Abstract
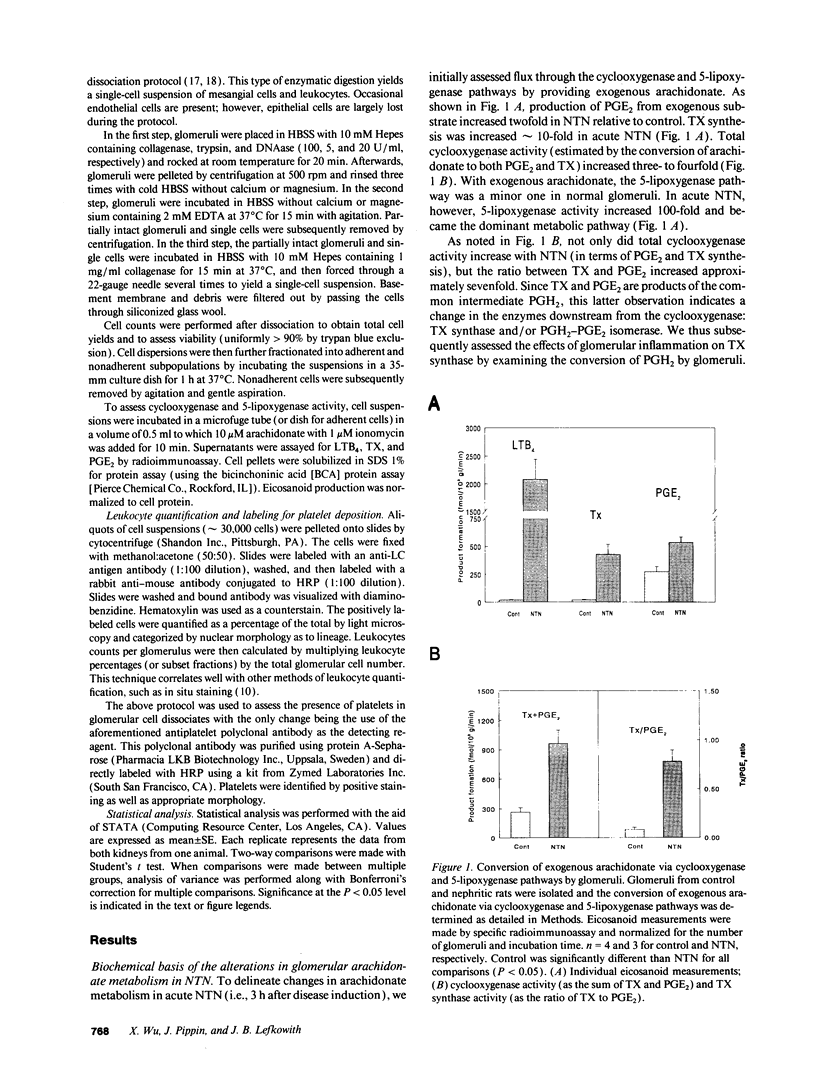
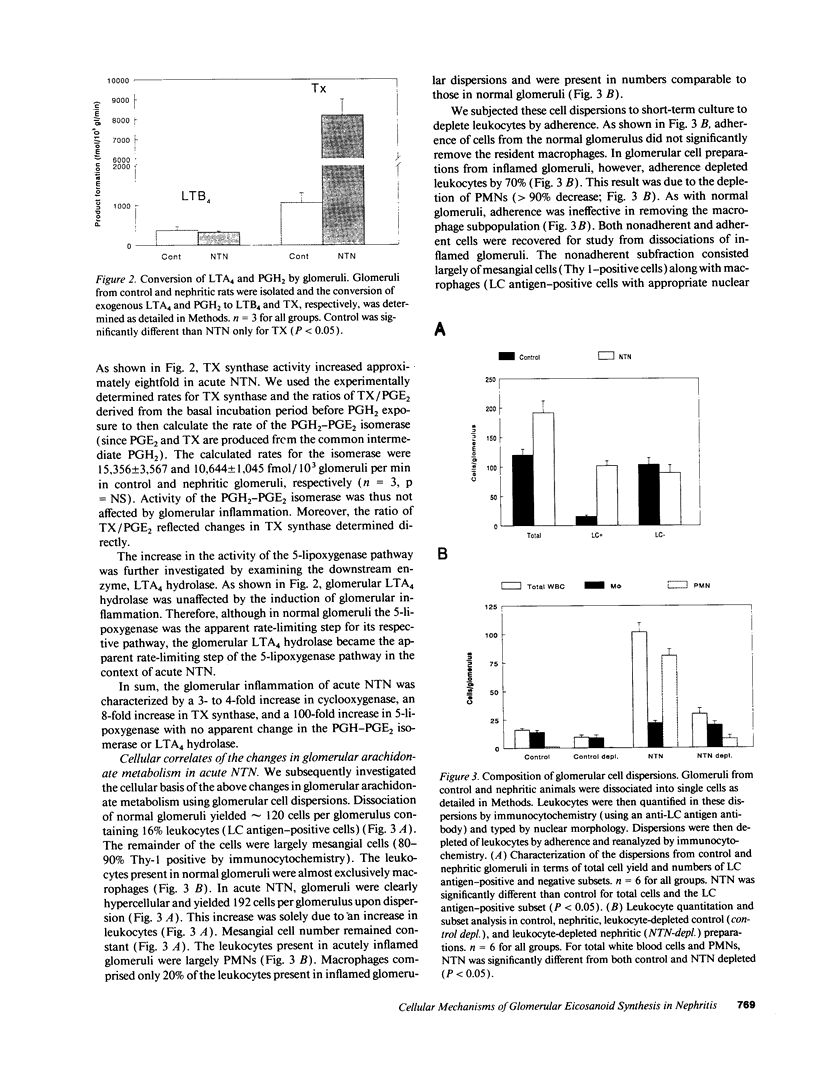
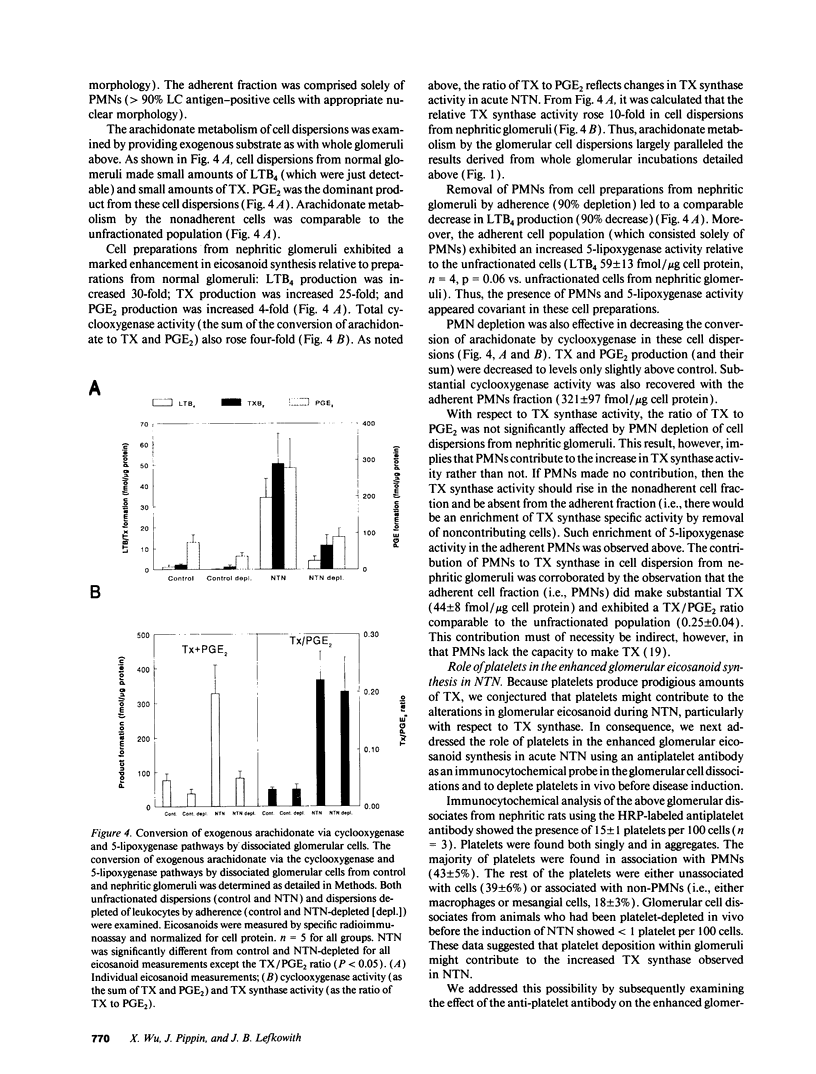
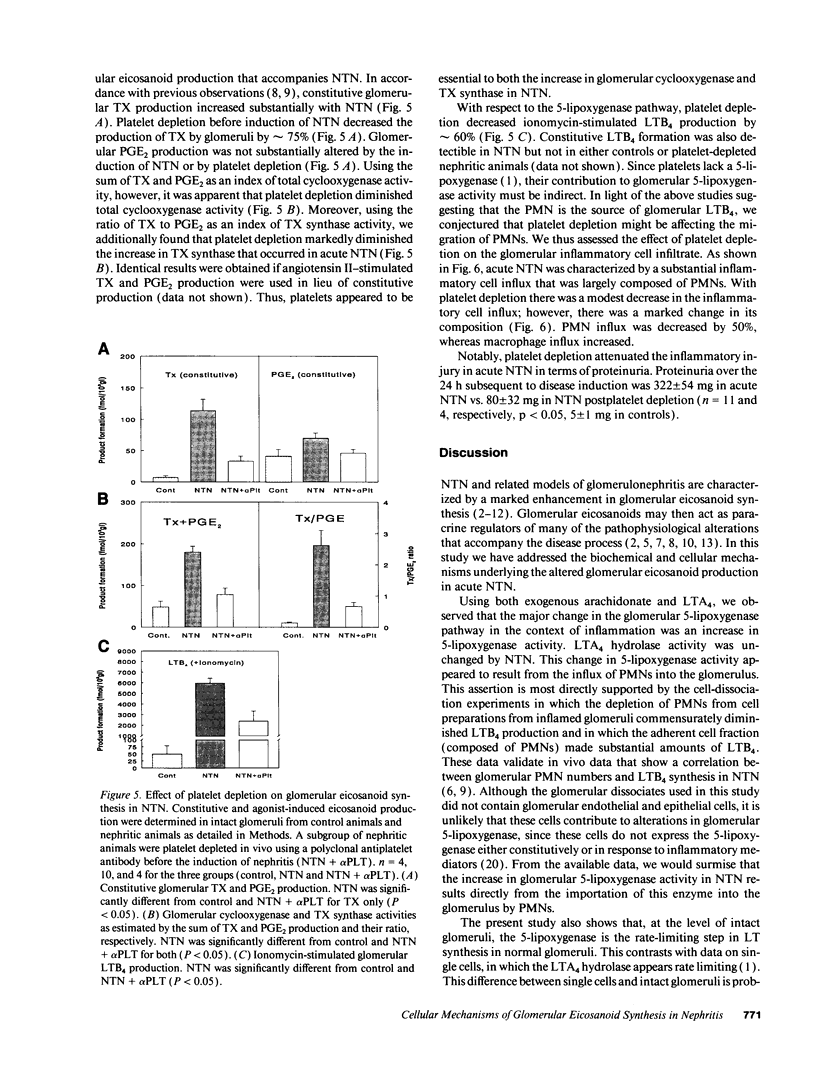
Nephrotoxic nephritis (NTN) is characterized by a marked increase in glomerular eicosanoid synthesis, which appears to play an important role in the pathophysiology of this disease model. In this study, we investigated the biochemical and cellular basis of this metabolic change. By examining the enzymatic conversion of exogenous substrates by intact glomeruli, we found that cyclooxygenase, TX synthase, and 5-lipoxygenase activities increased 4-, 8-, and 100-fold, respectively, in acute NTN. PGH2-PGE2 isomerase and leukotriene A4 hydrolase activities did not change. The cellular basis of these changes was examined using dissociated glomerular cells in vitro and by depleting platelets in vivo. Dissociated glomerular cells from nephritic glomeruli (largely mesangial cells and leukocytes) exhibited an enhanced arachidonate metabolism similar to intact nephritic glomeruli. Depletion of neutrophils (PMNs) from these cell preparations by 90% commensurately decreased 5-lipoxygenase and cyclooxygenase activity but had little effect on TX synthase activity. The recovered PMN fraction, however, did exhibit TX synthase activity. Immunocytochemical analysis of dissociated cells using an antiplatelet antibody demonstrated the presence of platelets, both adherent to cells and noncell associated. Depletion of platelets in vivo using this antibody substantially attenuated the increase in glomerular eicosanoid synthesis that accompanied NTN. Platelet depletion also decreased the influx of PMNs into the glomerulus by 50%. These data show that PMNs and platelets colocalize to the glomerulus in acute NTN and are coordinately essential to the increase in glomerular arachidonate metabolism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camazine S. M., Ryan G. B., Unanue E. R., Karnovsky M. J. Isolation of phagocytic cells from the rat renal glomerulus. Lab Invest. 1976 Oct;35(4):315–326. [PubMed] [Google Scholar]
- Cook H. T., Smith J., Cattell V. Isolation and characterization of inflammatory leukocytes from glomeruli in an in situ model of glomerulonephritis in the rat. Am J Pathol. 1987 Jan;126(1):126–136. [PMC free article] [PubMed] [Google Scholar]
- Cook H. T., Smith J., Salmon J. A., Cattell V. Functional characteristics of macrophages in glomerulonephritis in the rat. O2- generation, MHC class II expression, and eicosanoid synthesis. Am J Pathol. 1989 Feb;134(2):431–437. [PMC free article] [PubMed] [Google Scholar]
- Coyne D. W., Nickols M., Bertrand W., Morrison A. R. Regulation of mesangial cell cyclooxygenase synthesis by cytokines and glucocorticoids. Am J Physiol. 1992 Jul;263(1 Pt 2):F97–102. doi: 10.1152/ajprenal.1992.263.1.F97. [DOI] [PubMed] [Google Scholar]
- Fauler J., Wiemeyer A., Marx K. H., Kühn K., Koch K. M., Frölich J. C. LTB4 in nephrotoxic serum nephritis in rats. Kidney Int. 1989 Jul;36(1):46–50. doi: 10.1038/ki.1989.159. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Pritzl P., Schulze M., Baker P., Pruchno C., Couser W. G. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. J Clin Invest. 1988 Oct;82(4):1225–1235. doi: 10.1172/JCI113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Pritzl P., Iida H., Alpers C. E. Platelet-complement interactions in mesangial proliferative nephritis in the rat. Am J Pathol. 1991 Feb;138(2):313–321. [PMC free article] [PubMed] [Google Scholar]
- Lefkowith J. B., Jakschik B. A., Stahl P., Needleman P. Metabolic and functional alterations in macrophages induced by essential fatty acid deficiency. J Biol Chem. 1987 May 15;262(14):6668–6675. [PubMed] [Google Scholar]
- Lefkowith J. B., Morrison A. R., Schreiner G. F. Murine glomerular leukotriene B4 synthesis. Manipulation by (n-6) fatty acid deprivation and cellular origin. J Clin Invest. 1988 Nov;82(5):1655–1660. doi: 10.1172/JCI113777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowith J. B., Nagamatsu T., Pippin J., Schreiner G. F. Role of leukocytes in metabolic and functional derangements of experimental glomerulonephritis. Am J Physiol. 1991 Aug;261(2 Pt 2):F213–F220. doi: 10.1152/ajprenal.1991.261.2.F213. [DOI] [PubMed] [Google Scholar]
- Lefkowith J. B., Schreiner G. Essential fatty acid deficiency depletes rat glomeruli of resident macrophages and inhibits angiotensin II-induced eicosanoid synthesis. J Clin Invest. 1987 Oct;80(4):947–956. doi: 10.1172/JCI113187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianos E. A., Andres G. A., Dunn M. J. Glomerular prostaglandin and thromboxane synthesis in rat nephrotoxic serum nephritis. Effects on renal hemodynamics. J Clin Invest. 1983 Oct;72(4):1439–1448. doi: 10.1172/JCI111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianos E. A., Bresnahan B. A., Pan C. Mesangial cell immune injury. Synthesis, origin, and role of eicosanoids. J Clin Invest. 1991 Aug;88(2):623–631. doi: 10.1172/JCI115347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianos E. A. Synthesis of hydroxyeicosatetraenoic acids and leukotrienes in rat nephrotoxic serum glomerulonephritis. Role of anti-glomerular basement membrane antibody dose, complement, and neutrophiles. J Clin Invest. 1988 Aug;82(2):427–435. doi: 10.1172/JCI113615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita N., Funk C. D., Imai E., Hoover R. L., Badr K. F. Molecular cloning and functional expression of rat leukotriene A4 hydrolase using the polymerase chain reaction. FEBS Lett. 1992 Mar 16;299(3):273–277. doi: 10.1016/0014-5793(92)80130-9. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Yeh C. G., Rudolph A. R., Ward P. A. Protective effects of soluble CR1 in complement- and neutrophil-mediated tissue injury. J Immunol. 1992 Mar 1;148(5):1479–1485. [PubMed] [Google Scholar]
- Nagamatsu T., Pippin J., Schreiner G. F., Lefkowith J. B. Paradoxical exacerbation of leukocyte-mediated glomerulonephritis with cyclooxygenase inhibition. Am J Physiol. 1992 Aug;263(2 Pt 2):F228–F236. doi: 10.1152/ajprenal.1992.263.2.F228. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Osborne M. J., Droz B., Meyer P., Morel F. Angiotensin II: renal localization in glomerular mesangial cells by autoradiography. Kidney Int. 1975 Oct;8(4):245–254. doi: 10.1038/ki.1975.108. [DOI] [PubMed] [Google Scholar]
- Ramos B. F., Zhang Y., Jakschik B. A. Mast cells contribute to fibrin deposition in reverse passive Arthus reaction in mouse skin. Eur J Immunol. 1992 Sep;22(9):2381–2385. doi: 10.1002/eji.1830220930. [DOI] [PubMed] [Google Scholar]
- Remuzzi G., FitzGerald G. A., Patrono C. Thromboxane synthesis and action within the kidney. Kidney Int. 1992 Jun;41(6):1483–1493. doi: 10.1038/ki.1992.217. [DOI] [PubMed] [Google Scholar]
- Schifferli J. A., Taylor R. P. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989 Apr;35(4):993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Rovin B., Lefkowith J. B. The antiinflammatory effects of essential fatty acid deficiency in experimental glomerulonephritis. The modulation of macrophage migration and eicosanoid metabolism. J Immunol. 1989 Nov 15;143(10):3192–3199. [PubMed] [Google Scholar]
- Sindrey M., Marshall T. L., Naish P. Quantitative assessment of the effects of platelet depletion in the autologous phase of nephrotoxic serum nephritis. Clin Exp Immunol. 1979 Apr;36(1):90–96. [PMC free article] [PubMed] [Google Scholar]
- Stahl R. A., Thaiss F., Kahf S., Schoeppe W., Helmchen U. M. Immune-mediated mesangial cell injury--biosynthesis and function of prostanoids. Kidney Int. 1990 Aug;38(2):273–281. doi: 10.1038/ki.1990.196. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Metabolism of arachidonic acid in ionophore-stimulated neutrophils. Esterification of a hydroxylated metabolite into phospholipids. J Clin Invest. 1979 Nov;64(5):1457–1465. doi: 10.1172/JCI109604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork J. E., Dunn M. J. Hemodynamic roles of thromboxane A2 and prostaglandin E2 in glomerulonephritis. J Pharmacol Exp Ther. 1985 Jun;233(3):672–678. [PubMed] [Google Scholar]
- Takahashi K., Kato T., Schreiner G. F., Ebert J., Badr K. F. Essential fatty acid deficiency normalizes function and histology in rat nephrotoxic nephritis. Kidney Int. 1992 May;41(5):1245–1253. doi: 10.1038/ki.1992.186. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Schreiner G. F., Yamashita K., Christman B. W., Blair I., Badr K. F. Predominant functional roles for thromboxane A2 and prostaglandin E2 during late nephrotoxic serum glomerulonephritis in the rat. J Clin Invest. 1990 Jun;85(6):1974–1982. doi: 10.1172/JCI114661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yared A., Albrightson-Winslow C., Griswold D., Takahashi K., Fogo A., Badr K. F. Functional significance of leukotriene B4 in normal and glomerulonephritic kidneys. J Am Soc Nephrol. 1991 Jul;2(1):45–56. doi: 10.1681/ASN.V2145. [DOI] [PubMed] [Google Scholar]












