three blocks of trials. In one block, the stimulus location was always at the same location (completely predictable). In the other blocks, the stimulus could appear randomly in either two or four locations.
The second characteristic is stimulus duration. Some of the studies on saccadic eye movements used relatively long stimulus durations, whereas others presented the stimulus only very briefly (less than 200 ms). When saccades are made toward a location that can be seen throughout the preparation and execution of the saccade, there is ample time to determine an accurate end position for the saccade based on visual information. This will result in a small effect of the illusion (De Grave et al. 2006b). Furthermore, retinal error signals become available at the end of a saccade. These signals can be used to adapt saccadic amplitude over trials, which will result in a small effect of the illusion. Saccade adaptation will be most efficient if saccades are made repeatedly to the same location with about the same amplitude. However, adaptation can also occur when the stimulus is at different locations and when saccades have different amplitudes, although the adaptation process is slower, and the amount of adaptation is smaller (Albano and King 1989). When the stimulus location is only visible for a very short time, it may be more difficult to determine an accurate end position for the saccade. Therefore, participants may be forced to use a different way of coding the visual information (De Grave et al. 2004). Alternatively, they might use the remembered position of the stimulus (Westwood and Goodale 2003). Both alternatives will result in a large illusion effect. Note that with very short stimulus presentations, saccadic adaptation cannot occur as retinal error signals are never available at the end of a saccade. Indeed, the two studies (Bernardis et al. 2005; De Grave et al. 2006a) that used short stimulus durations (the stimulus disappeared before the saccade is finished) found larger illusion effects compared to the other studies. Here, we investigate within the same study how long or short stimulus presentation affects the illusion effect on saccadic eye movements.
The third, and final, characteristic is the latency of the saccades (time between stimulus onset and the start of a saccade). In a recent paper De’Sperati and Baud-Bovy (2008) reported that the illusion effect increased with increasing saccadic latencies. In that study, participants had to saccade to the location of a flashed stimulus that was presented on a moving arc. Short-latency saccades (100–250 ms) were minimally affected by the arcs motion, whereas saccades with longer latencies (up to 400 ms) showed substantial illusion effects. This finding was interpreted as a dissociation between a fast visuomotor mechanism, which uses the egocentric location of the target independent of contextual elements, and a slower, context-sensitive mechanism, which codes the target position in relation to the arc. However, other studies provide evidence that more accurate coding of the position of a target occurs with longer, not shorter latencies (Coëffé and O’Regan 1987). Additionally, Van Zoest and Hunt (2008) found a larger effect of the Judd illusion on saccades with short latencies (about 175 ms) than on ones with longer latencies (about 360 ms). Given these conflicting outcomes, it is interesting to assess the effect of saccade latency.
Materials and methods
Participants
Ten participants (age 32 ± 4 years; five males) took part in this study. Nine of them were employees at the VU University (including the first author). The remaining participant was the second author. All participants had normal or corrected-to-normal vision. The study was approved by the ethics committee of the Faculty of Human Movement Science.
Stimulus and apparatus
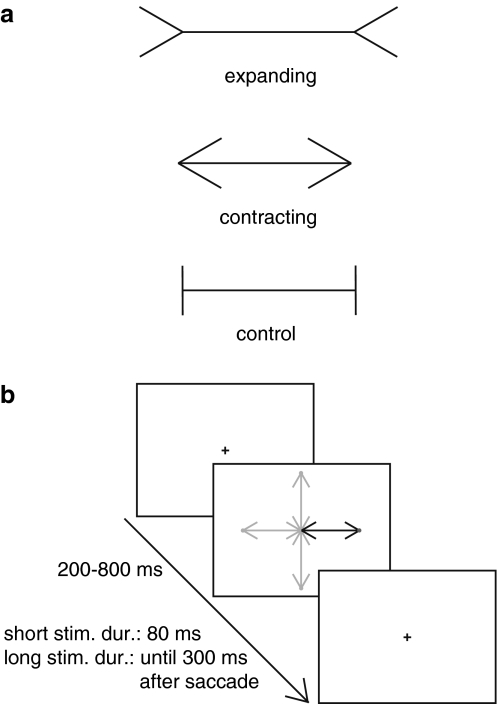
A chin-rest was placed in front of a computer screen (36 × 27 cm, 1,024 × 768 pixels, 85 Hz) to keep the participant’s head fixed at a viewing distance of 57.3 cm. In this case, 1 cm corresponds to 1 degree of visual angle. The stimulus consisted of a black Müller-Lyer illusion and a red target dot on a white background. The shaft had a length of either 6.5 cm or 7.0 cm. The length of the fins was 2.0 cm. The inclination of the fins with respect to the shafts was 30, 90 or 150 degrees, depending on the configuration: expanding, compressing or control (Fig. 1a). In each trial, one of these configurations was presented on a computer screen. In the middle of the screen, a fixation cross (0.5 cm) was presented. The stimulus always appeared with one end of the shaft at the position of the fixation cross. The target dot (diameter 0.15 cm) appeared on the other end of the shaft. Eye movements were recorded with an Eyelink eye tracker (SR Research Ltd.). This system records eye position by tracking the pupil center with a temporal resolution of 500 Hz and a spatial resolution of 0.2º.
Fig. 1.
a The three configurations of the Müller-Lyer illusion: expanding, contracting and control. b Basic trial structure: A fixation cross appears on the screen. After a random interval (range 200–800 ms), the fixation cross is replaced with one of the three configurations of the Müller-Lyer illusion. Depending on the predictability condition, the stimulus appeared to the right of the fixation cross (highly predictable), to the left or right of the fixation cross (moderately predictable) or in any of four possible locations: left, right, above or below the fixation cross (least predictable). In the “short stimulus duration” condition, the stimulus was visible for 80 ms, whereas in the “long stimulus duration” condition the stimulus remained visible until 300 ms after a saccade was made in the correct direction
Procedure
All participants performed two conditions in random order: short and long stimulus duration. Each condition consisted of three blocks of trials, which differed in spatial predictability of the stimulus. In one block, the stimulus always appeared to the right of the fixation cross (high predictability, see Fig. 1b). In another one, it appeared randomly on the left or the right side of the fixation cross (moderate predictability). In a third block, the stimulus appeared on either the left or the right side or above or below the fixation cross (low predictability). The order of blocks within a condition was chosen randomly. Each stimulus configuration was presented 10 times at each location. This resulted in 60 trials for the blocks with high predictability (1 location × 2 shaft lengths × 3 configurations × 10 repetitions), 120 trials for the blocks with moderate predictability (2 locations × 2 shaft lengths × 3 configurations × 10 repetitions) and 240 trials in the blocks with low predictability (4 locations × 2 shaft lengths × 3 configurations × 10 repetitions). Within a block, trials were presented randomly with the restriction that the same stimulus could not be presented on successive trials.
At the start of each trial, participants fixated the fixation cross in the middle of the screen and then pressed a key to correct for slippage of the head-band, drift in gaze or excessive head/body movements (drift correction). Then, the fixation cross disappeared after a random interval of 200–800 ms, and a stimulus was presented. The task of the participant was to make a saccade to the red dot. In the “short duration” condition, the stimulus was presented for 80 ms. The fixation cross reappeared 300 ms after participants made a saccade in the correct direction. In the “long duration” condition, the stimulus disappeared when the participants fixated a location for 300 ms after having made a saccade in the correct direction. At the moment, the stimulus disappeared the fixation cross reappeared. All trials containing no saccades or saccades not in the required direction were repeated at the end of a block.
Data analysis
We only analyzed primary saccades, that is, the first saccades occurring after fixation offset. Secondary saccades were relatively rare and occurred in 21% of all trials of which 14% occurred in the short stimulus durations and 86% in the long stimulus durations. If the gaze shifted within 50 ms after fixation offset, the trial was excluded from analysis, as were all trials resulting in saccadic amplitudes more than two standard deviations from the condition mean. This resulted in a total loss of 4.5% of all trials.
For each participant, we calculated a mean percent illusion effect for each combination of stimulus duration (long, short), spatial predictability of the stimulus (1, 2, or 4 directions), stimulus location (right, left, up or down) and shaft size (6.5 or 7.0 cm). The percent illusion effect was obtained by subtracting the average amplitude for the expanding configuration from the average saccadic amplitude for the compressing configuration. This difference was divided by average saccadic amplitude for the control configuration. The calculated illusion effects were pooled across the two shaft sizes. In addition, for each participant and each condition we calculated saccadic latencies, defined as the difference in time between the presentation of the stimulus and the start of a saccade. For testing the hypotheses, statistical tests are all performed on comparable conditions of equal sample sizes.
Results
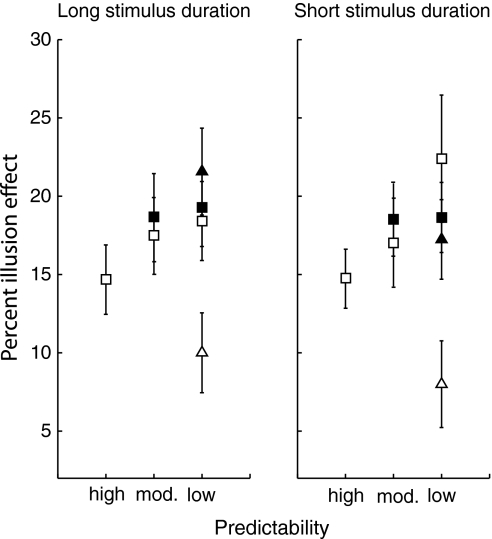
Illusion effects (in percentages) for each stimulus duration, spatial predictability and stimulus location are shown in Fig. 2. In all conditions, the Müller-Lyer illusion had a substantial effect on saccades. To check whether the illusion effect differs between the spatial predictability conditions and the stimulus durations, we performed a repeated measures ANOVA on the illusion effects of the right stimulus location (open squares in Fig. 2). The illusion affected saccades in both the short and the long stimulus duration conditions (illusion effects: 20.7 ± 1.7% and 18.5 ± 1.4%, respectively). The illusion effect on short stimulus durations was slightly larger, but not significantly different from the effect on the long stimulus durations (F(1,9) = 0.56, P = 0.47). Furthermore, a significant effect of spatial predictability was found (F(2,18) = 5.90, P = 0.01). The smallest illusion effect was present in the most predictable condition (16.5 ± 1.5%), and the illusion effect increased with decreasing predictability (moderately predictable condition: 19.4 ± 2.0%, least predictable condition: 22.8 ± 2.2%). Tukey’s post hoc analysis showed that the effect of the illusion differed between the least and the most predictable condition as well as between the least and the moderately predictable condition (both P’s < 0.01). There is no interaction between spatial predictability and stimulus duration (F(2,18) = 1.22, P = 0.32). Thus, knowledge about the spatial location of the stimulus in the upcoming trial reduces the effect the illusion has on saccadic eye movement. Additionally, we checked whether illusion effects on saccades for the left stimulus location showed a similar pattern of spatial predictability. Although non-significant, a similar trend is found for saccades to the left (F(1,9) = 2.18, P = 0.16). The illusion effect in the least predictable condition (19.2%) is larger than in the moderately predictable condition (18.4%).
Fig. 2.
Average percent illusion effect for each predictability condition for long stimulus durations (a) and for short stimulus durations (b). Open squares represent stimuli to the right of the fixation cross. Filled squares represent stimuli to the left of the fixation cross. Open and filled triangles represent stimuli below and above the fixation cross respectively. Error bars represent standard errors of the mean (between subjects)
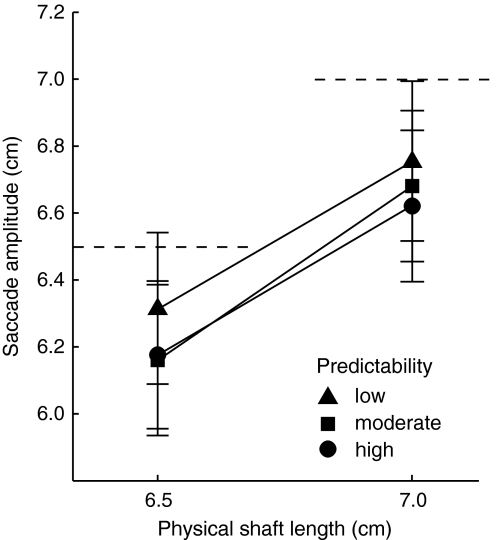
An alternative explanation for the different illusion effects in the spatial predictabilities also comes to mind. We used two shaft sizes (6.5 and 7.0 cm) to prevent participants from going to the same end position on each trial. However, participants might ignore this difference in physical shaft sizes and repeatedly make saccades of similar amplitude (to a location in between the two shaft’s end points). This would reduce or eliminate the effect of the illusion. Particularly, in the high predictability condition because subjects can pick one specific position on the screen as the endpoint for their saccades. If this alternative explanation is correct, saccades over the 6.5 and 7.0 cm shaft should have about the same amplitude (Fig. 3). This should be most clear in the trials with a high predictability. To check whether an effect of predictability can be ascribed to participants making eye movements of the same amplitude, we performed a repeated measures ANOVA on the saccadic amplitudes with the factors physical shaft size and spatial predictability. Saccadic amplitudes did significantly differ between the shaft sizes (F(1,9) = 379.68, P < 0.01), whereas no effect of predictability (F(2,9) = 1.77, P = 0.20) or an interaction (F(2,9) = 1.23, P = 0.32) could be found. Thus, we found no evidence that the effect of spatial predictability on illusion effect is caused by participants making saccades of similar amplitudes.
Fig. 3.
Saccadic amplitudes for the 6.5 and 7.0 cm shafts in the three predictability conditions. Dotted lines represent the physical shaft length. Error bars represent standard errors of the mean (between subjects)
Additionally, Fig. 2 shows that saccades toward a stimulus below the fixation point (in short and long stimulus durations) were less affected by the illusion than saccades to the other locations. To check whether stimulus location modulated the illusion effect on saccades, a repeated measures ANOVA with the factors stimulus duration (short, long) and stimulus location (right, left, up or down) was performed on the least predictable condition. Indeed a main effect of stimulus location was found (F(3,27) = 10.62, P < 0.01): right (22.8 ± 2.2%), left (21.3 ± 1.8%), down (9.4 ± 1.7%) and up (21.5 ± 1.8%). In a Tukey post hoc analysis, only the stimulus below the fixation point differed from all the others (P < 0.01). There was no main effect of stimulus duration (F(1,9) = 0.01, P = 0.93) or an interaction between stimulus duration and stimulus location (F(3,27) = 1.79, P = 0.17).
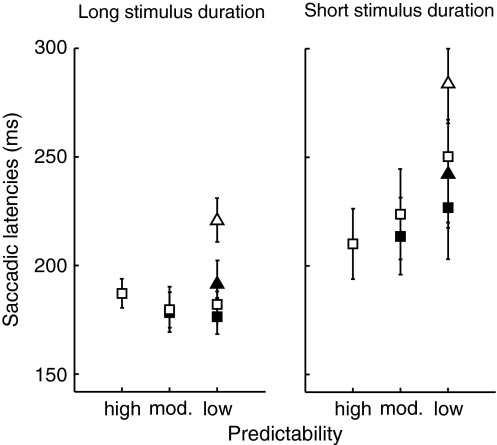
Figure 4 shows saccadic latencies for the long and short stimulus duration and for each predictability condition. A repeated measures ANOVA with the factors spatial predictability of the stimulus and stimulus duration was performed on the saccadic latencies for the right stimulus location. Saccadic latencies for long stimulus durations (183 ± 4 ms) were significantly shorter than those for short stimulus durations (228 ± 10 ms) (F(1,9) = 8.53, P = 0.02). No effect was found for spatial predictability (F(2,18) = 1.97, P = 0.17). Additionally, an interaction is found (F(2,18) = 4.07, P = 0.03). For short stimulus durations, saccadic latencies increased with decreasing predictability, whereas for long stimulus durations latencies did not significantly differ between predictability conditions.
Fig. 4.
Average saccadic latencies for each predictability condition for long stimulus durations (a) and for short stimulus durations (b). Open squares represent stimuli to the right of the fixation cross. Filled squares represent stimuli to the left of the fixation cross. Open and filled triangles represent stimuli below and above the fixation cross, respectively. Error bars represent standard errors of the mean (between subjects)
To check whether stimulus location affected saccadic latencies, a repeated measures ANOVA with the factors stimulus location and stimulus duration was performed on latencies in the least predictable condition. Saccadic latencies differed significantly between stimulus locations (F(3,27) = 16.79, P < 0.01). The latencies in all locations differed from each other (post hoc Tukey test: all P < 0.05), except the stimuli to the right (216 ± 14 ms) and above the fixation point (217 ± 10 ms). Stimuli on the left had the shortest latencies (202 ± 10 ms), whereas stimuli in the downward direction had the longest latencies (252 ms ± 9 ms). Furthermore, long stimulus durations revealed significantly shorter latencies (193 ms ± 4 ms) than short ones (251 ± 9 ms) (F(1,9) = 14.82, P < 0.01). No interaction between stimulus location and stimulus duration was found (F(3,27) = 0.81, P = 0.50).
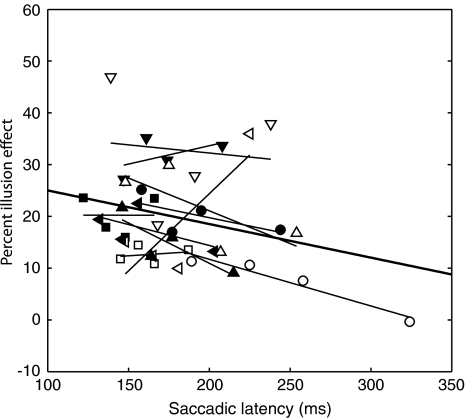
Finally, we investigated whether the illusion effect differed between saccadic latencies. To do so, latencies from conditions in which the stimulus was presented to the right of the fixation point with long stimulus durations were used. Only latencies from these conditions were included in the calculation as differences in illusion effect on saccadic latencies are confounded with stimulus duration and spatial predictability (see Fig. 4). For each participant, we divided the latency durations in quartiles. In each quartile an effect of the illusion was calculated. For each participant, a linear fit was computed on the illusion effects of the quartiles. Figure 5 shows the illusion effects in the quartiles of each participant (with fitted line). Seven participants have a negative slope (range: −0.03 to −0.15), one has a slope of 0.00 and two have a positive slope (0.10 and 0.19). For most participants, the illusion effect tends to decrease with increased saccadic latencies. This trend is summarized by a linear fit on all quartiles of all participants (thick black line in Fig. 5). The fitted line shows that for the fastest saccades (express saccades: about 80–120 ms) a maximum illusion effect of ±30% is to be expected (95% confidence interval of the intercept: 18–45%). The effect of the illusion will decrease with about 7% for every 100 ms increase in saccadic latency (95% confidence interval: 0.1–14%). A significant correlation was found between the effect of the illusion and saccadic latency (r = −0.30; P = 0.05).
Fig. 5.
Percent illusion effect plotted against saccadic latencies. The different symbols represent the average illusion effect for each participant in each of the four quartiles. The thin lines represent the linear fit over the quartiles of one participant. The thick black line is the overall fit for all data of all participants
Discussion and conclusion
In this study, we investigated whether the variability in reported effects on the effect of the Müller-Lyer illusion on saccades can be explained by differences in spatial predictability of the stimulus, stimulus duration and saccadic latencies. If the stimulus is constantly presented in the same location (high predictability) subjects only have to determine an end position for their saccades and the illusion effect will be relatively small. As predicted, spatial predictability of the stimulus modulated the effect of the illusion on saccades. We found the largest illusion effect in the least predictable condition and the illusion effect decreased with increasing predictability. This suggests that participants used more vector coding when the spatial location of the stimulus is unpredictable. It might be suggested that the effect of predictability is restricted to only one direction (saccades to the right side of the fixation point), as we performed statistical comparisons only for those displays. However, stimuli presented on the left of the fixation point (which were presented in the moderately and least predictable conditions) also showed a slightly larger illusion effect in the least predictable condition (19.2%) than in the moderately predictable condition (18.4%). Although this difference did not reach statistical significance, the pattern of results is similar to the stimuli on the right.
Conversely, stimulus duration did not clearly modulate illusion effects, although the illusion effect is slightly larger with short stimulus durations (20.7%) than with long ones (18.5%). Thus, we found no evidence for saccades being differently affected by the illusion due to stimulus duration. This lack of difference might be due to the longer latencies in the short stimulus duration conditions compared to the long stimulus duration conditions. Longer latencies provide participants more information regarding the end position of their saccades, which can reduce the illusion effect for the short stimulus durations and therefore the difference in illusion effect between short and long stimulus durations might be underestimated. Another suggestion for the lack of a significant difference in illusion effect between long and short stimulus durations might be that participants can hardly use the retinal error signal to adapt saccadic amplitude over trials (with long stimulus durations). In the introduction, we hypothesized that retinal error signals that become available at the end of a saccade (only in long stimulus durations) can be used to adapt saccadic amplitude over trials. This will result in a small effect of the illusion. When the stimulus location is only visible for a very short time, it may be more difficult to determine an accurate end position for the saccade, which will result in a large illusion effect. Here, we did not find a significant difference in illusion effect between long and short stimulus durations. This might be due to the way this study was set up: the randomization procedure prevented identical stimuli to appear on successive trials. This minimizes opportunities for saccadic adaptation. Thus, large illusion effects are found for both short and long stimulus durations. Finally, we found larger illusion effects for shorter saccadic latencies (see Fig. 5). The shortest saccades (express saccades) have the maximum illusion effect of about 30%. This amount decreased to about 11% for saccadic latencies up to 330 ms, which were the maximum latencies found in this study.
Our latency data are consistent with the results of Van Zoest and Hunt (2008), who reported larger illusion effects for shorter saccadic latencies. In contrast, De’Sperati and Baud-Bovy (2008) found that the illusion effect on saccades increased with increasing saccadic latencies. This was interpreted as evidence for a dissociation between vision-for-perception and vision-for-action. However, their results can be explained in a different way. Processing the trajectory of a moving stimulus requires the activation of motion detectors over time, which results in a longer temporal window for detecting and processing motion than for merely locating a stationary target dot. This suggests that the effect of a moving context will build up over time and thus illusory motion information is simply not available for short-latency saccades. Therefore, the motion illusion will only show up in saccades with longer latencies. In our experiment, the stimulus was static, and all information was already present before the start of the saccade. Given that we found the largest illusion effects on saccades with the shortest latencies, our data suggest that for short-latency saccades there is not enough time to determine an accurate target position. Therefore, participants are forced to use another source of information, such as perceived length. Thus, depending on the task constraints different information is used to perform the task, resulting in different illusion effects. Consistent with previous studies on pointing and grasping (Bruno et al. 2008; Bruno and Franz 2009), the findings in this study confirm that the large variability in the results of studies investigating illusion effects on saccades can be ascribed to specific experimental differences.
An additional and interesting finding of the current experiment is the smaller illusion effect for downward saccades compared to the other directions. A similar pattern of results on saccadic eye movements was reported by De Grave et al. (2006a, b). They suggested that the reduction in illusion effect may be caused by the hand partly occluding the visual stimulus, which may have prompted the eye to saccade to a predetermined position. However, this cannot be a valid explanation in this study as no hand movements or any other objects were present to occlude the stimulus below the fixation point. Another explanation for smaller illusion effects in the downward saccades might be the longer latencies for these saccades. According to Van Zoest and Hunt (2008), longer latencies result in smaller illusion effects as long latencies provide the participant with enough time to determine an accurate position of the target. We did find significantly longer saccadic latencies for downward saccades, similar to Dafoe et al. (2007) and Bell et al. (2000). Based on the overall fit in Fig. 5 (thick line), we were able to test whether the smaller illusion effect for the downward stimulus location could be ascribed to larger saccadic latencies. For the downward location in long stimulus durations, the estimated illusion effect was 19%. This estimated value was larger than the observed illusion effect for this condition (10%, see Fig. 2). Thus, the smaller illusion effect on downward saccades cannot be completely explained by an increase in saccadic latency. A third possibility involves differences in acuity between the lower and upper visual field. Outside the fovea, the superior retina projects to a larger area in the visual cortex (Van Essen et al. 1984) and has more ganglion cells (Curcio and Allen 1990) than the inferior retina. This difference may allow more accurate localization of the target in the lower visual field, at the cost of slightly slower processing. Interestingly, better performance for visually guided pointing in the lower visual field is reported by Danckert and Goodale (2001). Additionally, Krigolson and Heath (2006) found better aimpoint precision in the lower visual field compared to the upper field. Given these results, we speculate that better motor performance in the lower visual field occurs due to better retinal and cortical acuity.
In conclusion, we found that the large variety in illusion effects on saccades can be ascribed to several factors. Although saccades show a substantial illusion effect under all conditions, the amount of illusion effect is modulated by spatial predictability of the stimulus and saccadic latencies. These results argue against strong independence of vision-for-action and vision-for-perception (Goodale and Milner 1992) as in none of the conditions in this study saccades appeared to be immune to perceptual illusory effects. More specifically, we have provided evidence that a reduction in the illusion effect occurs when the stimulus is at a more predictable location, when saccadic latencies are long (>250 ms) and when the stimulus is presented below the fixation point.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aglioti S, DeSouza JF, Goodale MA. Size-contrast illusions deceive the eye but not the hand. Curr Biol. 1995;5(6):679–685. doi: 10.1016/S0960-9822(95)00133-3. [DOI] [PubMed] [Google Scholar]
- Albano JE, King WM. Rapid adaptation of saccadic amplitude in humans and monkeys. Investig Ophthalmol Vis Sci. 1989;30(8):1883–1893. [PubMed] [Google Scholar]
- Bell AH, Everling S, Munoz DP. Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J Neurophysiol. 2000;84(5):2595–2604. doi: 10.1152/jn.2000.84.5.2595. [DOI] [PubMed] [Google Scholar]
- Bernardis P, Knox P, Bruno N. How does action resist visual illusion? Uncorrected oculomotor information does not account for accurate pointing in peripersonal space. Exp Brain Res. 2005;162(2):133–144. doi: 10.1007/s00221-004-2121-9. [DOI] [PubMed] [Google Scholar]
- Binsted G, Elliott D. Ocular perturbations and retinal/extraretinal information: the coordination of saccadic and manual movements. Exp Brain Res. 1999;127(2):193–206. doi: 10.1007/s002210050789. [DOI] [PubMed] [Google Scholar]
- Bruno N. When does action resist visual illusions? Trends Cognit Sci. 2001;5(9):379–382. doi: 10.1016/S1364-6613(00)01725-3. [DOI] [PubMed] [Google Scholar]
- Bruno N, Franz VH. When is grasping affected by the Muller-Lyer illusion? A quantitative review. Neuropsychologia. 2009;47(6):1421–1433. doi: 10.1016/j.neuropsychologia.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Bruno N, Bernardis P, Gentilucci M. Visually guided pointing, the Muller-Lyer illusion, and the functional interpretation of the dorsal-ventral split: conclusions from 33 independent studies. Neurosci Biobehav Rev. 2008;32(3):423–437. doi: 10.1016/j.neubiorev.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Coëffé C, O’Regan JK. Reducing the influence of non-target stimuli on saccade accuracy: predictability and latency effects. Vis Res. 1987;27(2):227–240. doi: 10.1016/0042-6989(87)90185-4. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comput Neurol. 1990;300(1):5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Dafoe JM, Armstrong IT, Munoz DP. The influence of stimulus direction and eccentricity on pro- and anti-saccades in humans. Exp Brain Res. 2007;179(4):563–570. doi: 10.1007/s00221-006-0817-8. [DOI] [PubMed] [Google Scholar]
- Danckert J, Goodale MA. Superior performance for visually guided pointing in the lower visual field. Exp Brain Res. 2001;137(3–4):303–308. doi: 10.1007/s002210000653. [DOI] [PubMed] [Google Scholar]
- De Grave DDJ, Brenner E, Smeets JBJ. Illusions as a tool to study the coding of pointing movements. Exp Brain Res. 2004;155(1):56–62. doi: 10.1007/s00221-003-1708-x. [DOI] [PubMed] [Google Scholar]
- De Grave DDJ, Franz VH, Gegenfurtner KR. The influence of the Brentano illusion on eye and hand movements. J Vis. 2006;6(7):727–738. doi: 10.1167/6.7.5. [DOI] [PubMed] [Google Scholar]
- De Grave DDJ, Smeets JBJ, Brenner E. Why are saccades influenced by the Brentano illusion? Exp Brain Res. 2006;175(1):177–182. doi: 10.1007/s00221-006-0536-1. [DOI] [PubMed] [Google Scholar]
- De’Sperati C, Baud-Bovy G. Blind saccades: an asynchrony between seeing and looking. J Neurosci. 2008;28(17):4317–4321. doi: 10.1523/JNEUROSCI.0352-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresman C, Saucier D, Heath M, Binsted G. Online corrections can produce illusory bias during closed-loop pointing. Exp Brain Res. 2008;188(3):371–378. doi: 10.1007/s00221-008-1367-z. [DOI] [PubMed] [Google Scholar]
- Festinger L, White CW, Allyn MR. Eye movements and decrement in the Muller-Lyer illusion. Percept Psychophys. 3;3(5B):376–382. [Google Scholar]
- Franz VH. Action does not resist visual illusions. Trends Cognit Sci. 2001;5(11):457–459. doi: 10.1016/S1364-6613(00)01772-1. [DOI] [PubMed] [Google Scholar]
- Franz VH, Gegenfurtner KR. Grasping visual illusions: consistent data and no dissociation. Cognit Neuropsychol. 2008;25(7):920–950. doi: 10.1080/02643290701862449. [DOI] [PubMed] [Google Scholar]
- Franz VH, Gegenfurtner KR, Bulthoff HH, Fahle M. Grasping visual illusions: no evidence for a dissociation between perception and action. Psychol Sci. 2000;11(1):20–25. doi: 10.1111/1467-9280.00209. [DOI] [PubMed] [Google Scholar]
- Glover SR. Separate visual representations in the planning and control of action. Behav Brain Sci. 2004;27(1):3–24. doi: 10.1017/s0140525x04000020. [DOI] [PubMed] [Google Scholar]
- Goodale MA. Action without perception in human vision. Cognit Neuropsychol. 2008;25(7–8):891–919. doi: 10.1080/02643290801961984. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol. 2004;14:1–9. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Jacob P, Jeannerod M. Ways of seeing: the scope and limits of visual cognition. Oxford: Oxford University Press; 2003. [Google Scholar]
- Knox PC. The effect of Kanizsa’s compression illusion on reflexive saccades. Exp Brain Res. 2006;175(4):764–768. doi: 10.1007/s00221-006-0741-y. [DOI] [PubMed] [Google Scholar]
- Krigolson O, Heath M. A lower visual field advantage for endpoint stability but no advantage for online movement precision. Exp Brain Res. 2006;170(1):127–135. doi: 10.1007/s00221-006-0386-x. [DOI] [PubMed] [Google Scholar]
- Lavrysen A, Helsen WF, Elliott D, Buekers MJ, Feys P, Heremans E. The type of visual information mediates eye and hand movement bias when aiming to a Muller-Lyer illusion. Exp Brain Res. 2006;174:544–554. doi: 10.1007/s00221-006-0484-9. [DOI] [PubMed] [Google Scholar]
- McCarley JS, Kramer AF, DiGirolamo GJ. Differential effects of the Muller-Lyer illusion on reflexive and voluntary saccades. J Vis. 2003;3:751–760. doi: 10.1167/3.11.9. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. The visual brain in action. Oxford: Oxford University Press; 1995. [Google Scholar]
- Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46(3):774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Munoz DP (2002) Commentary: saccadic eye movements: overview of neural circuitry. In: Hyona J, Munoz DP, Heide W, Radach R (eds). Prog Brain Res 140:89–96 [DOI] [PubMed]
- Pavani F, Boscagli I, Benvenuti F, Rabuffetti M, Farne A. Are perception and action affected differently by the Titchener circles illusion? Exp Brain Res. 1999;127(1):95–101. doi: 10.1007/s002210050777. [DOI] [PubMed] [Google Scholar]
- Schenk T, McIntosh R (2010) Do we have independent visual streams for perception and action? Cognit Neurosci 1(1):52–62 [DOI] [PubMed]
- Smeets JBJ, Brenner E. Perception and action are based on the same visual information: distinction between position and velocity. J Exp Psychol Hum Percept Perform. 1995;21(1):19–31. doi: 10.1037/0096-1523.21.1.19. [DOI] [PubMed] [Google Scholar]
- Smeets JBJ, Brenner E. 10 years of illusions. J Exp Psychol Hum Percept Perform. 2006;32(6):1501–1504. doi: 10.1037/0096-1523.32.6.1501. [DOI] [PubMed] [Google Scholar]
- Tegetmeyer H, Wenger A. Influence of visual perception on spatial coding of saccadic eye movements and fixation. Neuro Ophthalmol. 2004;28(5–6):197–204. doi: 10.1080/01658100490887931. [DOI] [Google Scholar]
- Tegetmeyer H, Wenger A. Der Einfluss geometrisch-optischer Tauschungen auf sakkadische Augenbewegungen. Klinisches Monatsblat fuer Augenheilkunde. 2006;223:84–87. doi: 10.1055/s-2005-858870. [DOI] [PubMed] [Google Scholar]
- Thompson AA, Westwood DA. The hand knows something that the eye does not: reaching movements resist the Muller-Lyer illusion whether or not the target is foveated. Neurosci Lett. 2007;426(2):111–116. doi: 10.1016/j.neulet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Trevarthen CB. Two mechanisms of vision in primates. Psychologische Forschung. 1968;31(4):299–348. doi: 10.1007/BF00422717. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RWJ, editors. Analysis of visual behavior. Cambridge: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vis Res. 1984;24(5):429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- Van Zoest W, Hunt A. Visual illusions: saccadic and manual responses reveal a similar time-course. Perception. 2008;37:s152. [Google Scholar]
- Vishton PM, Rea JG, Cutting JE, Nunez LN. Comparing effects of the horizontal-vertical illusion on grip scaling and judgment: relative versus absolute, not perception versus action. J Exp Psychol Hum Percept Perform. 1999;25(6):1659–1672. doi: 10.1037/0096-1523.25.6.1659. [DOI] [PubMed] [Google Scholar]
- West GL, Welsh TN, Pratt J. Saccadic trajectories receive online correction: evidence for a feedback-based system of oculomotor control. J Mot Behav. 2009;41(2):117–127. doi: 10.3200/JMBR.41.2.117-127. [DOI] [PubMed] [Google Scholar]
- Westwood DA, Goodale MA. Perceptual illusion and the real-time control of action. Spat Vis. 2003;16(3–4):243–254. doi: 10.1163/156856803322467518. [DOI] [PubMed] [Google Scholar]
- Wong E, Mack A. Saccadic programming and perceived location. Acta Psychol. 1981;48(1–3):123–131. doi: 10.1016/0001-6918(81)90054-8. [DOI] [PubMed] [Google Scholar]