Abstract
Plasmodium falciparum surface protein 25 (Pfs25) is a candidate for transmission-blocking vaccines (TBVs). Anti-Pfs25 antibodies block the development of oocysts in membrane-feeding assays and we have shown the activity correlates with antibody titer. In this study, we purified Pfs25-specific IgGs to convert antibody titer to µg/mL and determined the amount of antibody required to inhibit 50 percent of oocyst development (IC50). The IC50 were, 15.9, 4.2, 41.2, and 85.6 µg/mL for mouse, rabbit, monkey and human, respectively, and the differences among species were significant. Anti-Pfs25 sera from rabbit, monkey and human showed different patterns of competition against 6 mouse monoclonal antibodies, and the avidity of antibodies among four species were also different. These data suggests that information obtained from animal studies which assess efficacy of TBV candidates may be difficult to translate to human immunization.
Keywords: Plasmodium falciparum, Transmission-blocking vaccine, Vaccine development
1. Introduction
Efforts to eradicate malaria have become the focus of the global community as malaria continues to be one of the deadliest diseases in tropical and sub-tropical countries worldwide. In 2006, there were estimates of 3.3 billion individuals at risk for developing malaria [1]. It is estimated that 610, 000–1, 212,000 malaria related deaths occurred in 2006 of which, 91 percent were in Africa and 85 percent of these were children under the age of five. The major cause of morbidity and mortality is attributed to Plasmodium falciparum, causing approximately 360.5 million clinical cases annually in Africa [2]. Current interventions include insecticide treated nets, anti-malarial drugs, and indoor insecticide spray. Although vaccination against malaria is considered to be the most cost-effective control method once applied, to date, only the RTS, S vaccine has shown partial clinical protection in several phase 2 trials in Africa [3, 4] and a phase 3 trial is underway.
Transmission-blocking vaccines (TBV) are designed to induce antibodies in human hosts against sexual stage malaria antigens [5]. The antibodies induced by a TBV are ingested by Anopheline mosquitoes along with the blood meal and subsequently inhibit development of the parasite in the mosquito host, resulting in the prevention of malaria transmission to another human. Therefore, an effective TBV would be expected to lead to the elimination of parasites in low transmission areas, and also prolong the lifetime of anti-malarial drugs by impeding the spread of drug-resistant parasite strains [6]. Plasmodium falciparum surface protein 25 (Pfs25) is a lead candidate for development of a TBV and one of only two TBVs which have been tested in humans. Pfs25 is expressed on zygote and ookinete stages of parasites within mosquitoes [7]. There is less sequence polymorphism in Pfs25 than other malaria vaccine candidate molecules [8, 9] as this molecule has not been under immune selection pressure [10]. Numerous animal studies conducted by us and other investigators have shown that Pfs25 vaccination elicits antibodies which effectively block development of parasites in mosquitoes judged by ex vivo membrane-feeding assay (MFA)[11–16]. A Phase 1 human trial of Pfs25 formulated with Montanide ISA 51 was conducted in malaria naive U.S. adults [17], and the vaccine was shown to elicit functional antibodies.
We have also shown that the percent inhibition of oocyst density in the mosquito is a function of antibody titer measured by enzyme-linked immunosorbent assay (ELISA) in previous studies [13, 15, 17, 18]. However, the level of anti-Pfs25 antibodies had been expressed in arbitrary ELISA units, so it was impossible to compare the biological activities of anti-Pfs25 antibodies from different species on the same scale or interpret the results from other laboratories in the context of efficacy assessment. Therefore, to overcome this limitation of comparison (either among species or among laboratories), we converted anti-Pfs25 antibody titers into a standard mass concentration read-out (i.e., µg/mL) for mice, rabbits, rhesus and humans in this study. We analyzed the amount of anti-Pfs25 antibody required to inhibit 50% of oocyst development (IC50) for each species, and demonstrated significant differences of IC50 among species.
2. Materials and Methods
2.1 Animal sera
BALB/c mice, New Zealand White rabbits and rhesus monkeys (Macaca mulatta) were immunized with Pfs25 formulated with several adjuvants including aluminum hydroxide (alum), Montanide ISA720, ISA51 and Outer Membrane Protein Complex from Neisseria meningitidis (OMPC). High ELISA titer sera were selected for preparing pools for each species. Table 1 describes in detail the animal experiments and sera used for this study. Non-immunized, normal sera were also collected for each species as a negative control. All animal studies were done in compliance with National Institutes of Health (NIH) guidelines and under the auspices of Animal Care and Use committee approved protocols.
Table 1.
Details of mouse, rabbit, monkey, and human sera used in this study
2.2 Human sera
Details of a phase 1 study using Pfs25 formulated with Montanide ISA51 were described elsewhere [17]. High ELISA titer sera were selected and 5 pools were made from 5 different individuals. Table 1 describes sera used for this study. Normal serum from a malaria naive US volunteer was also used as a negative control. The human trial was conducted under an Investigational New Drug Application reviewed by the U.S. Food and Drug Administration, and was reviewed and approved by the Institutional Review Boards at the National Institute of Allergy and Infectious Diseases, NIH and by the Committee on Human Research at the Johns Hopkins Bloomberg School of Public Health Institutional Review Board. Written informed consent was obtained from all participants.
2.3 Total and Pfs25-specific IgG preparation
Total and antigen-specific IgG purification followed procedures previously described [19], with small modifications where Pfs25 protein was used for the affinity purification of antigen-specific IgG. In brief, to obtain total IgG from the test serum, protein G purification columns were prepared. The eluted IgG was collected and neutralized with neutralizing buffer (1M Tris-HCl, pH 9). NHS-activated Sepharose 4 Fast Flow agarose matrix was coupled with Pfs25 antigen as instructed in the user’s manual. Pfs25-specific IgGs were purified from the total IgGs and the eluted IgGs were neutralized with 1M Tris-HCl (pH 9). Buffer exchange was carried out using 1XPBS in an Amicon Ultra-15 centrifugal filter device. Total and Pfs25-specific IgGs were filtered using 0.22um Millipore UltraFree Centrifugal filter units. Total and Pfs25-specific IgGs were concentrated and their protein concentration were determined (mg/mL) using a spectrometer.
2.4 Standard ELISA, competition ELISA and avidity test
Standard ELISA procedure has been described elsewhere [20]. The ELISA unit value of a standard was assigned as the reciprocal of the dilution giving an O.D. 405 = 1 in a standardized assay. The absorbance of individual test samples was converted into ELISA units using a standard curve generated by serially diluting the standard in the same plate.
For competition ELISA, 6 mouse monoclonal antibodies (mAbs: 4B7, 4F7, 1A6, 1G2, 4D10 and 2A7), which were diluted to 120 ELISA units, and 2-fold dilutions of rabbit, rhesus monkey or human anti-Pfs25 serum starting with 1500 ELISA units were prepared. The mAbs and the antiserum were mixed in 1:1 (vol.) ratio and the mixture was applied to a Pfs25-coated ELISA plate. After 2 hours incubation, the plate was washed extensively and the mouse mAbs which bound to the plate antigens were detected by goat anti-mouse antibodies conjugated with alkaline phosphatase (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). Other procedures were the same as the standard ELISA, except the final readout was the actual OD value, instead of ELISA units calculated from a standard curve.
To determine the avidity of anti-Pfs25 antibody, an avidity test was performed as described elsewhere [19] with minor modification. In brief, 6 (mouse, rabbit and monkey) or 5 (human) individual antisera were diluted to give around OD of 2.0. A 15-min incubation step with varying concentrations of urea (from 0 to 10 M) was performed between the primary and secondary antibody incubation steps. The concentration of urea resulting in 50% of the original ELISA units (EC50) was calculated for each serum.
2.5 Membrane-Feeding Assay (MFA)
Membrane-Feeding assay was previously described in detail [21]. Briefly, test samples (either serum, total IgG or Pfs25-specific IgG) from animals and humans were diluted and mixed with a gametocyte culture of P. falciparum (NF54 strain). The mixture was fed to Anopheles stephensi (Nijmegen strain) mosquitoes through a membrane feeding apparatus. Mosquitoes were kept for 8 days and dissected to enumerate the oocysts in the midgut.
2.6 Statistical analysis
For each species, a conversion factor (mass concentration of antibody which gives 1 ELISA unit) was estimated using a linear regression model and the variance of the log transformed conversion factors were estimated by a delta method [22]. To model a relationship between the antibody level and the mean oocyst count per mosquito, we used a non-linear logistic-type model allowing for random effects for individual feeds. Specifically, we model the expected number of oocysts for the jth test sample on the ith feed, say E(Zij), when antibody is Xij (ELISA units):
Where ui is the random effect for the ith feed, a is the Hill coefficient of the Hill model and b is the antibody level needed to give 50% reduction in oocysts per mosquito (IC50). When this model is written in terms of the percent inhibition of oocyst, it becomes the Hill model as has been used previously [17]. We solved for the parameters using weighted least squares with inverse weights proportional to means raised to a power parameter [23] and solved by iterating with a Nelder-Mead algorithm [24]. To test for differences in IC50 by type of sample (sera, total IgG or Pfs25-specific IgG sample) within species, we did a Monte Carlo permutation test, where the test statistic was the sample variance of the 3 different IC50 values, so that a significant difference would indicate significantly different IC50 by type of sample. To compare IC50 across species, the IC50 in ELISA units was multiplied by the corresponding conversion factor. We can treat the sum of the log (IC50) parameters and the log (conversion factor) as normal using standard normality assumptions for the log of each parameter, and compare the IC50 (in µg/mL) of all four species by a chi-square distribution with 3 degree of freedom, followed by each pair comparison using a Z-test.
Avidity of antibody (EC50) in different species was compared by Kruskal-Wallis test, followed by Dunn’s multiple comparison test.
All statistical tests were performed by R (version 2.9.1) or Prism 5, and probability values less than 0.05 were considered significant.
3. Results
3.1 Conversion factor
To compare level of antibodies among species, we first made an attempt to convert arbitrary ELISA units to a standard mass concentration scale (i.e., µg/mL). We affinity purified Pfs25-specific IgGs, then determined ELISA units and protein concentration of each specific IgG. Figure 1 demonstrates that there is a linear relationship between ELISA units and protein concentration. Based on the linear regression analysis, we determined the conversion factor (CF: mass concentration of antibody which gives 1 ELISA unit) for mouse, rabbit, monkey and human as 40, 11, 17 and 45 ng/mL per 1 ELISA unit, respectively.
Fig. 1.
Relationship between ELISA units and mass concentration of Pfs25-specific IgG in each species. Pfs25-specific IgG from mouse (A) rabbit (B), monkey (C) and human (D) antisera were prepared and ELISA units (x-axis) and mass concentration (y-axis) were measured for each IgG preparation. Individual data points (close circles) and the best-fit line (dotted line) for each species are shown.
3.2 Determine IC50 in ELISA units
The MFA confirmed that Pfs25 antisera from all species blocked oocyst development in mosquitoes (Fig. 2A–D). There was a strong correlation between ELISA units and oocyst reduction activity for each species. To determine whether the purification procedures altered the biological activity, we prepared sufficient volume of total IgGs and Pfs25-specific IgGs from rabbit and monkey sera for testing by MFA. As shown in Fig. 2B and 2C, both total and Pfs25-specific IgGs showed similar transmission-blocking activity when the ELISA units were normalized. There were no significant differences in IC50 among sera, total IgG and Pfs25-specific IgG samples for these two species by a permutation test (rabbit, p=0.235; monkey, p>0.8). Thus for simplicity, a non-linear curve fit was applied including total and Pfs25-specific IgGs data for rabbit and monkey. In case of mouse and human, only anti-Pfs25 serum data was used to determine the IC50 in ELISA units.
Fig. 2.
Biological activity of mouse, rabbit, monkey and human anti-Pfs25 antibodies judged by MFA. (A–D): Anti-Pfs25 antisera (open circle) from mouse (A), rabbit (B), monkey (C), and human (D) were tested by MFA. Total IgGs (open reverse triangle) and Pfs25-specific IgGs (open squire) from rabbits and monkeys were also tested by MFA. The amount of anti-Pfs25 antibody in the membrane feeder (x-axis) is plotted against % inhibition of oocysts (y-axis). Individual data points represent % inhibition of a test sample, regardless of whether multiple samples were tested against a single control in the same feeding assay or a single sample was tested against a single control. A line represents the best-fit curve of the data for each species taking into consideration feed-to-feed variation as described in Materials and Methods section. (E): the amount of Pfs25 antibodies (µg/mL) required to inhibit 50 percent of oocyst development (IC50) in the MFA is shown for each species with the 95% confidence interval. There is a significant difference of IC50 among the four species (p<0.0001, by chi-square test)
3.3 IC50 Comparison
IC50 in ELISA units was transformed into IC50 in µg/mL using a conversion factor for each species (Fig. 2E). IC50 of rabbit was the lowest with 4.2 (95% Confidence Interval, 2.7–7.0) µg/mL of anti-Pfs25 antibody. The mouse IC50 was 15.9 (95%CI, 13.3–19.0) µg/mL, the monkey’s was 41.2 (95%CI, 33.9–50.1) µg/mL, while the human’s was 85.6 (95%CI, 58.1–126.0) µg/mL. There was a significant difference of IC50 in µg/mL among the four species (p<0.0001, chi-square test). When we compared the difference of IC50 between any two species, there were significant differences for all combinations (p<0.0001 for all combinations except for the case of monkey vs. human comparison, which was significant at p=0.0009).
3.4 Mechanisms responsible for the differences among species
To investigate the basis for the differences among the species, we first performed a competition ELISA with mouse monoclonal antibodies (mAbs) against polyclonal anti-Pfs25 serum of rabbit, monkey and human (Fig. 3). Five of the mAbs have shown transmission-blocking activities in MFA (4B7, 4F7, 1A6, 1G2 and 4D10) and the other mAb (2A7) only recognize a reduced Pfs25 protein and has no activity in MFA (data not shown). A fixed amount of mouse mAbs was tested with 2-fold dilutions of polyclonal anti-Pfs25 serum as competitors. The O.D. value reflects only the mouse mAbs bound to Pfs25 antigen on the ELISA plate. The patterns of competition were different depending on the mAb tested and the species of the antiserum. For all mAbs, the monkey antiserum competed most effectively, while rabbit and human antisera varied depending on the mAb. For 4B7 (Fig. 3A), rabbit antiserum was the least competitive and human antiserum was middle. For 4F7 (Fig. 3B) and 1A6 (Fig. 3C), human was the least and rabbit antiserum was intermediate; rabbit and human antisera were similar for 1G2 (Fig. 3D) and 4D10 (Fig. 3E). The pattern of competition against 2A7 (Fig. 3F) was similar to the case of 4B7. The same dilution of normal rabbit, monkey or human serum did not change the OD values of the mouse mAbs in this assay (data not shown).
Fig. 3.
Competition ELISA with mouse monoclonal antibodies against rabbit, monkey, or human anti-Pfs25 serum. A fixed amount of mouse monoclonal anti-Pfs25 antibodies 4B7 (A), 4F7 (B), 1A6 (C), 1G2 (D), 4D10 (E) and 2A7 (F) were tested with 2-fold dilutions of anti-Pfs25 serum from rabbit, monkey and humans. The amount of Pfs25-specific antibodies in the diluted serum (competitor) is shown on the x-axis and the O.D. value from the individual mouse monoclonal antibody is shown on the y-axis. The mean and standard deviation were calculated from two independent experiments. For some data points, the standard deviations are too small and are not visible.
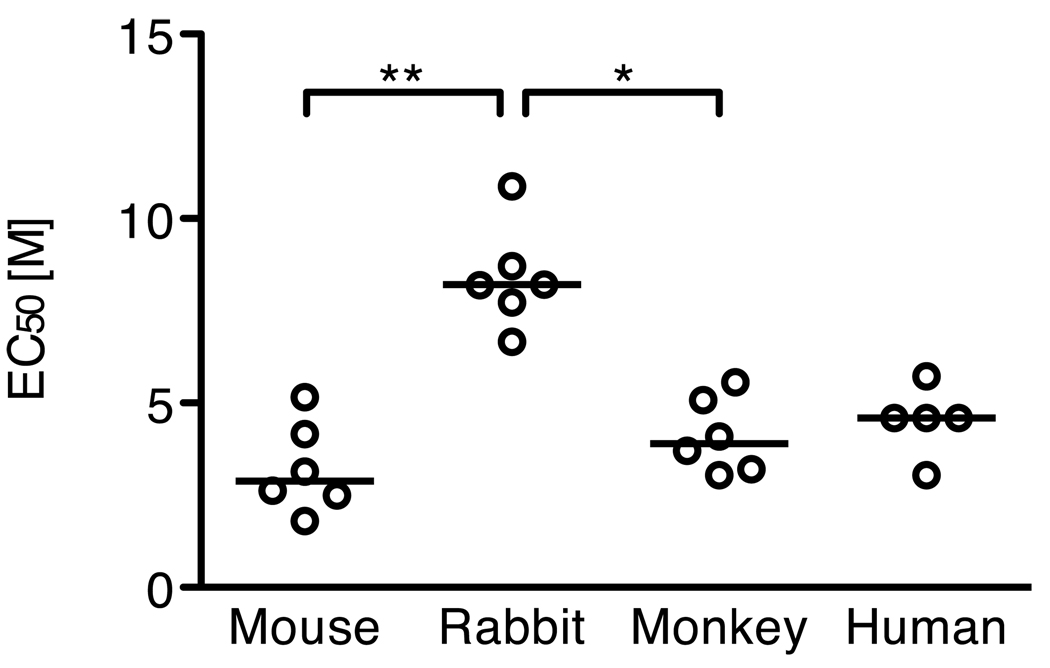
Second, we examined avidity of antibodies for each species. There was a significant difference in EC50 among the four species (p=0.0022, Kruskal-Wallis test), and the EC50 in rabbit was significantly higher than that in mouse and monkey.
4. Discussion
This is the first study to compare directly the functional activity (IC50) of antibodies to a single transmission-blocking vaccine candidate tested in various species. We report here that anti-Pfs25 antibodies from mouse, rabbit, monkey and human require significantly different amounts of antibody to inhibit 50 percent of oocyst development (IC50) in the MFA. The IC50 of mouse was 15.9 µg/mL while, rabbit, monkey and human, were 4.2, 41.2, and 85.6 µg/mL, respectively.
An ELISA unit is an arbitrary number and 1 ELISA unit in one species is not the same amount of antibody as 1 ELISA unit in other species. This is primarily due to the use of different secondary antibodies. Therefore, converting ELISA units to mass concentration (i.e., µg/mL) is necessary to compare IC50 among species. In order to convert ELISA units to mass concentration, Pfs25-specific IgGs were affinity purified from immunized mouse, rabbit, monkey and human sera. One potential caveat of this method was that the quality of Pfs25-specific IgGs (with which we determined the CFs) might be different from the original antibody in serum (with which we determined IC50 in ELISA units). As shown in Fig. 2, purified total and Pfs25-specific IgGs had the same biological activity as anti-Pfs25 sera at the same ELISA units in case of rabbit and monkey. Because only a limited volume of antiserum was available for mouse and human, we did not perform MFA with mouse and human total and/or Pfs25-specific IgGs in this study. However, we have shown with human antibody against another malaria antigen that the purification process did not change the biological activity judged by an in vitro growth inhibition assay [19]. These data suggest that the purification process did not affect (denature) the antibodies. Hence, we believe it is valid to calculate IC50 for each species in ELISA units, and then convert the IC50 to µg/mL using CFs.
In previous study [17], we reported IC50 as 1093 (95%CI, 683–1565) ELISA units, and the number differs from the one calculated in this study. In the Hill model formulation used in the previous study, we didn’t consider feed-to-feed variation (i.e., the differences in percent inhibition that could result if the same sample was tested in a different day on a different feed experiment). On the other hand, as described in Materials and Methods section, the new model allows random effects for individual feeds (i.e., includes a feed-to-feed variation effect). Therefore, we believe the new model provides better estimate of IC50 values.
In this study, we demonstrated that the IC50 are statistically different among species. We hypothesized that the difference (or part of it) is due to different recognition of Pfs25 epitopes among species and/or avidity of antibodies. To test the hypothesis, we first performed competition ELISA using 6 mouse mAbs to Pfs25. Five of the mAbs (4B7, 4F7, 1A6, 1G2 and 4D10) have shown transmission-blocking activities in MFA and the other mAb (2A7) has no activity in MFA (unpublished data). While, specific epitopes recognized by these mAbs were not known, beside 4B7, they have different strength of transmission-blocking activities in MFA. Fixed amounts of mouse mAbs were tested against several concentrations of polyclonal anti-Pfs25 serum from rabbit, monkey and human. Although all of the polyclonal antibodies inhibited binding of all 6 mouse mAbs tested, the competition patterns were different. The data suggest that each species recognizes Pfs25 epitopes in a distinct manner. Second, we measured avidity of antibodies for the four species. As shown in Fig 4, rabbit antibodies, which show the lowest IC50 in MFA, show the highest avidity to Pfs25 antigen. On the other hand, human antibodies, which show the highest IC50 in MFA, show lower avidity compared with rabbit, but similar level as mouse and monkey antibodies. These data suggest that avidity of antibody may contribute to the difference in IC50, but cannot explain all of the mechanistic differences among species.
Fig. 4.
Avidity of anti-Pfs25 antibody. The avidity of anti-Pfs25 antibody was tested using 6 (mouse, rabbit and monkey) or 5 (human) individual antisera by incubating ELISA plates with increasing concentrations of urea. The EC50 of individual samples and the median of each group are shown. The EC50 in different species was compared by Kruskal-Wallis test, followed by Dunn’s multiple comparison test (*, P<0.05; **, p<0.01).
One possible mechanism that might contribute to the difference in IC50 values was quality of antibody; i.e., antibody induced by vaccines with different adjuvants and/or different serum collection days. This possibility was dismissed because 1) rabbit and monkey studies were conducted with Pfs25 formulated with two different adjuvants in each species (Table 1), but all data points follow a single dose-response curve within a species (Fig. 2A–D); 2) there was no obvious impact of the bleed days on the transmission-blocking activity measured by MFA for all 4 species (Fig. 2A–D). Our previous study also showed that antibody concentration alone was the major determinant of the transmission-blocking activity, regardless of adjuvant used or time after immunization [15]. One could also speculate that differences in IgG subclasses might also contribute to the differences in IC50, but we did not investigate it in this study where we are comparing IC50 in mouse, rabbit, rhesus monkey and human. The reasons are 1) there is no IgG subclass (rabbit) or no commercially available reagents to detect subclasses (rhesus monkey), and 2) there is no direct matching of subclasses between mouse and human in functionality (e.g., mouse IgG2a is not exactly same as human IgG1, etc). One may consider another hypothesis that a difference of proportion of Pfs25- specific IgG in total IgGs causes the difference in IC50. However, we believe it is unlikely, because 1) while it is true that mouse and rabbit usually showed a higher proportion of specific IgG in total IgG, Pfs25-specific IgGs is less than 10% in most of the cases (usually less than 5%) of total IgG regardless of species (data not shown). This means that more than 90% of antibodies in MFA test samples are non-specific antibody in most cases, except when we tested purified Pfs25-specific antibodies. Moreover, 2) in each species, it is usual to see a more than 20–30 fold difference of Pfs25 antibody level among serum samples. In those cases, the proportion of specific antibody would also have very large fold-changes, assuming the amount of total IgG does not change as drastically. However, all data points in the same species follow a single dose-response curve (Fig.2A–D), which indicates that there is no qualitative difference among samples in the same species even when they have a totally different proportion of specific antibody. Taken together, it seems difficult to explain the IC50 differences among the species only by a single mechanism. Further study is required to uncover all the factors responsible for the IC50 differences.
In general, two different endpoints have been utilized to express transmission-blocking activity: 1) a reduction in the oocyst density per mosquito (% inhibition of oocysts, which is used in this paper) or 2) an increase in the proportion of mosquitoes that have no parasites (% inhibition of infected mosquitoes). The latter is thought to be the best predictor of vaccine efficacy under field conditions, as it is suggested that one oocyst contributes to a number of sporozoites [25]. However, we choose to use % inhibition of oocysts in this study, because the number of oocysts per mosquito in this experimental setting is different from field conditions. In direct mosquito feed assays where mosquitoes fed on blood directly from malaria patient's skin [26–28] or in a study where mosquitoes were caught in the field [29], most of the mosquitoes had less than 5–6 oocysts for each. On the other hand, the average number of oocysts per mosquito in a control group varied between study to study in our MFA: ranging from 1 to 300 oocysts per mosquito with an average of 34.4 (95%CI, 33.0–35.8) for all control groups in this study. The level of antibody that requires reducing oocyst numbers from 1 to 0 is much lower than the amount of antibody required for reduction from 300 to 0, so even though the same sample is tested, % inhibition of infected mosquitoes may change depending on the number of oocysts in the control. Technically it is very difficult to maintain the average number of oocysts per mosquito in a control feed. In addition, reducing number of oocysts in a control may be problematic; van der Kolk et al have shown that a minimum of 35 oocysts per mosquito in control feeds gave optimal reproducibility between experiments [30]. Similarly, we observed that if the average number of oocysts per mosquito in the control feed was lower than 4, reproducible results were not obtained (unpublished data). Although these two readouts are different, Medley et al have shown that there is a significant correlation between intensity of oocysts and percent of infected mosquitoes [31]. In addition, we have shown that % inhibition of infected mosquitoes can be reasonably estimated from intensity of oocysts in a control feed and the degree of oocyst reduction (calculated from % inhibition of oocysts) of a test sample [15]. Therefore, determining antibody levels giving 50% reduction of oocyst numbers per mosquito, instead of 50% reduction of infected mosquitoes, in MFA is valuable for evaluating the efficacy of a TBV candidate.
This study suggests that information obtained from animal studies which assess efficacy of TBV candidates may be difficult to translate to human immunization. More importantly, although the value of IC50 determined in this study might be different when other investigators use other Pfs25 antigens and/or other TBV candidate antigens (e.g., Pf48, Pf230, etc), the methodology described in this paper can be applied not only to Pfs25-based vaccines, but also to other antigen-based TBV. By determining IC50 in µg/mL, results from different species, antigens and/or laboratories can be compared on the same scale. Therefore we believe this study supports and facilitates future TBV development.
Acknowledgements
We are very grateful to all volunteers who participated in the clinical trial. We are also very grateful to Lynn Lambert and her team for meticulous execution of animal immunization studies. The study was supported by the intramural program of the National Institute of Allergy and Infectious Diseases/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. World Malaria Report. 2008
- 2.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434(7030):214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Milman J, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 4.Aponte JJ, Aide P, Renom M, Mandomando I, Bassat Q, Sacarlal J, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370(9598):1543–1551. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 5.Girard MP, Reed ZH, Friede M, Kieny MP. A review of human vaccine research and development: Malaria. Vaccine. 2007;25(9):1567–1580. doi: 10.1016/j.vaccine.2006.09.074. [DOI] [PubMed] [Google Scholar]
- 6.Phillips RS. Current status of malaria and potential for control. Clin Microbiol Rev. 2001;14(1):208–226. doi: 10.1128/CMR.14.1.208-226.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaslow DC, Quakyi IA, Syin C, Raum MG, Keister DB, Coligan JE, et al. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333(6168):74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 8.Kaslow DC, Quakyi IA, Keister DB. Minimal variation in a vaccine candidate from the sexual stage of Plasmodium falciparum. Mol Biochem Parasitol. 1989;32(1):101–103. doi: 10.1016/0166-6851(89)90134-5. [DOI] [PubMed] [Google Scholar]
- 9.Shi YP, Alpers MP, Povoa MM, Lal AA. Single amino acid variation in the ookinete vaccine antigen from field isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1992;50(1):179–180. doi: 10.1016/0166-6851(92)90254-h. [DOI] [PubMed] [Google Scholar]
- 10.Carter R, Graves PM, Quakyi IA, Good MF. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J Exp Biol. 1989;169(1):135–147. doi: 10.1084/jem.169.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr PJ, Green KM, Gibson HL, Bathurst IC, Quakyi IA, Kaslow DC. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174(5):1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy PE, Kaslow DC. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65(3):1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Y, Przysiecki C, Flanagan E, Bello-Irizarry SN, Ionescu R, Muratova O, et al. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc Natl Acad Sci U S A. 2006;103(48):18243–18248. doi: 10.1073/pnas.0608545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubler-Kielb J, Majadly F, Wu Y, Narum DL, Guo C, Miller LH, et al. Long-lasting and transmission-blocking activity of antibodies to Plasmodium falciparum elicited in mice by protein conjugates of Pfs25. Proc Natl Acad Sci U S A. 2007;104(1):293–298. doi: 10.1073/pnas.0609885104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura K, Keister DB, Muratova OV, Sattabongkot J, Long CA, Saul A. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/Pvs25 is a direct and predictable function of antibody titer. Malaria J. 2007;6:107. doi: 10.1186/1475-2875-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeBlanc R, Vasquez Y, Hannaman D, Kumar N. Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation. Vaccine. 2008;26(2):185–192. doi: 10.1016/j.vaccine.2007.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS ONE. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian F, Wu Y, Muratova O, Zhou H, Dobrescu G, Duggan P, et al. Conjugating recombinant proteins to Pseudomonas aeruginosa ExoProtein A: a strategy for enhancing immunogenicity of malaria vaccine candidates. Vaccine. 2007;25(20):3923–3933. doi: 10.1016/j.vaccine.2007.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura K, Zhou H, Moretz SE, Diouf A, Thera MA, Dolo A, et al. Comparison of biological activity of human anti-apical membrane antigen-1 antibodies induced by natural infection and vaccination. J Immunol. 2008;181(12):8776–8783. doi: 10.4049/jimmunol.181.12.8776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26(2):193–200. doi: 10.1016/j.vaccine.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol. 1987;139(12):4213–4217. [PubMed] [Google Scholar]
- 22.Lehmann EL. Elements of large-sample theory. Springer-Verlag; 1999. [Google Scholar]
- 23.Carroll RJ, Ruppert D. Transformation and Weighting in Regression. Chapman and Hall; 1988. [Google Scholar]
- 24.Nelder JA, Mead R. A simplex algorithm for function minimization. Computer Journal. 1965;7:308–313. [Google Scholar]
- 25.Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology. 1991;102(Pt 1):27–31. doi: 10.1017/s0031182000060303. [DOI] [PubMed] [Google Scholar]
- 26.Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, et al. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology. 1988;96(Pt 2):251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- 27.Githeko AK, Brandling-Bennett AD, Beier M, Atieli F, Owaga M, Collins FH. The reservoir of Plasmodium falciparum malaria in a holoendemic area of western Kenya. Trans R Soc Trop Med Hyg. 1992;86(4):355–358. doi: 10.1016/0035-9203(92)90216-y. [DOI] [PubMed] [Google Scholar]
- 28.Toure YT, Doumbo O, Toure A, Bagayoko M, Diallo M, Dolo A, et al. Gametocyte infectivity by direct mosquito feeds in an area of seasonal malaria transmission: implications for Bancoumana, Mali as a transmission-blocking vaccine site. Am J Trop Med Hyg. 1998;59(3):481–486. doi: 10.4269/ajtmh.1998.59.481. [DOI] [PubMed] [Google Scholar]
- 29.Billingsley PF, Medley GF, Charlwood D, Sinden RE. Relationship between prevalence and intensity of Plasmodium falciparum infection in natural populations of Anopheles mosquitoes. Am J Trop Med Hyg. 1994;51(3):260–270. doi: 10.4269/ajtmh.1994.51.260. [DOI] [PubMed] [Google Scholar]
- 30.van der Kolk M, De Vlas SJ, Sauls A, van de Vegte-Bolmer M, Eling WM, Sauerwein W. Evaluation of the standard membrane feeding assay (SMFA) for the determination of malaria transmission-reducing activity using empirical data. Parasitology. 2005;130(Pt 1):13–22. doi: 10.1017/s0031182004006067. [DOI] [PubMed] [Google Scholar]
- 31.Medley GF, Sinden RE, Fleck S, Billingsley PF, Tirawanchai N, Rodriguez MH. Heterogeneity in patterns of malarial oocyst infections in the mosquito vector. Parasitology. 1993;106(Pt 5):441–449. doi: 10.1017/s0031182000076721. [DOI] [PubMed] [Google Scholar]