Abstract
Purpose
We aimed at determining the cutoff value of waist circumference with respect to its ability to reflect insulin resistance in a Korean population.
Materials and Methods
A total of 8,817 subjects aged 40 years and over were analyzed. Insulin resistant individuals were defined as those who had the highest quartile value of the homeostasis model assessment of insulin resistance (HOMA-IR) in a non-diabetic population. Receiver operating characteristic (ROC) curve analysis and multiple logistic regression analysis were applied.
Results
The cutoff value of waist circumference reflecting insulin resistance from the ROC analysis was 84.4 cm for men and 80.6 cm for women. Sensitivity and specificity were 70.0% and 54.2% in men and 71.1% and 59.3% in women, respectively. After being controlled for other covariates, the odds ratio for the risk of insulin resistance using < 70 cm of waist circumference as a reference increased significantly in the category of 85.0-89.9 cm for men and 80.0-84.9 cm for women. In addition, statistically significant associations were consistently observed over the category of 85.0-89.9 cm for men and 80.0-84.9 cm for women.
Conclusion
The optimal cutoff value for waist circumference reflecting insulin resistance is considered to be 85 cm for men and 80 cm for women, suggesting that the Asian criterion of abdominal obesity (90 cm for men and 80 cm for women) as a component of metabolic syndrome (MetS) might not be applicable for middle-aged to older men in Korea.
Keywords: Metabolic syndrome, waist circumference, insulin resistance
INTRODUCTION
Metabolic syndrome (MetS) is a clustering of abdominal obesity, elevated blood pressure, elevated blood glucose, and dyslipidemia,1 and it is associated with an increased risk of type 2 diabetes and cardiovascular disease (CVD).2 Various kinds of definitions have been suggested since the World Health Organization (WHO) defined MetS in 1999.3
In 2009, a joint statement of the International Diabetes Federation (IDF), the National Heart, Lung, and Blood Institute (NHLBI), the American Heart Association (AHA), the World Heart Federation, the International Atherosclerosis Society, and the International Association for the Study of Obesity proposed a harmonized definition of MetS in which the presence of any three of five risk factors comprises a diagnosis of MetS.4 This new definition of MetS recommends that cutoff points of waist circumference (WC) representing abdominal obesity be advocated for different countries and ethnic groups, and that the IDF cutoff points be used for non-Europeans until more data are available.4 Based on the IDF recommendation, the WC cutoff for Asians including Chinese and Japanese was suggested as 90 cm for men and 80 cm for women, respectively, which was different from that of other ethnic groups such as Europeans.5 However, the WC cutoff point for Koreans has not been specified yet. Although a few studies in the Korean population propose the cutoff values of WC related to the diagnosis of metabolic syndrome, the optimal cutoffs of WC are different from those of the IDF.6-8
Insulin resistance is an important risk factor for type 2 diabetes and CVD.9,10 In addition, insulin resistance is known to play a major role in the pathophysiology of MetS,11,12 although it is not a component of diagnosing MetS. Much evidence suggests that there is a positive association between abdominal obesity and insulin resistance.13-16 In the subjects with visceral obesity, it has been suggested that a defective free-fatty acid (FFA) metabolism17 or an altered profile of proinflammatory markers18 could contribute to insulin resistance. Therefore, the WC cutoff value reflecting insulin resistance as an intermediate outcome of type 2 diabetes or CVD may be quite clinically relevant and will be useful to identify the population at high risk for MetS. The purpose of this study was to determine the optimal WC cutoff value to reflect insulin resistance as a criterion of MetS in a Korean population aged 40 years and over.
MATERIALS AND METHODS
The Chungju Metabolic Disease Cohort (CMC) study is an ongoing community-based cohort study of metabolic disease including type 2 diabetes and metabolic syndrome in a population aged 40 years and over.19,20 We conducted a baseline study using stratified random cluster sampling between 2003 and 2006 on the rural area of Chungju City in the middle of South Korea. Subjects were selected and investigated using random cluster sampling each year after being stratified by the residential areas of 13 health subcenters and 16 community health clinics, thus examining the whole population aged 40 years and over.
A total of 11,718 subjects (4,802 men and 6,916 women) participated in the study and gave written informed consent. This study was approved by the Institutional Review Board of the Catholic University of Korea. The following exclusion criteria were applied in the data analysis. Individuals with type 2 diabetes (n = 1,469) were excluded because the homeostatic model assessment of insulin resistance (HOMA-IR) correlates well with insulin resistance in a nondiabetic population.21,22 In addition, individuals who already had cardiovascular outcomes such as ischemic heart disease and CVD (n = 349) were excluded. Lastly, individuals with a missing value of waist circumference, insulin, or glucose (n = 1,083) were excluded from the analysis. When all the exclusions were considered, 8,817 subjects (3,574 men and 5,243 women) were available for final analyses.
All the measurements were performed by trained investigators between 8 am and 10 am. General characteristics assessed by a questionnaire included life style factors such as smoking, drinking alcohol, exercise, and dietary habits. Non-smokers were defined as individuals who had not smoked over 100 cigarettes, and ex-smokers were defined as individuals who had not smoked for at least 6 months. Non-drinkers were defined as individuals who answered that they had never drunk. Regular exercisers were defined as individuals who had engaged in exercise three or more times a week for at least 30 minutes per session. High fat diet eaters were defined as individuals who regularly consumed animal products such as meat and dairy products every day.
WC was measured to the nearest 0.1 cm midway between the upper margin of the iliac crest and the lower margin of the last rib in the late exhalation phase while subjects were standing and wearing no clothes. Other anthropometric parameters including height, weight, and hip circumference were measured using standard protocols. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. We measured blood pressure twice on the right upper arm with a mercury manometer while the subject was sitting in the upright position after 5 minutes of rest, thus using the average of two measurements. Since the examination was performed in various health sub-centers, we tried to reduce the inter- and intra-observer variability in measuring anthropometic parameters. This reduction was accomplished by standardizing all the processes of measurements by training investigators before the survey. For instance, inter- and intra-observer variability of measuring WC in a sampled population was less than 1% altogether, respectively. After an overnight 12-hour fast, the subjects' fasting plasma glucose (FPG), insulin, and lipids were consistently measured in a central laboratory. Total serum cholesterol and triglycerides were measured by an enzymatic calorimetric test, high-density lipoprotein (HDL) cholesterol was measured with a selective inhibition method, and low-density lipoprotein (LDL) cholesterol was calculated with the Friedewald formula. FPG was measured with the hexokinase method using sodium fluoride tubes. Serum insulin was measured with the RIA kit (Dainabot, Tokyo, Japan).
Type 2 diabetes was defined according to the American Diabetes Association criteria23 of FPG ≥ 126 mg/dL, or if the subjects had a diabetes history or had been using antidiabetic medication. Insulin resistance was measured using the HOMA index (HOMA-IR = fasting insulin (µU/mL)×FPG (mmol/L)/22.5).24 Insulin resistant individuals were defined as those who had the highest quartile value of the HOMA-IR in a non-diabetic population.21 The prevalence of metabolic syndrome in several cutoffs of WC was estimated according to the joint criterion of AHA/NHLBI and IDF,4 and the IDF criterion,25 respectively.
Data are presented as a mean ± standard deviation (SD) or a median (5th and 95th percentile) or in percentages. Skewed data such as HOMA-IR, insulin, and triglycerides were analyzed after logarithmic transformation. Receiver operating characteristic (ROC) curve analysis was applied to determine the optimal cutoff value of WC in subjects with insulin resistance. Sensitivity and specificity for predicting insulin resistance were the values producing maxima in men and women separately. Associations of WC measures (in 5 cm increments using < 70 cm as a WC reference) with insulin resistance were analyzed using multiple logistic regression analysis after adjusting for age, BMI, and lifestyle related factors. Data were analyzed using SAS v. 9.01 (SAS, Inc., Cary, NC, USA).
RESULTS
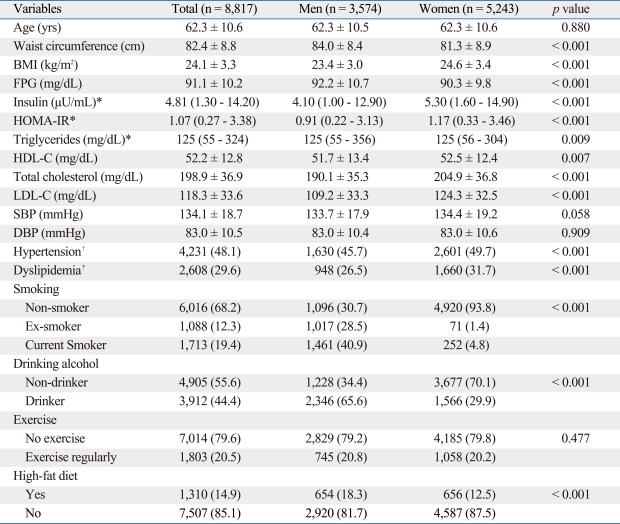
The mean age (± SD) of the study subjects was 62.3 ± 10.5 in men and 62.3 ± 10.6 in women, and the mean WC was 84.0 ± 8.4 cm in men and 81.3 ± 8.9 cm in women. When other clinical and lifestyle characteristics were compared between men and women, FPG, triglycerides, smoking, drinking alcohol, and high fat diet were statistically significantly higher for men, whereas BMI, insulin, HOMA-IR, HDL cholesterol, total cholesterol, LDL cholesterol, hypertension, and dyslipidemia were higher for women (Table 1).
Table 1.
Baseline Characteristics of the Participants
BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Data are summarized as a mean ± SD, a median (5 - 95%) or n (%).
*t-test was performed between men and women after logarithmic transformation.
†SBP ≥ 140 or DBP ≥ 90 or previously diagnosed hypertension.
‡Total cholesterol ≥ 240 or LDL-C ≥ 160 or HDL-C < 40 or specific treatment for lipid abnormality.
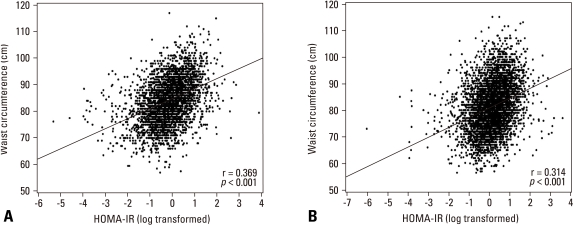
The relationship between HOMA-IR and WC was clearly found as depicted in Fig. 1. The degree of correlation was slightly higher in men than women (p = 0.004), and it was also significant when the correlation was additionally adjusted by BMI, smoking, drinking alcohol, and dietary habit (p = 0.002).
Fig. 1.
Scatter plots with regression lines for age-adjusted correlations between waist circumference and homeostasis model assessment of insulin resistance (HOMA-IR) in men (A) and women (B). The degree of correlation was higher in men than women (p= 0.0041).
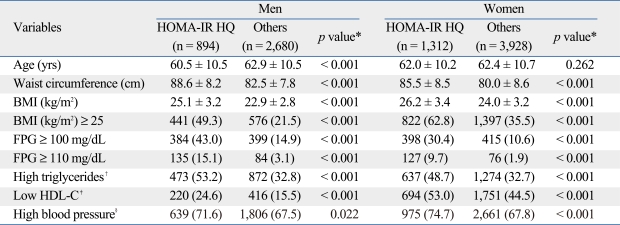
The highest quartile value of the HOMA-IR representing insulin resistance was 1.38 for men and 1.89 for women. When we compared the metabolic risk factors between insulin resistant subjects and others by gender, we found that all of the metabolic risk factors including waist circumference, BMI, high FPG, high triglycerides, low HDL cholesterol, and high blood pressure were significantly higher in insulin resistant subjects of both sexes (Table 2).
Table 2.
Comparison of Metabolic Risk Factors between Insulin Resistant Subjects and Others by Gender
HOMA-IR HQ, homeostatic model assessment of insulin resistance highest quartile; BMI, body mass index; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol.
Data are summarized as a mean ± SD or n (%).
*p values were obtained using t-test or chi-square test.
†Triglycerides ≥ 150 mg/dL.
‡HDL-C < 50 for men and HDL-C < 40 for women.
§Systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg or antihypertensive medication of previously diagnosed hypertension.
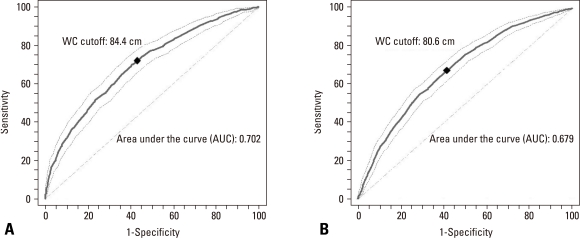
The optimal cutoff value of WC producing maximum sensitivity plus specificity for reflecting the presence of insulin resistance from the ROC analysis was 84.4 cm for men and 80.6 cm for women. Sensitivity and specificity were 70.0% and 54.2% in men and 71.1% and 59.3% in women, respectively (Fig. 2).
Fig. 2.
Receiver operating characteristic (ROC) curves for waist circumference (WC) for reflecting insulin resistance in men (A) and women (B). The cutoff value of WC producing maximum sensitivity plus specificity was 84.4 cm for men and 80.8 cm for women. Sensitivity and specificity were 70.0% and 54.2% in men and 71.1% and 59.3% in women.
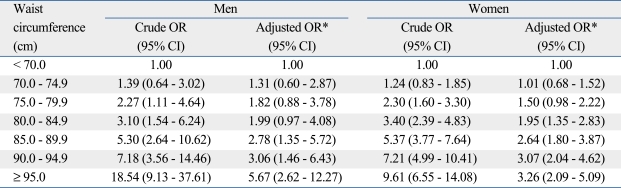
As the WC increased, the odds ratio (OR) for the association between the highest quartile of the HOMA-IR and categories of WC (in 5 cm units) increased in men and women, as shown in Table 3. After being controlled for age, BMI, smoking, drinking alcohol, exercise, and dietary habits, the association was statistically significant in the category of 85-89.9 cm for men and 80-84.9 cm for women using < 70 cm of WC as a reference. Statistically significant associations were also consistently observed over the category of 85-89.9 cm for men and 80-84.9 cm for women.
Table 3.
Odds Ratios (ORs) of Insulin Resistance according to the Categories of Waist Circumference in Men and Women
CI, confidence interval.
*Adjusted for age, body mass index (BMI), smoking, drinking alcohol, exercise, and high fat diet.
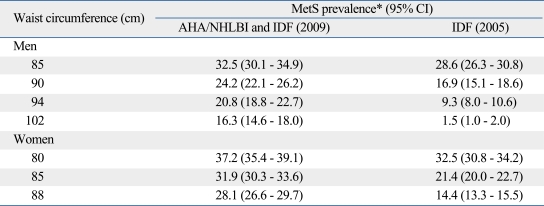
When we compared the prevalence of metabolic syndrome based on several cutoffs of waist circumference, the prevalence of our proposed cutoff was 32.5% and 37.2% in men and women according to the joint criterion of AHA/NHLBI and IDF, and 28.6% and 32.5% in men and women according to the IDF criterion, respectively (Table 4).
Table 4.
Comparison of Prevalence of Metabolic Syndrome in Several Cutoffs of Waist Circumference
AHA, American Heart Association; NHLBI, National Heart, Lung, and Blood Institute; IDF, International Diabetes Federation.
*Metabolic syndrome (MetS) prevalence was adjusted by Korean standard population in 2005.
DISCUSSION
The aim of this study was to establish the optimal cutoff value of WC to reflect insulin resistance in a community-based Korean population aged 40 years and over, allowing identification of high risk subjects for MetS. We found that the optimal cutoff value for WC predicting insulin resistance was 84.4 cm for men and 80.6 cm for women based on the cutoff value producing the maximal sensitivity and specificity of ROC analysis. The value produced by multiple logistic regression analysis was consistent with that of ROC analysis, based on the fact that the OR for the risk of insulin resistance increased significantly in the category of 85-89.9 cm of WC for men and 80-84.9 cm of WC for women. Our results demonstrated a lower WC cutoff value in men compared with that of other Asians proposed by the IDF (men ≥ 90 cm and women ≥ 80 cm).25
Even at a lower level of WC, Asians tend to have more obesity-related metabolic risks than Caucasians.26 Many studies on other Asian people have consistently shown that WC cutoffs predicting obesity-related co-morbidities are lower than those of Caucasians. For a Chinese population comprising 111,411 subjects aged 20-70 years, the cutoff values of WC in regard to the risk of diabetes mellitus and lipoprotein disorders are 85 cm in men and 80 cm in women, which are similar to those in our results.27 In a group of 5,305 Thai subjects aged ≥ 35 years, the cutoff values of WC predicting two or more cardiovascular risk factors are 84 cm for both men and women.28 In a Japanese study on 329 subjects aged ≥ 35 years where insulin resistance is used as an outcome for ROC analysis for WC like in our study, the cutoff values of WC are 83 cm in men and 75 cm in women.29
There have been several studies estimating WC cutoff values for diagnosing MetS in Korea. A study of 6,561 adults, nationally representative samples of Koreans, demonstrates that the appropriate WC cutoff value is ≥ 90 cm for men and ≥ 85 cm for women.8 However, this study differs from the present study in that they estimate the WC cutoff value for predicting two or more risk factors of MetS that the subjects have at the same time. Other hospital-based cross-sectional studies using CT scan for measuring the visceral fat area or visceral adipose tissue have determined the WC cutoff values: ≥ 89.8 cm for men and ≥ 86.1 cm for women for 413 subjects;7 ≥ 88.1 cm for men and ≥ 84.0 cm for women for 816 subjects,6 although these studies have a limitation of small samples recruited for hospitals where a selection bias may exist.30 All these studies in Korea show that the WC cutoff for men is around 5 cm higher than that for women, and our results are consistent with them. However, we found that our WC cutoff value is lower than those of other studies for both sexes. This phenomenon may be explained by the fact that the study population in this study is older than those in other studies in which the mean ages of the subjects were distributed in early to mid-forties for both sexes. Aging is generally associated with decreases in lean body mass and increases in body fat, and these age-related alterations contribute to insulin resistance.31,32 Considering the importance of abdominal obesity as a risk factor for insulin resistance in the elderly,33 the WC cutoff value for diagnosing MetS might need to be lowered in middle-aged to older individuals in Korea.
In order to investigate the impact of changing the WC cutoff value, MetS prevalence was calculated according to the joint criterion of AHA/NHLBI and IDF,4 and the IDF criterion.25 The difference of MetS prevalence between the two diagnostic criteria was minimized when our WC cutoff was applied. Higher MetS prevalence in women was also observed in the same age group in the 3rd Korea National Health and Nutrition Examination Survey (KNHANES III).34
In the present study, HOMA-IR was correlated more with WC in men than in women, which is consistent with the results of other studies.35,36 However, the degree of association was found to be similar when we observed the association between the highest quartile of the HOMA-IR representing insulin resistance and categories of WC (in 5 cm units). In addition, we confirmed that the risk of insulin resistance is positively associated with increasing WC for both men and women, which is consistent with other studies.15,37 Interestingly, the risk of insulin resistance associated with WC was sharply increased over the category of WC (≥ 95 cm) in men compared with women [adjusted OR, 95% confidence inteval (CI): 5.67 (2.62-12.27) in men vs. 3.26 (2.09-5.09) in women]. This discrepancy has been shown in another study for Asians where the risk of diabetes was sharply increased over the category of WC (≥ 100 cm) in men.38 This finding suggests that more vigorous efforts to reduce WC might be needed for men with higher WC over 95 cm than for women to help prevent CVD.
In addition to the association of WC with insulin resistance, other metabolic risk factors including BMI, high FPG, high triglycerides, low HDL cholesterol, and high blood pressure were higher in insulin resistant subjects for both sexes in this study. These findings were consistent with those of other studies,15,36 which suggest that insulin resistance is closely related to all of the metabolic syndrome components.
The present study does have limitations that should be considered. We have used the highest quartile of HOMA-IR to estimate the insulin resistance instead of using the hyperinsulinaemic clamp method, which could result in lowering validity. However, much consensus suggests that HOMA-IR is applicable to the large epidemiologic study especially in the nondiabetic population21,22 and can predict CVD.39,40 The cutoff of HOMA-IR to define insulin resistance might be controversial. When we analyzed these data again using the definition of insulin resistance as the highest quintile and tertile values of the HOMA-IR, however, the optimal cutoff values for WC were quite similar to those of the present study. When insulin resistant individuals were defined as those who had the highest quintile value of the HOMA-IR, the optimal cutoff value of WC from the ROC analysis was 85.5 cm for men and 81.0 cm for women. Sensitivity and specificity were 67.2% and 62.9% in men and 68.9% and 56.7% in women, respectively. In addition, when insulin resistant individuals were defined as those who had the highest tertile value of the HOMA-IR, the optimal cutoff value of WC from the ROC analysis was 83.5 cm for men and 79.8 cm for women. Sensitivity and specificity were 71.9% and 57.9% in men and 73.1% and 51.8% in women, respectively.
In addition, the results in this study may not be representative of all of the Korean population, and the cross-sectional study design limits the ability to make broad inferences from the results of this study. However, to the best of our knowledge, this is the first report proposing cutoff values of WC predicting insulin resistance as a diagnostic criterion of MetS in Korea based on a large-scale, community-based population.
In conclusion, the results of the present study show that the optimal cutoff value for WC reflecting insulin resistance is considered to be 85 cm for men and 80 cm for women, suggesting that the Asian criterion of abdominal obesity (90 cm for men and 80 cm for women) might not be applicable for middle-aged to older men in Korea. Determining the cutoff value of WC as a diagnostic criterion of metabolic syndrome in well-designed prospective cohort studies is important in identifying high-risk individuals who might develop CVD, and will help Korean health professionals to instigate intervention strategies.
ACKNOWLEDGEMENTS
This study was supported by the grants from the "2003-2005 Korea Health Promotion Research Program" of the Ministry of Health and Welfare, Republic of Korea. We would like to thank Larenda Mielke at Washington University in St. Louis for assistance in revising the manuscript. We would also like to thank the personnel in the Chungju Public Health Center for recruiting the participants as well as for collecting the data for this study. In addition we thank Hee Sung Ha, MPH for her assistance in data analysis and management.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Bloomgarden ZT. Definitions of the insulin resistance syndrome: the 1st World Congress on the Insulin Resistance Syndrome. Diabetes Care. 2004;27:824–830. doi: 10.2337/diacare.27.3.824. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 6.Han JH, Park HS, Kim SM, Lee SY, Kim DJ, Choi WH. Visceral adipose tissue as a predictor for metabolic risk factors in the Korean population. Diabet Med. 2008;25:106–110. doi: 10.1111/j.1464-5491.2007.02317.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim JA, Choi CJ, Yum KS. Cut-off values of visceral fat area and waist circumference: diagnostic criteria for abdominal obesity in a Korean population. J Korean Med Sci. 2006;21:1048–1053. doi: 10.3346/jkms.2006.21.6.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Howard G, O'Leary DH, Zaccaro D, Haffner S, Rewers M, Hamman R, et al. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation. 1996;93:1809–1817. doi: 10.1161/01.cir.93.10.1809. [DOI] [PubMed] [Google Scholar]
- 10.Haffner SM, Miettinen H. Insulin resistance implications for type II diabetes mellitus and coronary heart disease. Am J Med. 1997;103:152–162. doi: 10.1016/s0002-9343(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med. 1997;50:191–197. doi: 10.1016/s0300-2977(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 12.Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 13.Garg A. Regional adiposity and insulin resistance. J Clin Endocrinol Metab. 2004;89:4206–4210. doi: 10.1210/jc.2004-0631. [DOI] [PubMed] [Google Scholar]
- 14.Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diabetes Care. 2005;28:2322–2325. doi: 10.2337/diacare.28.9.2322. [DOI] [PubMed] [Google Scholar]
- 15.Park SH, Lee WY, Rhee EJ, Jeon WK, Kim BI, Ryu SH, et al. Relative risks of the metabolic syndrome according to the degree of insulin resistance in apparently healthy Korean adults. Clin Sci (Lond) 2005;108:553–559. doi: 10.1042/CS20040331. [DOI] [PubMed] [Google Scholar]
- 16.Poirier P, Lemieux I, Mauriège P, Dewailly E, Blanchet C, Bergeron J, et al. Impact of waist circumference on the relationship between blood pressure and insulin: the Quebec Health Survey. Hypertension. 2005;45:363–367. doi: 10.1161/01.HYP.0000155463.90018.dc. [DOI] [PubMed] [Google Scholar]
- 17.Bergman RN, Kim SP, Catalano KJ, Hsu IR, Chiu JD, Kabir M, et al. Why visceral fat is bad: mechanisms of the metabolic syndrome. Obesity (Silver Spring) 2006;14(Suppl 1):16S–19S. doi: 10.1038/oby.2006.277. [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 19.Kwon HS, Park YM, Lee HJ, Lee JH, Choi YH, Ko SH, et al. Prevalence and clinical characteristics of the metabolic syndrome in middle-aged Korean adults. Korean J Intern Med. 2005;20:310–316. doi: 10.3904/kjim.2005.20.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park YM, Kwon HS, Lim SY, Lee JH, Kim SR, Yoon KH, et al. Clustering characteristics of risk variables of metabolic Syndrome in Korean rural populations. Korean Diabetes J. 2006;30:177–189. [Google Scholar]
- 21.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 22.Resnick HE, Bergman RN, Henderson JA, Nez-Henderson P, Howard BV. Utility of a surrogate measure of insulin resistance in American Indians: the Strong Heart Study. Ethn Dis. 2002;12:523–529. [PubMed] [Google Scholar]
- 23.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.The IDF consensus worldwide definition of the metabolic syndrome. International Diabetes Federation (IDF) [Accessed 1 September 2009]. Available from http://www.idf.org/webdata/docs/MetS_def_update2006.pdf.
- 26.Iwao N, Iwao S, Muller DC, Koda M, Ando F, Shimokata H, et al. Differences in the relationship between lipid CHD risk factors and body composition in Caucasians and Japanese. Int J Obes (Lond) 2005;29:228–235. doi: 10.1038/sj.ijo.0802615. [DOI] [PubMed] [Google Scholar]
- 27.Zhou BF Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 28.Aekplakorn W, Kosulwat V, Suriyawongpaisal P. Obesity indices and cardiovascular risk factors in Thai adults. Int J Obes (Lond) 2006;30:1782–1790. doi: 10.1038/sj.ijo.0803346. [DOI] [PubMed] [Google Scholar]
- 29.Ohkubo T, Kikuya M, Asayama K, Imai Y. A proposal for the cutoff point of waist circumference for the diagnosis of metabolic syndrome in the Japanese population. Diabetes Care. 2006;29:1986–1987. doi: 10.2337/dc06-1018. [DOI] [PubMed] [Google Scholar]
- 30.Kenneth JR, Sander G, Timothy LL. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 31.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. [PubMed] [Google Scholar]
- 32.Ryan AS, Nicklas BJ, Elahi D. A cross-sectional study on body composition and energy expenditure in women athletes during aging. Am J Physiol. 1996;271:E916–E921. doi: 10.1152/ajpendo.1996.271.5.E916. [DOI] [PubMed] [Google Scholar]
- 33.Racette SB, Evans EM, Weiss EP, Hagberg JM, Holloszy JO. Abdominal adiposity is a stronger predictor of insulin resistance than fitness among 50-95 year olds. Diabetes Care. 2006;29:673–678. doi: 10.2337/diacare.29.03.06.dc05-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korea Center for Disease Control and Prevention. The Third Korea National Health and Nutrition Examination Survey (KNHANES III), 2005. 2007. [Google Scholar]
- 35.Kuo CS, Hwu CM, Chiang SC, Hsiao LC, Weih MJ, Kao WY, et al. Waist circumference predicts insulin resistance in offspring of diabetic patients. Diabetes Nutr Metab. 2002;15:101–108. [PubMed] [Google Scholar]
- 36.Meshkani R, Taghikhani M, Larijani B, Khatami S, Khoshbin E, Adeli K. The relationship between homeostasis model assessment and cardiovascular risk factors in Iranian subjects with normal fasting glucose and normal glucose tolerance. Clin Chim Acta. 2006;371:169–175. doi: 10.1016/j.cca.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Wannamethee SG, Shaper AG, Morris RW, Whincup PH. Measures of adiposity in the identification of metabolic abnormalities in elderly men. Am J Clin Nutr. 2005;81:1313–1321. doi: 10.1093/ajcn/81.6.1313. [DOI] [PubMed] [Google Scholar]
- 38.Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in asian Indian adults. Diabetes Care. 2003;26:1380–1384. doi: 10.2337/diacare.26.5.1380. [DOI] [PubMed] [Google Scholar]
- 39.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Meigs JB, et al. Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care. 2007;30:318–324. doi: 10.2337/dc06-0919. [DOI] [PubMed] [Google Scholar]
- 40.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25:1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]