Abstract
Purpose
The incidence of differentiated thyroid cancer is increasing in young adults and females in Korea. Some of them experience short-term hypothyroidism in preparation for radioiodine (RAI) therapy, which can have a deleterious effect on the cardiovascular system. However, it is not clear if short-term hypothyroidism induces endothelial dysfunction in patients with low cardiovascular risk. Therefore, the aim of this study was to investigate whether short-term hypothyroidism is associated with endothelial dysfunction in patients with low cardiovascular risk.
Materials and Methods
To evaluate the effect of short-term hypothyroidism on endothelial function in this group, we recruited fifteen female patients with low cardiovascular risk. We analyzed clinical, biochemical, and cardiovascular parameters at four time points: the last day on levothyroxine (LT4) at their usual thyroid-stimulating hormone (TSH)-suppressive doses (P1), 7 days (P2) & 4 weeks (P3) after withdrawal of LT4, and 8 weeks (P4) after replacement of the previous dose of LT4. A high resolution ultrasound was used to measure brachial artery diameter at rest, after reactive hyperemia, and after sublingual nitroglycerin.
Results
During short-term hypothyroidism (P3), serum concentrations of total cholesterol and low-density lipoprotein (LDL)-cholesterol were increased (p < 0.001 for each period). In spite of having worsened lipid states, serum high sensitivity C-reactive protein or flow-mediated vasodilatation, which is one of the surrogate markers of the endothelial function, did not change during short-term hypothyroidism.
Conclusion
Short-term hypothyroidism induced worsening of metabolic parameters, but not enough to induce the endothelial dysfunction in patients with low cardiovascular risk.
Keywords: Short-term hypothyroidism, endothelial dysfunction, thyroid cancer, radioactive iodine, flow-mediated vasodilatation
INTRODUCTION
Radioactive iodine (RAI) therapy is widely used in differentiated thyroid cancer for thyroid remnant ablation and remnant disease treatment.1,2 A high level of thyroid-stimulating hormone (TSH) is required to stimulate sufficient radioiodine uptake for therapy. This can be obtained either by withdrawal of thyroid hormone or by the injection of recombinant human TSH (Thyrogen®, Genzyme, Allston, MA, USA); the prior method is more commonly used. Therefore, during RAI therapy, most patients experience varied states of thyroid disease, ranging from subclinical hyperthyroidism to short-term hypothyroidism.
Atherosclerosis is a chronic disease that makes arterial walls thick and less elastic. The resulting restriction of blood flow can lead to various problems such as heart attacks, stroke, and other serious cardiovascular complications. Overt hypothyroidism is associated with cardiovascular disease mortality and it is a well-known risk factor of atherosclerosis. The atherosclerosis is induced through the aggravation of lipid profiles, insulin resistance, hypertension, arterial stiffness, and endothelial dysfunction in hypothyroid states.3-5 However, the effect of short-term hypothyroidism on atherosclerosis or cardiovascular disease is not clear yet.
Erbil, et al.6 reported that short-term hypothyroidism induces endothelial dysfunction and recovers after correcting the hypothyroid state, although there were no clinical cardiovascular events during their study. In the study, the Turkish people were overweight [body mass index (BMI) 28.8 ± 3.7 kg/m2] and had higher levels of cholesterol at the baseline compared with our patients.6 Considering that cardiovascular disease is affected by many environmental and genetic factors, further studies are warranted to apply the previous results to the Korean population. The incidence of thyroid cancer is increasing in Korea, especially in women, who have a relatively lower cardiovascular risk than men.7 Thus, we evaluated endothelial function and metabolic parameters before and after levothyroxine (LT4) withdrawal prior to RAI therapy in a single cohort of Korean female patients with differentiated thyroid cancer who did not have well-established metabolic risk factors.
MATERIALS AND METHODS
Subjects and study protocols
Fifteen female patients with differentiated thyroid cancer treated by bilateral total or near-total thyroidectomy were included in this prospective study (age 49.1 ± 7.2 years; range 38-62 years). All patients were non-smokers. Before the recruitment, none of the patients had clinically detectable diabetes, hypertension, coronary artery disease, renal disease, liver disease, or familial hypercholesterolemia. They also had no family history of cardiovascular disease.
All patients were treated by withdrawal of LT4 for 4 weeks. This procedure resulted in serum TSH levels higher than 30 mU/L in all patients. Each patient was evaluated at four time points: the last day on LT4 at their usual TSH-suppressive doses (P1), 7 days after withdrawal (P2), the day of RAI therapy (P3), and 8 weeks after RAI therapy (P4). The previous dose of LT4 restarted after RAI therapy. Evaluations included measurements of physical, biochemical, and cardiovascular parameters.
After a 12-hour fast, blood was collected from participants and immediately centrifuged. Sera were stored at -70℃ and measured after all patients had completed their follow-up (P4). The Institutional Review Board of the Seoul National University Bundang Hospital approved the study protocol, and written informed consent was obtained from all subjects.
Anthropometric and body fat measurements
The subjects' height and weight were measured using an automatic height-weight scale while they wore light clothing. BMI was then calculated as the ratio between weight and height squared (kg/m2). Blood pressure was measured four times on each occasion using a standard mercury sphygmomanometer after 10 minutes rest during which the subject was sitting. Waist circumference was measured at the narrowest point between the lower border of the rib cage and the iliac crest. Hip circumference was measured at the level of the symphysis pubis and the greatest gluteal protuberance. The waist to hip ratio was then calculated by dividing waist circumference by hip circumference.
The percentages of body fat and lean body mass were measured using a tetrapolar bioelectrical impedance analyzer (Inbody 3.0®, Biospace, Seoul, Korea). Bioelectrical impedance measures two parameters, fat and lean tissue, using empirically derived formulas that have been validated, and yield they estimates that correlate with estimates obtained using underwater weighing.
Measurements of thyroid function and metabolic parameters
The serum TSH and free T4 concentrations were measured by immunoradiometric assays using commercial kits (TSH, CIS bio international, Gif-sur-Yvette, France; free T4, DiaSorin S.p.A, Saluggia, Italy). The normal ranges were 0.4-4.1 mIU/L for serum TSH and 0.7-1.8 ng/dL for free T4, as reported by the Central Laboratory of Seoul National University Bundang Hospital. Total cholesterol, triglyceride, and high-density lipoprotein (HDL)-cholesterol concentrations were measured enzymatically using the TBA-200FR system (Toshiba, Tokyo, Japan) according to the manufacturer's instructions. Serum low-density lipoprotein (LDL)-cholesterol levels were calculated using Friedewald's equation: LDL = total cholesterol-HDL-triglyceride/5. The apolipoprotein A and apolipoprotein B concentrations were measured using immunoturbidimetric assays (Randox, Crumlin, UK) on the 502X system (A&T, Tokyo, Japan). High sensitive-C-reactive protein (hs-CRP) was measured using a highly sensitive particle enhanced light-scattering immunoassay (Denka Seiken, Tokyo, Japan) on the TBA-200FR system according to the manufacturer's instructions.
Vascular studies
The vascular studies of the brachial artery were performed noninvasively, as described previously,8,9 at each of the four stages (P1, 2, 3, 4). Patients were investigated by high-resolution imaging of the brachial artery of the dominant arm using a 15 MHz linear array probe (Sonos 7500, Philips Medical Systems, Andover, MA, USA) to assess endothelial responses. A sphygmomanometer cuff was placed between the elbow and hand. The brachial artery internal diameter was measured from the anterior to the posterior "m" line, the interface between media and adventia, at the end-diastole gated on the R wave of the electrocardiogram (ECG). For the reactive hyperemia scan, another diameter measurement was taken 45-60 s after cuff deflation. Four cardiac cycles were analyzed for each scan and the measurements were averaged. In each patient, scans were taken at rest, during reactive hyperemia (with increased flow induced by inflation and then deflation of a sphygmomanometer cuff around the limb, distal to the scanned part of the artery), again at rest, and after administration of sublingual nitroglycerin (an endothelium-independent smooth muscle mediated vasodilatation). The subjects rested in supine position for at least 10 min before the first resting scan was recorded. Increased flow was then induced by inflating a pneumatic tourniquet to a pressure of 300 mmHg for 5 min. The second scan was taken 45-60 s after cuff deflation. A time interval of 15 min was allowed for vessel recovery, and consecutive resting scans were taken. Sublingual nitroglycerin (0.6 mg) was then administered, and 3 min later, the final scan was performed. Each vessel diameter in scans after reactive hyperemia, 15 min at rest, and with nitroglycerin was calculated in percentages of the first control scan, that is, % diameter changes = [Vessel Diameter (during reactive hyperemia or after nitroglycerin) - Vessel Diameter (resting)]/vessel diameter (resting). Each Doppler examination was performed by the same investigator who was unaware of the laboratory values at the time of the examination.
Statistical analysis
Data are reported as mean (s.d.). Differences between continuous variables before and after treatment were compared by means of repeated measures ANOVA [general linear model (GLM) procedure in SPSS]. Prior to ANOVA procedures, the assumption of sphericity was tested with Mauchly's test. When the sphericity assumption was not met, the multivariate test statistic was determined using Wilks' lambda test for significance testing. For comparing the profiles at P1 and P4, the nonparametric Wilcoxon's rank test was used for paired samples. Results were considered statistically significant at p < 0.05. All data were analyzed using the Statistical Package for the Social Sciences (SPSS® 12.0 for Windows, SPSS, Chicago, IL, USA).
RESULTS
Changes of thyroid function
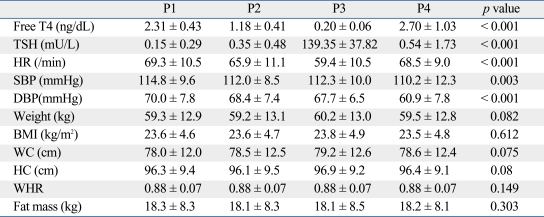
As expected with chronic treatment with supraphysiological doses of LT4, the mean serum thyroid hormone levels were in the mild hyperthyroid range at the first evaluation (P1) (Table 1).
Table 1.
Effect of Thyroid Dysfunction on Physical Parameters
BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist-hip ratio.
Data are presented as means ± SD.
At the second visit (P2: 1 week after LT4 withdrawal), the mean TSH level was below the normal range, whereas the mean serum free T4 was within the normal range. At the third time point (P3), all patients presented with an increased serum TSH (139.35 ± 37.82 mU/L) and low serum free T4 (0.20 ± 0.06 ng/dL) levels (p < 0.001, respectively).
At the fourth time point (P4: 8 weeks after LT4 resumption), all patients had recovered from the short-term hypothyroidism. Some patients did not show suppressed TSH levels despite supraphysiological LT4 treatment. However, there was no difference in serum TSH (p = 0.556) and free T4 (p = 0.177) levels between P1 and P4.
Changes in anthropometric parameters
As shown in Table 1, the heart rate significantly decreased during short-term hypothyroidism (P3) compared to P1 (59.4 ± 10.5 vs. 69.3 ± 10.5 beats/min, p < 0.001). Systolic and diastolic blood pressures significantly decreased as the study went on (i.e., blood pressure was highest at P1, lowest at P4), but the differences in blood pressure at each time were negligible. There was a trend that short-term hypothyroidism increased body weight, waist circumference, and hip circumferences, but the changes were not statistically significant. The other physiologic findings of fat mass, BMI, and waist-hip ratio did not change throughout the study in spite of thyroid dysfunction.
Changes in lipid profiles
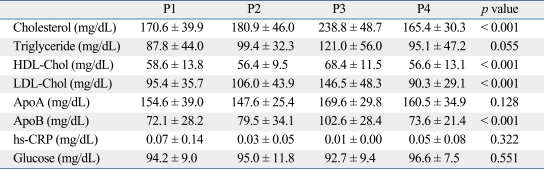
The levels of total cholesterol, apolipoprotein B, and LDL-cholesterol significantly increased during the short-term hypothyroidism and they improved after reversal of the acute hypothyroidism (Table 2). However, the level of HDL-cholesterol significantly increased during short-term hypothyroidism (p < 0.001).
Table 2.
Effect of Thyroid Dysfunction on Lipid Profiles
HDL, high-density lipoprotein; LDL, low-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein.
Serum LDL levels were calculated using Friedewald's equation.
Data are presented as means ± SD.
Changes in parameters representing endothelial functions
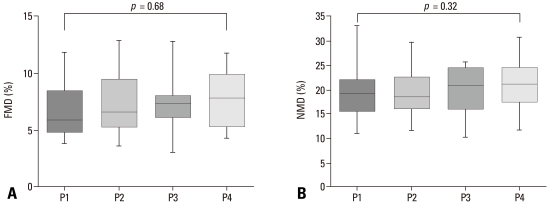
Serum hsCRP levels did not change throughout the study (p = 0.322). Both flow-mediated dilatation (FMD) and nitroglycerin-mediated dilatation (NMD) were measured at each time point; however, there were no differences according to thyroid status (Fig. 1). There were no clinical cardiovascular events during the study.
Fig. 1.
Effect of thyroid dysfunction on endothelial function. (A) Flow-mediated arterial dilatation (FMD) (endothelin-dependent vasodilatation) and (B) nitroglycerin-mediated dilatation (NMD) (endothelin-independent vasodilatation) was measured at each of the four stages (P1, 2, 3, 4). Differences between continuous variables before and after treatment were compared by means of repeated measures ANOVA. Median values, interqurtile ranges, and ranges are denoted by horizontal bars, boxes, and error bars.
DISCUSSION
This study was designed to show whether the changes of metabolic parameters during short-term hypothyroidism resulting from LT4 withdrawal could affect endothelial function in patients with low cardiac risk. This study showed that short-term hypothyroidism had deleterious effects on lipid metabolism, but not endothelial function in patients with low cardiovascular risk.
It is very important for clinicians to know whether LT4 withdrawal for RAI in differentiated thyroid cancer patients can aggravate or initiate ischemic insults to the heart, because ischemic heart disease causes serious morbidity and mortality. Ischemic heart disease is mainly caused by atherosclerotic changes in arteries, and atherosclerosis begins from the innermost layer of the artery (the intima) by inflammatory processes, i.e. endothelial dysfunction.10 Thus, endothelial functions are used to assess cardiovascular risk by many researchers. FMD is known to be a reliable and reproducible noninvasive measure of vascular endothelial function.11 Although our study limited because we measured endothelial function not by intraarterial pharmacologic stimulation but by FMD, endothelial function assessed by FMD correlates significantly with invasive testing of coronary endothelial function as well as with the severity and extent of coronary atherosclerosis.11 Thus, noninvasive FMD provides valuable information into vascular changes associated with early atherogenesis and this merit led to our study.
It is well known that overt hypothyroidism increases the total cholesterol level and LDL-cholesterol because of decreased fractional clearance of LDL due to a reduced number of LDL receptors in the liver.12 It is also established that endothelial dysfunction acts as a promoter of atherosclerosis and is associated with an increased risk of cardiovascular events.13,14 Many studies have demonstrated that thyroid hormone replacement improves intima-media thickness, arterial stiffness, and endothelial dysfunction in chronically hypothyroid patients,5,15-18 including patients with subclinical hypothyroidism.18,19 Thyroid hormones are considered to have direct vasodilatory effects on vascular smooth muscle cells, and may exert part of their vascular effects through an endothelium-mediated mechanism.6 Thus, these findings suggest that overt hypothyroidism is associated with atherosclerosis initiating by endothelial dysfunction.
There are data about the effects of short-term hypothyroidism on the cardiovascular system; however, most studies focused on the functions relating to ECG changes, pump performance, and echocardiographic changes.3,20-22 Only two studies reported the association between short-term hypothyroidism and endothelial dysfunction, and showed that short-term hypothyroidism aggravated lipid profiles and FMD.6,23
As expected, the results of this study correspond with the earlier studies that short-term hypothyroidism increased total and LDL-cholesterol.3,6,22-24 In addition, these abnormalities improved after LT4 treatment, as seen in other studies. However, our results failed to show changes in endothelial function according to the degree of thyroid function. There was no difference in FMD (%) between P3 (7.32 ± 2.26%) and P4 (7.61 ± 2.48%). We estimated the sample size (the number of patients = 15) from the previous data6,23 (i.e., mean FMD = 12.0, SD = 3.0, expected difference = 0.2) at α= 0.05 and power (1-β) = 0.8. We could not find any FMD change during hypothyroid states due to a discrepancy between expected data and obtained data. Lesser changes could have been missed due to a small FMD (%) difference between hypothyroid and hyperthyroid states.
One of the possible reasons of the discrepancy is the different metabolic risk among these groups. Compared to our patients, the Turkish patients (n = 22) had higher levels of total and LDL-cholesterol at baseline. These differences were maintained during overt hypothyroidism in the Turkish group,6 which means that the Turkish group was exposed to more atherogenic conditions. Furthermore, our group had higher HDL-cholesterol (68.4 ± 11.5 mg/dL) during short-term hypothyroidism than those of Turkish group (43.9 ± 8.8 mg/dL). As higher HDL-cholesterol is known for numerous beneficial effects including improvement of endothelial function, anti-inflammatory, anti-thrombotic, antioxidative effects, and the stimulation of endothelial regeneration,25 it may reverse the endothelial dysfunction during short-term hypothyroidism. Being overweight and relatively hypercholesterolemia might influence endothelial dysfunction, even when a clinical disease has not yet developed. In addition, the small number of subjects and their ethnicity may have contributed to the differing results. The difference between the Chinese group and ours can be explained by HDL-cholesterol concentration. The Chinese group (n = 12)23 that showed a negative correlation between TSH and FMD had a relatively lower concentration of HDL-cholesterol compared to ours.
Based on large-scale epidemiological and prospective hormone treatment trials, CRP, a marker of inflammation, has been reported as a strong predictor of cardiovascular risk.26 However, the impact of hypothyroidism on CRP is still controversial. Some studies have reported that CRP levels are higher in patients with hypothyroidism than in healthy controls,27,28 whereas others have reported no changes in CRP levels.6,29 In this study, we could not find any significant differences in CRP levels according to the thyroid function status. This finding is consistent with our FMD results.
It is also consistent with other studies which indicated that short-term hypothyroidism does not induce changes in the more atherogenic small LDL particles30 and the antiatherogenic protein adiponectin,24 suggesting that transient lipid changes during short-term hypothyroidism may not induce endothelial dysfunction in patients with low cardiac risk.
In conclusion, we demonstrated that short-term hypothyroidism after T4 withdrawal induced deleterious effects on lipid profiles; however, it does not induce significant changes in endothelial function. Thus, RAI using LT4 withdrawal can be safely used on patients with low cardiovascular risk.
ACKNOWLEDGEMENTS
We are thankful for the support of the Medical Research Collaborating Center of the Seoul National University for the statistical analysis.
Footnotes
Abstract presented at the 78th Annual Meeting of the American Thyroid Association, New York, USA, October 4-7, 2007.
The authors have no financial conflicts of interest.
References
- 1.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 3.Duntas LH, Biondi B. Short-term hypothyroidism after Levothyroxine-withdrawal in patients with differentiated thyroid cancer: clinical and quality of life consequences. Eur J Endocrinol. 2007;156:13–19. doi: 10.1530/eje.1.02310. [DOI] [PubMed] [Google Scholar]
- 4.Flynn RW, Macdonald TM, Jung RT, Morris AD, Leese GP. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab. 2006;91:2159–2164. doi: 10.1210/jc.2005-1833. [DOI] [PubMed] [Google Scholar]
- 5.Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, et al. Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab. 2003;88:3731–3737. doi: 10.1210/jc.2003-030039. [DOI] [PubMed] [Google Scholar]
- 6.Erbil Y, Ozbey N, Giriş M, Salmaslioğlu A, Ozarmağan S, Tezelman S. Effects of thyroxine replacement on lipid profile and endothelial function after thyroidectomy. Br J Surg. 2007;94:1485–1490. doi: 10.1002/bjs.5915. [DOI] [PubMed] [Google Scholar]
- 7.The annual report of national cancer registration program. Ministry for health welfare, Korea Central Cancer Registry. 2007. Ref Type: Report ( www.cancer.go.kr/cms/statics/incidence/index.html)
- 8.Chang HJ, Chung J, Choi SY, Yoon MH, Hwang GS, Shin JH, et al. Endothelial dysfunction in patients with exaggerated blood pressure response during treadmill test. Clin Cardiol. 2004;27:421–425. doi: 10.1002/clc.4960270713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 10.Fuster V. Atherosclerosis, thrombosis, and vascular biology. In: Goldman, Ausiello, editors. Cecil Textbook of Medicine. 22nd ed. Philadelphia: Saunders; 2004. pp. 383–389. [Google Scholar]
- 11.Raitakari OT, Celermajer DS. Flow-mediated dilatation. Br J Clin Pharmacol. 2000;50:397–404. doi: 10.1046/j.1365-2125.2000.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biondi B, Klein I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine. 2004;24:1–13. doi: 10.1385/ENDO:24:1:001. [DOI] [PubMed] [Google Scholar]
- 13.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 14.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 15.Papaioannou GI, Lagasse M, Mather JF, Thompson PD. Treating hypothyroidism improves endothelial function. Metabolism. 2004;53:278–279. doi: 10.1016/j.metabol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Monzani F, Caraccio N, Kozàkowà M, Dardano A, Vittone F, Virdis A, et al. Effect of levothyroxine replacement on lipid profile and intima-media thickness in subclinical hypothyroidism: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2004;89:2099–2106. doi: 10.1210/jc.2003-031669. [DOI] [PubMed] [Google Scholar]
- 17.Owen PJ, Rajiv C, Vinereanu D, Mathew T, Fraser AG, Lazarus JH. Subclinical hypothyroidism, arterial stiffness, and myocardial reserve. J Clin Endocrinol Metab. 2006;91:2126–2132. doi: 10.1210/jc.2005-2108. [DOI] [PubMed] [Google Scholar]
- 18.Owen PJ, Sabit R, Lazarus JH. Thyroid disease and vascular function. Thyroid. 2007;17:519–524. doi: 10.1089/thy.2007.0051. [DOI] [PubMed] [Google Scholar]
- 19.Razvi S, Ingoe L, Keeka G, Oates C, McMillan C, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:1715–1723. doi: 10.1210/jc.2006-1869. [DOI] [PubMed] [Google Scholar]
- 20.Botella-Carretero JI, Gómez-Bueno M, Barrios V, Caballero C, García-Robles R, Sancho J, et al. Chronic thyrotropin-suppressive therapy with levothyroxine and short-term overt hypothyroidism after thyroxine withdrawal are associated with undesirable cardiovascular effects in patients with differentiated thyroid carcinoma. Endocr Relat Cancer. 2004;11:345–356. doi: 10.1677/erc.0.0110345. [DOI] [PubMed] [Google Scholar]
- 21.Di Paola R, Alagona C, Pezzino V, Mangiameli S, Regalbuto C. Left ventricular function in acute hypothyroidism: a Doppler echocardiography study. Ital Heart J. 2004;5:857–863. [PubMed] [Google Scholar]
- 22.Regalbuto C, Alagona C, Maiorana R, Di Paola R, Cianci M, Alagona G, et al. Acute changes in clinical parameters and thyroid function peripheral markers following L-T4 withdrawal in patients totally thyroidectomized for thyroid cancer. J Endocrinol Invest. 2006;29:32–40. doi: 10.1007/BF03349174. [DOI] [PubMed] [Google Scholar]
- 23.Guang-da X, Hong-yan C, Xian-mei Z. Changes in endothelium-dependent arterial dilation before and after subtotal thyroidectomy in subjects with hyperthyroidism. Clin Endocrinol (Oxf) 2004;61:400–404. doi: 10.1111/j.1365-2265.2004.02112.x. [DOI] [PubMed] [Google Scholar]
- 24.Botella-Carretero JI, Alvarez-Blasco F, Sancho J, Escobar-Morreale HF. Effects of thyroid hormones on serum levels of adipokines as studied in patients with differentiated thyroid carcinoma during thyroxine withdrawal. Thyroid. 2006;16:397–402. doi: 10.1089/thy.2006.16.397. [DOI] [PubMed] [Google Scholar]
- 25.Warnholtz A, Wild P, Ostad MA, Elsner V, Stieber F, Schinzel R, et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis. 2009;204:216–221. doi: 10.1016/j.atherosclerosis.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Miller VM, Redfield MM, McConnell JP. Use of BNP and CRP as biomarkers in assessing cardiovascular disease: diagnosis versus risk. Curr Vasc Pharmacol. 2007;5:15–25. doi: 10.2174/157016107779317251. [DOI] [PubMed] [Google Scholar]
- 27.Nagasaki T, Inaba M, Shirakawa K, Hiura Y, Tahara H, Kumeda Y, et al. Increased levels of C-reactive protein in hypothyroid patients and its correlation with arterial stiffness in the common carotid artery. Biomed Pharmacother. 2007;61:167–172. doi: 10.1016/j.biopha.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Christ-Crain M, Meier C, Guglielmetti M, Huber PR, Riesen W, Staub JJ, et al. Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis. 2003;166:379–386. doi: 10.1016/s0021-9150(02)00372-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee WY, Suh JY, Rhee EJ, Park JS, Sung KC, Kim SW. Plasma CRP, apolipoprotein A-1, apolipoprotein B and Lpa levels according to thyroid function status. Arch Med Res. 2004;35:540–545. doi: 10.1016/j.arcmed.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Pearce EN, Wilson PW, Yang Q, Vasan RS, Braverman LE. Thyroid function and lipid subparticle sizes in patients with short-term hypothyroidism and a population-based cohort. J Clin Endocrinol Metab. 2008;93:888–894. doi: 10.1210/jc.2007-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]