Abstract
Purpose
A retrospective study was conducted to review the treatment and outcomes of mainly melanomas in acral location in a single institution in Korea, and to evaluate the prognostic significance of anatomic locations of the tumor.
Materials and Methods
A retrospective review was completed on 40 patients between 2001 and 2006 to obtain pertinent demographic data, tumor data, treatment characteristics, and follow-up data.
Results
Forty melanoma patients were identified and analyzed. Of these, 18 were male and 22 were female patients and the mean age at the time of diagnosis was 55.9 years. Of the tumors, 65% were located on the hands and feet with acral lentiginous melanoma being the most common histological subtype. Univariate analysis for the overall melanoma survival revealed that the thickness of the tumor and the clinical stage have prognostic significances. The most significant factor as analyzed by a multivariate analysis was shown to be the advanced clinical stage. Acral melanomas did not show statistically significant differences in the age at diagnosis, thickness of the tumor, stage, ulceration, and survival rates compared to non-acral melanomas. There was also no significant difference in the survival rate between the patients treated by amputation versus wide local excision in acral melanomas.
Conclusion
In Korean melanoma patients, thickness and advanced stages are significant factors for poorer prognosis. However, the location of melanoma did not have a significant prognostic value. In treating the melanomas in acral location, local wide excisions resulted in a similar prognosis compared to amputations.
Keywords: Melanoma, acral locations, prognosis, treatment
INTRODUCTION
Malignant melanoma is the leading cause of death among skin cancers in western countries. The incidence of cutaneous melanoma has increased more than tenfold over the last fifty years and its incidence is increasing more rapidly than any other malignancies in Caucasian populations.1 Exposure to ultraviolet rays of the sun is known to be an important risk factor for developing cutaneous melanoma.2 Therefore, cutaneous melanoma is largely considered to be a disease of fair skinned, blue-eyed individuals in latitudes with increased sun exposure. However, in Asian populations, there are only a few published reports since the incidence is much lower.3-8 Cutaneous melanomas in non-Caucasian populations are frequently manifested differently than in Caucasian populations. In comparison to the cutaneous melanomas arising in Caucasians, they occur more commonly in unusual sites (e.g., hands and feet) in the form of acral melanomas.3-6 Due to the rarity of their occurrences and acral presentations, diagnosis is often delayed resulting in more advanced stages of disease at presentation.9 Cutaneous melanoma is frequently curable with simple wide surgical excision when diagnosed at an early stage. As a result, efforts to enhance early diagnosis by educational programs represent one of the primary strategies to improve outcomes. It is generally believed that melanomas arising in acral locations may be initially diagnosed at a more advanced stage and it may account for the grave prognosis.9 However, it is unclear whether the worse prognosis of melanomas arising in hands and feet is due to a delay in diagnosis or the disease being a biologically distinct entity, or a combination of both. To confirm this issue, we reviewed our experience in the management of cutaneous melanomas patients in a single institution in Korea by dividing them according to the anatomic sites of acral and non-acral locations.
MATERIALS AND METHODS
Study population
The computerized database in the dermatology department of Yonsei University Health System in Seoul, Korea was used for the study. There were 48 patients diagnosed as malignant melanoma in the dermatology department from 2001 to 2006. However, 5 patients with insufficient data and 3 patients with metastatic melanoma with an unknown primary origin were excluded in the analysis. The age of onset, duration of lesion until diagnosis, sex, site, histologic subtype, Breslow thickness, presence of ulceration, clinical staging [American Joint Committee on Cancer (AJCC) 2002],10 treatment method, and survival (from the date of histologic diagnosis to the date of last follow-up or death) were extracted for the analysis.
Statistical analysis
The method of Kaplan and Meier was used to calculate survival curves, and significant differences were determined by the log-rank test. Univariate analysis was used to calculate the relative risk of each parameter and multivariate analyses of prognostic variables were performed using Cox regression model and the t-test analysis was used when appropriate. A p value less than 0.05 was considered significant.
RESULTS
Patient characteristics
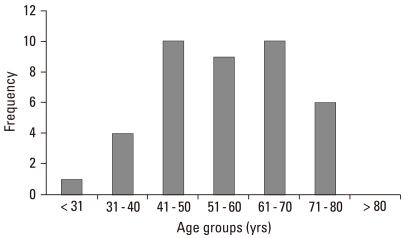
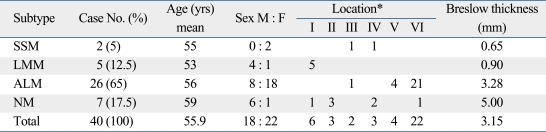
A total of 40 patients with cutaneous melanoma were analyzed in this study. Of these, 18 were men and 22 were women; the mean age was 55.9 years (range, 29-79 years) (Fig. 1). The mean duration of lesion until time of diagnosis was 6.2 years (range, 0.2-40 years). Hands and feet were the most commonly involved locations, with 65% of the patients having primary tumors at these sites. The mean tumor thickness was 3.15 mm and the local tumor (stage I and II) comprised 87.5%. Acral lentiginous melanoma (ALM) was the most common histological subtype (26/40, 65%), followed by nodular melanoma (NM) (7/40, 17.5%), lentigo maligna melanoma (5/40, 12.5%), and superficial spreading melanoma (2/40, 5%). The mean ages of the patients in each subtype were 55, 53, 56, and 59 years, respectively. The mean Breslow thickness of the tumors was also recorded (Table 1).
Fig. 1.
Distribution of cutaneous melanoma by age.
Table 1.
Subtypes of Melanoma
SSM, superficial spreading melanoma; LMM, lentigo maligna melanoma; ALM, acral lentiginous melanoma; NM, nodular melanoma.
*Location I, head and neck; II, upper limbs; III, lower limbs; IV, trunk; V, hands / finger; VI, feet / toe.
Characteristics of patients with melanoma in acral location
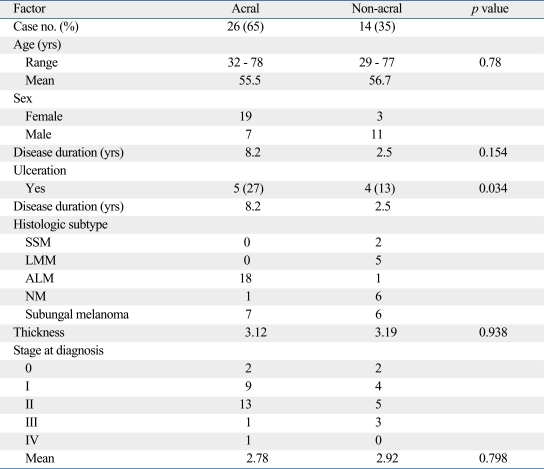
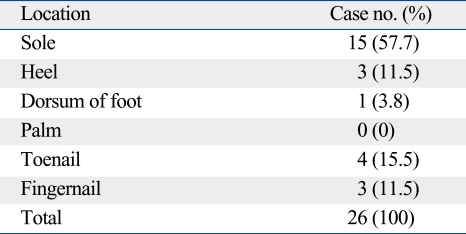
Table 2 summarizes the clinical characteristics of the melanoma according to the location. The locations were divided into two groups which are melanoma arising in acral locations, such as hand, foot, fingernail, and toenail, and non-acral locations. Twenty-six melanomas were located on acral locations. In these cases, there were more women than men (men : women ratio, 1 : 2.7). Subtypes of the melanoma occurring in this location were acral lentiginous melanoma in 18 cases, subungal melanoma in 7 cases, and nodular melanoma in one case on the dorsum of the foot. The sole (57.7%) was the most common location among the acral subtype (Table 3). The incidence of ulceration was not significantly different compared to non-acral locations. Age at the time of diagnosis was not statistically different between the two groups. Also, the thickness of the tumor and the mean stage were not statistically different between the two groups. The duration of the lesion until the time of diagnosis of melanomas in acral locations was 8.2 years and 2.5 years for melanomas in non-acral locations. However, they were statistically insignificant (Table 2). For the treatment, 7 patients (4 subungal melanomas, 2 melanomas on sole, 1 melanoma on heel) were treated by amputation and the others (n = 19) by local wide excision with safety margins determined according to the thickness of the tumor. The mean tumor thickness of the group treated with wide excision was 2.76 mm and 4.11 mm in the group treated with amputation. However, the difference was not statistically significant (p = 0.256). The mean stage of the group treated with wide excision was 1.82 and 1.86 in the group treated with amputation. This difference was also statistically insignificant (p = 0.919).
Table 2.
Characteristics of Melanoma According to Anatomic Location
SSM, superficial spreading melanoma; LMM, lentigo maligna melanoma; ALM, acral lentiginous melanoma; NM, nodular melanoma.
Table 3.
Specific Locations of Acral Melanoma
Overall survival and prognosis
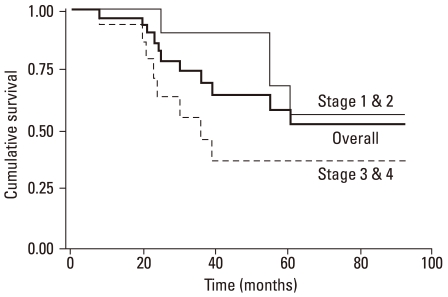
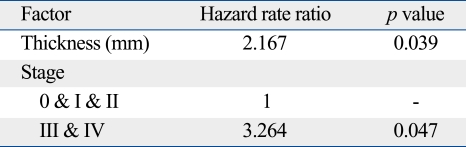
With univariate analysis, thicker tumors (> 2 mm) and clinical stage IV were found to predict a poorer survival; hazard ratios (HR) were 5.668 and 3.554 which were statistically significant (Table 4). In the multivariate analysis, we evaluated the tumor thickness and clinical staging for genuine decisive factors. An advanced clinical stage was shown to be the most significant factor in predicting survival. The HR was 3.264 for those with stage III and IV diseases (p < 0.05). Thickness remained in the model with a significant HR of 2.167 (p < 0.05) (Table 4). The overall survival curve was plotted according to the clinical stage (Fig. 2) and there was a significant difference between stages I/II and stages III/IV. The overall survival curve was also plotted according to the treatment method for the melanomas of hands and feet. There was no significant difference in the survival rate between the patients treated by wide excision and amputation (p = 0.39) (Fig. 3).
Table 4.
Univariate Analysis by Cox Proportional Hazard Model (Overall Survival)
SSM, superficial spreading melanoma; LMM, lentigo maligna melanoma; ALM, acral lentiginous melanoma; NM, nodular melanoma.
Fig. 2.
Survival curves by stages. Advanced stages had poorer prognosis (p < 0.05).
Fig. 3.
Survival curves of melanoma in acral location by treatment method (p = 0.39).
DISCUSSION
Cutaneous melanoma is a rare disease in the non-Caucasian populations, with an average annual age-adjusted incidence of 1.0 per 100,000 persons in Asians.11 In Korea, the incidence of malignant melanoma increased from 1.02% of all skin malignancies in the 1980s to 15.6% in the 1990s.7,8 In Japan, malignant melanoma is the 3rd most common cutaneous malignancy, representing 19% of all skin cancers.6 In our study, the incidence of malignant melanoma was 0.073% which was similar to previous reports of incidence (from 0.038% to 0.161%) of malignant melanoma in Korea.7,12-14 The estimated annual percentage increase in melanoma incidence of 6.1% was noted for Asians compared to 5.0% for Caucasians, which suggests that melanoma is a cancer with an increase in its incidence in Asian populations.11 Melanoma is known to affect young and middle-aged people, unlike other solid tumors, which mainly affect older adults. The median age at the diagnosis of melanoma was 55.9 years in our study which was similar to other studies.15 In Caucasian populations, males are approximately 1.5 times more likely to develope melanoma than females.15 The distribution of favored sites of occurrence is sex-dependent; the most common areas being the back for men and the arms and legs for women.16,17 In our study, there were more females diagnosed with melanoma overall, and females predominated in cases of melanoma occurring on hands and feet which corresponds to previous reports.
There are many predictors of survival proposed according to different stages. Tumor thickness determined by the Breslow method and associated depth of invasion by Clark's classification have been reported to be the most important independent prognostic variables in stage I, which includes locally invasive melanoma.18-21 In our study, tumor thickness (< 2 mm vs. > 2 mm) by univariate analysis and as a continuous variable in multivariate analysis showed significant difference in the prediction of survival. However, when the thickness was evaluated by 4 mm, we did not achieve a significant difference in prediction of survival due to a small number of cases with thick melanomas. For melanomas with regional metastases, the number of lymph nodes positive for melanomas and the presence of extranodal diseases were the most important prognostic factors for survival.20,22 For melanomas with distant metastases, the location of metastasis, presence of resectable metastatic lesions, and duration of remission has all been shown to be prognostic factors.19 Our study suggests that tumor thickness and stage are the important predicting factors for melanoma in Korean patients, with advanced stage being the more significant of the two. Acral lentiginous melanoma is the most common type in Asian populations.3,5,6 Our results correlated with the previous reports in which the acral lentiginous melanoma was the most common histologic subtype compromising of 56.3% to 58.4%,12-14 and the sole was the most common location.
The risk factors for melanoma vary among ethnicity ultraviolet radiation (UVR) exposures, specifically intense early sunburns, are closely associated with the development of melanoma in Caucasian patients.23-26 Other risk factors for Caucasian patients include atypical and multiple nevi, family or personal history of melanoma, intermittent sun exposure, and Fitzpatrick skin types I and II.23-27 However, UVR does not appear to be a significant risk factor for melanoma in patients with darker skin color, who tend to develop melanomas on non-sun-exposed areas such as palmar, plantar, and mucosal surfaces.28 However, although less significant than Caucasians, UVR still plays a role in the development of melanomas in non-Caucasian population. Several studies have shown evidence that migrant population who move closer to the equator develop high rates of melanoma compared to people in their country of origin.29-31 Some suggested that trauma may be a significant predisposing factor for melanomas of hands and feet.32,33 However, in our study, there were no patients with evident histories of previous trauma on the sites of melanoma. The prognostic significance of the anatomic site of origin remains controversial. Many consider tumors that arise on the palms, soles, and subungal locations to be particularly ominous.34 However, Wells, et al.35 suggested that the differences in outcome were largely explained by a difference in tumor thickness at the time of diagnosis. Therefore, we conducted our study in order to verify the prognostic significance according to the anatomic locations in Korean patients. In our study, 65% of the melanoma was located on the acral area (palm, sole and subungal area). However, when compared with melanomas arising in other locations, the age of patients at diagnosis, duration of lesions until diagnosis, stage, presence of ulceration, and thickness of tumor were not significantly different. Nor did the survival rate significantly differ between the two groups. Our report provides further evidence that melanomas arising in non-Caucasian populations do indeed occur more frequently in acral areas. However, melanomas occurring in acral areas have a similar prognosis as those occurring in other areas.
The standard therapy for primary cutaneous melanoma is wide local excision. Current recommendations regarding the optimal surgical management of the primary melanoma are based on randomized clinical trials that principally have evaluated patients with cutaneous melanomas of the trunk and extremities. Treatment of melanomas on hands and feet is particularly challenging for the surgeons due to the functional requirements of these body parts and the difficulty of obtaining conventionally recommended margins. Local recurrence rates have been reported to be two to five times higher than those in other areas because of the tendency to use smaller margins in treating lesions of the hands and feet.36 Ever since Hutchinson first propagated a radical surgical approach (amputation) for subungal melanomas, this radical surgical strategy has been recommended.37 However, amputations of the hands and feet result in impaired function and cosmesis. Therefore, moderate amputations were proposed to preserve the extremities as long as possible without compromising safety margins.36 Moehrle, et al.38 reported a recent experience with "functional" surgery, avoiding amputation, and found a similar incidence of local recurrence and improved survival in the group of patients undergoing limited excision compared with patients undergoing amputation. Our study results also showed that the group treated by amputation did not have a statistically significant survival benefit than the group treated by local wide excision on melanomas of hands and feet. However, in cases of subungal melanoma, amputation is usually required due to the paucity of soft tissue between the tumor and the bone beneath the nail. Therefore, although amputation should not be recommended in general, it should be the first recommendation in cases of subungal melanomas without enough safety margins. In general, acral lentiginous melanomas are known to have poorer prognosis due to a delay in diagnosis because of the atypical locations and lack of awareness of this disease entity in the non-Caucasian populations.39-41 However, our study shows no difference in the prognosis of melanomas of the hands and feet compared to melanomas of other areas in Korean patients. Kato, et al.41 have observed improved survival rates for patients with acral melanoma during the last 22 years and attributed this improvement to efforts by Japanese dermatologists to educate the general public. Early detection programs impact the outcome of melanoma. Therefore, increased educational efforts focusing on populations' anatomically distinct distribution of primary sites should be made to increase the early and accurate diagnosis of melanoma.
Table 5.
Multivariate Analysis by Cox Proportional Hazard Model (Overall Survival)
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Dooley TP. Recent advances in cutaneous melanoma oncogenesis research. Oncol Res. 1994;6:1–9. [PubMed] [Google Scholar]
- 3.Chen YJ, Wu CY, Chen JT, Shen JL, Chen CC, Wang HC. Clinicopathologic analysis of malignant melanoma in Taiwan. J Am Acad Dermatol. 1999;41:945–949. doi: 10.1016/s0190-9622(99)70251-3. [DOI] [PubMed] [Google Scholar]
- 4.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Luk NM, Ho LC, Choi CL, Wong KH, Yu KH, Yeung WK. Clinicopathological features and prognostic factors of cutaneous melanoma among Hong Kong Chinese. Clin Exp Dermatol. 2004;29:600–604. doi: 10.1111/j.1365-2230.2004.01644.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara K, Saida T, Yamamoto A Japanese Skin Cancer Society Prognosis and Statistical Investigation Committee. Updated statistical data for malignant melanoma in Japan. Int J Clin Oncol. 2001;6:109–116. doi: 10.1007/pl00012091. [DOI] [PubMed] [Google Scholar]
- 7.Lee MW, Koh JK, Kwon KS, Kim NI, Kim SW, Kim SN, et al. Clinical and histopathological study of cutaneous melanoma in Korea. Korean J Dermatol. 2003;41:43–47. [Google Scholar]
- 8.Moon SE, Cho KH, Hwang JH, Kim JA, Youn JI. A statistical study of cutaneous malignant tumors. Korean J Dermatol. 1998;36:7–15. [Google Scholar]
- 9.Hemmings DE, Johnson DS, Tominaga GT, Wong JH. Cutaneous melanoma in a multiethnic population: is this a different disease? Arch Surg. 2004;139:968–972. doi: 10.1001/archsurg.139.9.968. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Cascinelli N. The new melanoma staging system. Tumori. 2001;87:S64–S68. [PubMed] [Google Scholar]
- 11.Cormier JN, Xing Y, Ding M, Lee JE, Mansfield PF, Gershenwald JE, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907–1914. doi: 10.1001/archinte.166.17.1907. [DOI] [PubMed] [Google Scholar]
- 12.Jang KA, Kim JH, Choi JH, Sung KJ, Moon KC, Koh JK. A Clinico-histopathological study of malignant melanoma. Korean J Dermatol. 2000;38:1435–1443. [Google Scholar]
- 13.Park KD, Lee SJ, Lee WJ, Kim DW, Chung HY, Cho BC. Clinicopathological features of cutaneous malignant melanoma. Korean J Dermatol. 2007;45:149–158. [Google Scholar]
- 14.Lee DH, Kim YC, Cho SH, Lee MG. Clinicopathologic analysis of malignant melanoma. Korean J Dermatol. 2002;40:914–923. [Google Scholar]
- 15.Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Boyle P, Maisonneuve P, Doré JF. Epidemiology of malignant melanoma. Br Med Bull. 1995;51:523–547. doi: 10.1093/oxfordjournals.bmb.a072978. [DOI] [PubMed] [Google Scholar]
- 17.Tsai T, Vu C, Henson DE. Cutaneous, ocular and visceral melanoma in African Americans and Caucasians. Melanoma Res. 2005;15:213–217. doi: 10.1097/00008390-200506000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Morton DL, Davtyan DG, Wanek LA, Foshag LJ, Cochran AJ. Multivariate analysis of the relationship between survival and the microstage of primary melanoma by Clark level and Breslow thickness. Cancer. 1993;71:3737–3743. doi: 10.1002/1097-0142(19930601)71:11<3737::aid-cncr2820711143>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Garbe C, Büttner P, Bertz J, Burg G, d'Hoedt B, Drepper H, et al. Primary cutaneous melanoma. Identification of prognostic groups and estimation of individual prognosis for 5093 patients. Cancer. 1995;75:2484–2491. doi: 10.1002/1097-0142(19950515)75:10<2484::aid-cncr2820751014>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Vollmer RT. Malignant melanoma. A multivariate analysis of prognostic factors. Pathol Annu. 1989;24(Pt 1):383–407. [PubMed] [Google Scholar]
- 21.Breslow A. Tumor thickness, level of invasion and node dissection in stage I cutaneous melanoma. Ann Surg. 1975;182:572–575. doi: 10.1097/00000658-197511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bevilacqua RG, Coit DG, Rogatko A, Younes RN, Brennan MF. Axillary dissection in melanoma. Prognostic variables in node-positive patients. Ann Surg. 1990;212:125–131. doi: 10.1097/00000658-199008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu S, Ma F, Collado-Mesa F, Kirsner RS. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch Dermatol. 2004;140:819–824. doi: 10.1001/archderm.140.7.819. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes AR, Weinstock MA, Fitzpatrick TB, Mihm MC, Jr, Sober AJ. Risk factors for cutaneous melanoma. A practical method of recognizing predisposed individuals. JAMA. 1987;258:3146–3154. [PubMed] [Google Scholar]
- 26.Holly EA, Aston DA, Cress RD, Ahn DK, Kristiansen JJ. Cutaneous melanoma in women. I. Exposure to sunlight, ability to tan, and other risk factors related to ultraviolet light. Am J Epidemiol. 1995;141:923–933. doi: 10.1093/oxfordjournals.aje.a117359. [DOI] [PubMed] [Google Scholar]
- 27.Elwood JM. Melanoma and sun exposure. Semin Oncol. 1996;23:650–666. [PubMed] [Google Scholar]
- 28.Gloster HM, Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741–760. doi: 10.1016/j.jaad.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 29.McCredie M, Coates MS, Ford JM. Cancer incidence in migrants to New South Wales from England, Wales, Scotland and Ireland. Br J Cancer. 1990;62:992–995. doi: 10.1038/bjc.1990.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke KR, Fraser J. Migration and death from malignant melanoma. Int J Cancer. 1985;36:175–178. doi: 10.1002/ijc.2910360208. [DOI] [PubMed] [Google Scholar]
- 31.Titus-Ernstoff L. An overview of the epidemiology of cutaneous melanoma. Clin Plast Surg. 2000;27:305–316. vii. [PubMed] [Google Scholar]
- 32.Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667–673. doi: 10.1002/1097-0142(19950115)75:2+<667::aid-cncr2820751409>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 33.Stevens NG, Liff JM, Weiss NS. Plantar melanoma: is the incidence of melanoma of the sole of the foot really higher in blacks than whites? Int J Cancer. 1990;45:691–693. doi: 10.1002/ijc.2910450421. [DOI] [PubMed] [Google Scholar]
- 34.Kuchelmeister C, Schaumburg-Lever G, Garbe C. Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol. 2000;143:275–280. doi: 10.1046/j.1365-2133.2000.03651.x. [DOI] [PubMed] [Google Scholar]
- 35.Wells KE, Reintgen DS, Cruse CW. The current management and prognosis of acral lentiginous melanoma. Ann Plast Surg. 1992;28:100–103. doi: 10.1097/00000637-199201000-00025. [DOI] [PubMed] [Google Scholar]
- 36.Tseng JF, Tanabe KK, Gadd MA, Cosimi AB, Malt RA, Haluska FG, et al. Surgical management of primary cutaneous melanomas of the hands and feet. Ann Surg. 1997;225:544–550. doi: 10.1097/00000658-199705000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly JM, Berlin R, Urmacher C. Subungual melanoma: a 25-year review of cases. J Surg Oncol. 1987;35:107–112. doi: 10.1002/jso.2930350209. [DOI] [PubMed] [Google Scholar]
- 38.Moehrle M, Metzger S, Schippert W, Garbe C, Rassner G, Breuninger H. "Functional" surgery in subungual melanoma. Dermatol Surg. 2003;29:366–374. doi: 10.1046/j.1524-4725.2003.29087.x. [DOI] [PubMed] [Google Scholar]
- 39.Franke W, Neumann NJ, Ruzicka T, Schulte KW. Plantar malignant melanoma--a challenge for early recognition. Melanoma Res. 2000;10:571–576. doi: 10.1097/00008390-200012000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Bennett DR, Wasson D, MacArthur JD, McMillen MA. The effect of misdiagnosis and delay in diagnosis on clinical outcome in melanomas of the foot. J Am Coll Surg. 1994;179:279–284. [PubMed] [Google Scholar]
- 41.Kato T, Suetake T, Sugiyama Y, Tanita Y, Kumasaka K, Takematsu H, et al. Improvement in survival rate of patients with acral melanoma observed in the past 22 years in Sendai, Japan. Clin Exp Dermatol. 1993;18:107–110. doi: 10.1111/j.1365-2230.1993.tb00988.x. [DOI] [PubMed] [Google Scholar]