Abstract
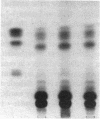
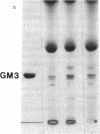
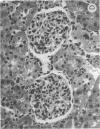
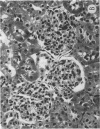
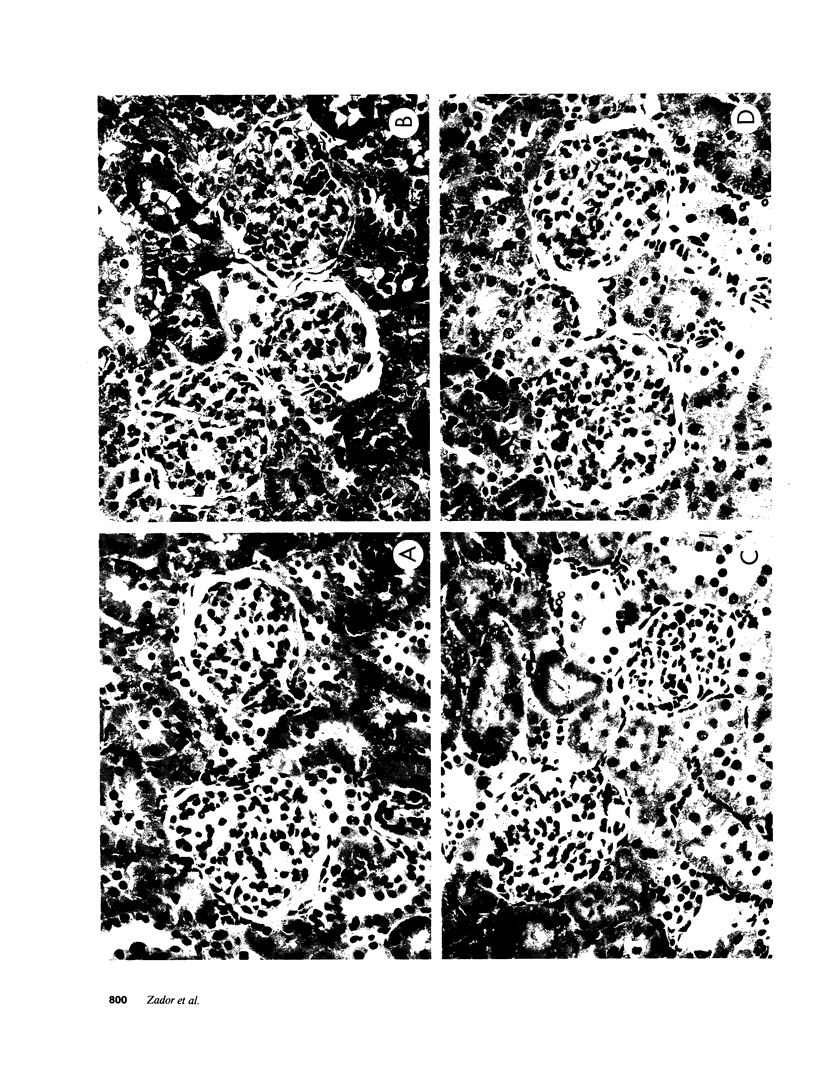
Glucosylceramide (GlcCer) and related glycosphingolipids have been implicated as causal elements in both the growth of cells and in the regulation of hormonal signaling. We therefore studied whether the renal hypertrophy induced by diabetes was associated with enhanced synthesis of glycosphingolipids. 16 d after the induction of diabetes, increases in renal size and concentration of glucocerebroside and ganglioside GM3 were observed paralleling an increase in UDP-Glc concentration. GlcCer synthase and beta-glucosidase-specific activities were no different between control and diabetic kidneys. The apparent Km of the GlcCer synthase with respect to UDP-Glc was 250 microM and was unchanged in the diabetic kidneys. The observed concentrations of UDP-Glc were 149 and 237 microM in control and diabetic kidneys, respectively. The UDP-Glc level is thus rate limiting with regard to GlcCer synthesis. To determine whether the changes in glycolipid content were functionally significant, diabetic and control groups were treated with the GlcCer synthase inhibitor, D-threo-1-phenyl-2-decanoyl-amino-3-morpholino-1- propanol, 2 wk after the induction of diabetes. Kidney weights in the diabetic rats treated with D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol were no different than the control groups. Morphometric analysis of glomerular volumes paralleled changes in renal growth. Glycosphingolipid formation may therefore represent a significant pathway for glucose utilization in early diabetic nephropathy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer-Mears A., Ku L., Cohen M. P. Glomerular polyol accumulation in diabetes and its prevention by oral sorbinil. Diabetes. 1984 Jun;33(6):604–607. doi: 10.2337/diab.33.6.604. [DOI] [PubMed] [Google Scholar]
- Bremer E. G., Schlessinger J., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986 Feb 15;261(5):2434–2440. [PubMed] [Google Scholar]
- Broulik P. D., Schreiber V. Effect of alloxan diabetes on kidney growth in intact and castrated mice. Acta Endocrinol (Copenh) 1982 Jan;99(1):109–111. doi: 10.1530/acta.0.0990109. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Glycosylation products as toxic mediators of diabetic complications. Annu Rev Med. 1991;42:159–166. doi: 10.1146/annurev.me.42.020191.001111. [DOI] [PubMed] [Google Scholar]
- Chatterjee S. Lactosylceramide stimulates aortic smooth muscle cell proliferation. Biochem Biophys Res Commun. 1991 Dec 16;181(2):554–561. doi: 10.1016/0006-291x(91)91225-2. [DOI] [PubMed] [Google Scholar]
- Cortes P., Dumler F., Levin N. W. Glomerular uracil nucleotide synthesis. Am J Physiol. 1988 Oct;255(4 Pt 2):F635–F646. doi: 10.1152/ajprenal.1988.255.4.F635. [DOI] [PubMed] [Google Scholar]
- Cortes P., Dumler F., Sastry K. S., Verghese C. P., Levin N. W. Effects of early diabetes on uridine diphosphosugar synthesis in the rat renal cortex. Kidney Int. 1982 May;21(5):676–682. doi: 10.1038/ki.1982.80. [DOI] [PubMed] [Google Scholar]
- Coste H., Martel M. B., Got R. Topology of glucosylceramide synthesis in Golgi membranes from porcine submaxillary glands. Biochim Biophys Acta. 1986 Jun 13;858(1):6–12. doi: 10.1016/0005-2736(86)90285-3. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Davidson C. M., DeRubertis F. R. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990 Jun;39(6):667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- Dinur T., Grabowski G. A., Desnick R. J., Gatt S. Synthesis of a fluorescent derivative of glucosyl ceramide for the sensitive determination of glucocerebrosidase activity. Anal Biochem. 1984 Jan;136(1):223–234. doi: 10.1016/0003-2697(84)90329-4. [DOI] [PubMed] [Google Scholar]
- Futerman A. H., Pagano R. E. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991 Dec 1;280(Pt 2):295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 1989 Jan 27;243(4890):500–507. doi: 10.1126/science.2643164. [DOI] [PubMed] [Google Scholar]
- Hirose K., Osterby R., Nozawa M., Gundersen H. J. Development of glomerular lesions in experimental long-term diabetes in the rat. Kidney Int. 1982 May;21(5):689–695. doi: 10.1038/ki.1982.82. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Keppler D., Rudigier J., Decker K. Enzymic determination of uracil nucleotides in tissues. Anal Biochem. 1970 Nov;38(1):105–114. doi: 10.1016/0003-2697(70)90160-0. [DOI] [PubMed] [Google Scholar]
- Mahdiyoun S., Deshmukh G. D., Abe A., Radin N. S., Shayman J. A. Decreased formation of inositol trisphosphate in Madin-Darby canine kidney cells under conditions of beta-glucosidase inhibition. Arch Biochem Biophys. 1992 Feb 1;292(2):506–511. doi: 10.1016/0003-9861(92)90023-p. [DOI] [PubMed] [Google Scholar]
- Merrill A. H., Jr, Jones D. D. An update of the enzymology and regulation of sphingomyelin metabolism. Biochim Biophys Acta. 1990 May 1;1044(1):1–12. doi: 10.1016/0005-2760(90)90211-f. [DOI] [PubMed] [Google Scholar]
- Needleman P., Passonneau J. V., Lowry O. H. Distribution of glucose and related metabolites in rat kidney. Am J Physiol. 1968 Sep;215(3):655–659. doi: 10.1152/ajplegacy.1968.215.3.655. [DOI] [PubMed] [Google Scholar]
- Radin N. S. Preparative isolation of cerebrosides (galactosyl and glucosyl ceramide). J Lipid Res. 1976 May;17(3):290–293. [PubMed] [Google Scholar]
- Shayman J. A., Deshmukh G. D., Mahdiyoun S., Thomas T. P., Wu D., Barcelon F. S., Radin N. S. Modulation of renal epithelial cell growth by glucosylceramide. Association with protein kinase C, sphingosine, and diacylglycerol. J Biol Chem. 1991 Dec 5;266(34):22968–22974. [PubMed] [Google Scholar]
- Shayman J. A., Mahdiyoun S., Deshmukh G., Barcelon F., Inokuchi J., Radin N. S. Glucosphingolipid dependence of hormone-stimulated inositol trisphosphate formation. J Biol Chem. 1990 Jul 25;265(21):12135–12138. [PubMed] [Google Scholar]
- Shayman J. A., Radin N. S. Structure and function of renal glycosphingolipids. Am J Physiol. 1991 Mar;260(3 Pt 2):F291–F302. doi: 10.1152/ajprenal.1991.260.3.F291. [DOI] [PubMed] [Google Scholar]
- Shukla A., Radin N. S. Metabolism of D-[3H]threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol, an inhibitor of glucosylceramide synthesis, and the synergistic action of an inhibitor of microsomal monooxygenase. J Lipid Res. 1991 Apr;32(4):713–722. [PubMed] [Google Scholar]
- Shukla A., Shukla G. S., Radin N. S. Control of kidney size by sex hormones: possible involvement of glucosylceramide. Am J Physiol. 1992 Jan;262(1 Pt 2):F24–F29. doi: 10.1152/ajprenal.1992.262.1.F24. [DOI] [PubMed] [Google Scholar]
- Shukla G. S., Radin N. S. Glucosyceramide synthase of mouse kidney: further characterization with an improved assay method. Arch Biochem Biophys. 1990 Dec;283(2):372–378. doi: 10.1016/0003-9861(90)90657-k. [DOI] [PubMed] [Google Scholar]
- Shukla G. S., Shukla A., Inokuchi J., Radin N. S. Rapid kidney changes resulting from glycosphingolipid depletion by treatment with a glucosyltransferase inhibitor. Biochim Biophys Acta. 1991 Apr 24;1083(1):101–108. doi: 10.1016/0005-2760(91)90130-a. [DOI] [PubMed] [Google Scholar]
- Spiro M. J. Effect of diabetes on the sugar nucleotides in several tissues of the rat. Diabetologia. 1984 Jan;26(1):70–75. doi: 10.1007/BF00252267. [DOI] [PubMed] [Google Scholar]
- Steer K. A., Sochor M., Gonzalez A. M., McLean P. Regulation of pathways of glucose metabolism in kidney. Specific linking of pentose phosphate pathway activity with kidney growth in experimental diabetes and unilateral nephrectomy. FEBS Lett. 1982 Dec 27;150(2):494–498. doi: 10.1016/0014-5793(82)80797-7. [DOI] [PubMed] [Google Scholar]
- Steer K. A., Sochor M., McLean P. Renal hypertrophy in experimental diabetes. Changes in pentose phosphate pathway activity. Diabetes. 1985 May;34(5):485–490. doi: 10.2337/diab.34.5.485. [DOI] [PubMed] [Google Scholar]
- Touchstone J. C., Levin S. S., Dobbins M. F., Matthews L., Beers P. C., Gabbe S. G. (3-sn-Phosphatidyl)cholines (lecithins) in amniotic fluid. Clin Chem. 1983 Nov;29(11):1951–1954. [PubMed] [Google Scholar]
- Weinberg J. M., Harding P. G., Humes H. D. Alterations in renal cortex cation homeostasis during mercuric chloride and gentamicin nephrotoxicity. Exp Mol Pathol. 1983 Aug;39(1):43–60. doi: 10.1016/0014-4800(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Zatz R., Brenner B. M. Pathogenesis of diabetic microangiopathy. The hemodynamic view. Am J Med. 1986 Mar;80(3):443–453. doi: 10.1016/0002-9343(86)90719-9. [DOI] [PubMed] [Google Scholar]