Abstract
In this study, we set out to investigate whether introducing molecular genetic measures into an analysis of sexual partner variety will yield novel sociological insights. The data source is the white male DNA sample in the National Longitudinal Study of Adolescent Health. Our empirical analysis has produced a robust protective effect of the 9R/9R genotype relative to the Any10R genotype in the dopamine transporter gene (DAT1). The gene-environment interaction analysis demonstrates that the protective effect of 9R/9R tends to be lost in schools in which higher proportions of students start having sex early or among those with relatively low levels of cognitive ability. Our genetics-informed sociological analysis suggests that the “one size” of a single social theory may not fit all. Explaining a human trait or behavior may require a theory that accommodates the complex interplay between social contextual and individual influences and genetic predispositions.
Risky sexual behavior often plays a critical role in the spread of sexually transmitted diseases (STD), including HIV among adolescents and young adults (IOM 1997). Sociological work on risky sexual behavior has demonstrated the importance of social contexts at the levels of individual, family, neighborhood, and school (Brewster 1994; Browning et al 2005; Furstenberg et al 1987). In this study, we set out to investigate whether introducing molecular genetic measures into analysis of sexual partner variety will lead to novel sociological insights.
The notion that individuals may differ with respect to genetic predispositions is not new. Twins and siblings have long been used to estimate the proportion of variance in an outcome due to innate influences, but the findings have frequently been questioned because the two fundamental assumptions of equal environment and assortative mating remain controversial.
Earlier efforts linking genetic variants and human outcomes often suffer from small sample sizes, population admixture, multiple testing, inappropriate controls, and non-replication (Cardon and Palmer 2003; Ioannidis et al 2001; Ioannidis et al 2003). Convincing evidence arrived recently. The year 2007 saw an unprecedented succession of discoveries in the genomics of complex traits (e.g., Frayling et al 2007; Pennisi 2007; Scott et al 2007; Sladek et al 2007; Steinthorsdottir et al 2007; Zeggini et al 2007). These discoveries identified genetic variants associated with acute lymphoblastic leukemia, obesity, type 2 diabetes mellitus, prostate cancer, breast cancer, and coronary heart disease. All of the evidence has been replicated multiple times in large independent samples. These discoveries in human genetic variation were chosen by the American Association for the Advancement of Science as Science’s breakthrough of the year (Pennisi 2007).
If individuals do differ in genetic susceptibilities for diseases, it will be a small stretch to suggest that individuals may also differ in genetic propensities for other traits and behaviors that are of interest to sociologists. The important question, however, is whether bringing in genetics will advance sociology. In this study, we develop the theoretical concept of genetics-informed sociology, outlining specific ways sociological analysis may benefit from genetic information. Our empirical investigation, carried out in an analysis of multilevel social contexts, has two objectives: establishing the main effect of genotype on number of sexual partners and exploring how the influences of social contexts may be exacerbated or mitigated by genotype.
Consequences of Pattern of Sexual Partnering
A large number of sexual partners is an important indicator of an elevated risk of contracting a sexually transmitted disease (STD), including HIV (Cates and Stone 1992; Kost and Forrest 1992). With a pair of sexual partners potentially “exposing” themselves to every person with whom the two have ever had sex and every person with whom the two’s previous partners have ever had sex, and so on, the risk of STD tends to increase exponentially (Moody 2002).
In the United States, STDs disproportionately affect youth. Of the estimated 18.9 million new STD infections that occur in 2000, 9.1 (48%) millions occur among people aged 15–24 (Weinstock et al 2004). A recent CDC study reveals that around 26% of young American women aged 14–19 are infected with a sexually transmitted infection (CDC 2008). Chlamydia, gonorrhea, vaginitis, and pelvic inflammatory disease all have the highest prevalence rates among adolescents and these diseases become dramatically less prevalent with increasing age (Hatcher and Hagan 1998). Adolescents are more susceptible to STDs than adults because they have a higher probability of having multiple sexual partners (IOM 1997).
Multi-levels of Social Contexts and Pattern of Sexual Partnering
Sociologists have had a long-standing interest in the influences of multilevel social contexts on youth sexual behavior. Structural deficits in families, neighborhoods, and schools are generally considered having negative impacts on adolescents’ sexual activity. It is also essential to include demographic and other individual factors as controls. In this section, we identify, describe, and justify social contextual factors that are used to build a sociological model of number of sexual partners. The model serves as groundwork for testing effects of genetic variants.
Family structure and household socioeconomic status are two main family characteristics that are routinely used to predict adolescent behaviors. There are many theoretical reasons to expect weaker parental controls over adolescents in non-intact and lower SES families (Hetherington 1979; Thornton 1991). Single-headed households reduce the number of parents available for monitoring and supervising children by 50%. On average, single parents’ capacity to influence children’s behavior is likely to be lower. With one earner, more often the non-primary earner, of the family, a non-intact family also experiences more financial difficulties, which frequently lead to the mother’s entry into the labor force or to an increase in the hours of employment. This could further reduce a single parent’s capacity to interact and supervise children.
Families with high socioeconomic status have more resources to support and promote their children’s healthy development. These families typically provide their children with better neighborhoods, schools, and peer groups. All of these advantages may help reduce risks for early sexual activity.
Recent years have witnessed markedly increased efforts to investigate the influences of neighborhoods and schools on sexual activity during adolescence and young adulthood (Billy et al 1994; Brewster 1994; Browning et al 2005; Browning and Olinger-Wilbon 2003; South and Baumer 2000). Neighborhoods of residence and schools are particularly important social contexts for youth because their social space is limited to certain geographic areas. Concentrated poverty in a neighborhood affects the effectiveness of local institutions including schools, voluntary organizations, and informal neighbor networks. These contextual deficits, in turn, tend to impact negatively on adolescent outcomes (Leventhal and Brooks-Gunn 2000; Sampson et al 1997).
Religiosity at the contextual level is emphasized in the “moral community” thesis (Stark 1996; Stark and Bainbridge 1996; Stark et al 1982). The thesis argues that religion should be understood not only as an individual trait, but also a group property. Regardless of an individual’s own religiosity, living in a religious community protects teens from “deviant” behaviors.
Furstenberg and colleagues (1987) highlight the importance of the prevalence of sexual activity in a community. These authors noted that African American youth attending predominantly black schools were nearly four times more likely to report having had sexual intercourse than their counterparts in predominantly white schools. They concluded that the tendency toward early sexual initiation among African Americans had primarily been a consequence of their divergent sexual norms.
Several authors including Furstenberg (1987) noted that evaluating the normative explanation is not a straightforward matter (Brewster 1994; Lauritsen 1994; Moore 1986). School race composition and neighborhood race segregation generally reflect structural conditions and opportunity factors. The structural socioeconomic conditions could still be the underlying causes for the normative differences even if evidence is available for the normative explanation.
Social control theory has often been invoked to explain youth sexual behavior, but it is also recognized that social control is not the only mechanism through which social contexts influence sexual behavior (Lauritsen 1994; Thornton 1991; Udry 1988). Social control theory assumes a universal human tendency to pursue pleasure and postulates that the pursuit tends to go excessive unless checked by painful consequences. The theory is prominent in the study of criminal behavior (Gottfredson and Hirschi 1990; Hirschi 1969).
Sexual activity, however, differs from most criminal activity in a number of ways (Udry 1988). It is by and large pleasurable. Many criminal acts are for economic gains rather than pleasure. It is age-graded. Sexual activity is discouraged or prohibited in adolescence, but after adolescence it increasingly becomes accepted and even expected. Unlike much criminal activity, adolescent sexual activity is largely victimless. Though we consider social control theory useful, it will not be the overarching theory for our analysis.
Demographic and individual characteristics such as age, marital and cohabitation experience, religiosity, cognitive test score, and physical maturity are also included as controls. Individuals who marry young and who are in a stable marriage are expected to have fewer sexual partners. Religion is considered affecting beliefs, attitudes, and behaviors through the mechanisms of social control, social support, and values/identity (Wallace and Williams 1997). Religious influences coming from families, church youth groups, and community norms can impact on adolescent behavior.
Traditionally, religious institutions in the United States promote certain types of sexual ideologies, which are characterized by abstinence, procreation, and celibacy and which are intended to assert social influences and control over sexual behavior by prescribing and proscribing behavioral standards of sexual activity. In contrast to these more traditional sexual ideologies, American popular culture and mass media are perceived to actively promote a sexual ideology characterized by pleasure (DeLamater 1989).
Higher levels of cognitive development generally operates as a protective factor against early sexual activity during adolescence (Halpern et al 2000). Physical maturity is found to predict early sexual debut among adolescents (Capaldi et al 1996; Udry and Billy 1987). Gagnon and Simon (1973) argue that when an adolescent male develops musculature, beard, body hair, and mature genitals, he responds to social expectations of peers and parents by developing a role as a potential sexual partner.
Genetics-Informed Sociology
Advances in Molecular Genetics and Consensus on Importance of Environment
Intense efforts in molecular genetics over the past two decades have discovered more than a thousand genes responsible for Mendelian human outcomes—outcomes mostly determined by alleles1 of a single gene (Botstein and Risch 2003; Risch 2000). Examples of such human outcomes include Huntington’s disease, cystic fibrosis, hereditary non-polyposis colon cancer, and heritable breast cancers. Molecular genetic efforts have been much less successful on Non-Mendelian or complex human outcomes. Many of these outcomes, including reading disability, smoking, alcohol use, drug use, and obesity, are of interest to sociologists. The links between genetic heritage and complex human outcomes are enormously complicated, typically involving multiple genes, numerous environmental factors, and the interactions between the two.
As reviewed briefly earlier, it was not until recently that the genetics community produced persuasive evidence for the association between genetic variants and complex human outcomes. Two fundamental technological breakthroughs had paved the way for the discoveries: the HapMap data that reduce genotyping of redundant SNPs and high throughput genotyping.
The genetics community increasingly expressed the consensus that social scientists’ expertise in social contexts is essential for understanding many complex human diseases. The success of the Human Genome Project (Collins et al 2003) and the HapMap Project2 (The_International_HapMap_Consortium 2005) is improving the design and effectiveness of genetic studies of complex outcomes. These advances, however, do not lessen the need for understanding the social/environmental component of the puzzles. On the contrary, inadequate understanding of social environments has become a bottleneck for the rapid technological advances in molecular genetics. The HapMap project (The_International_HapMap_Consortium 2005), the National Human Genome Research Institute (Collins et al 2003), and the Committee on Gene-Environment Interactions for Health Outcomes at the Institute of Medicine in the National Academies of Sciences (Hernandez and Blazer 2006) called for heavy investment in information on social and cultural exposures and in longitudinal studies of adequate size that could obtain such information.
In What Ways can Genetics Inform Sociology?
The fundamental insight of genetics-informed sociology is that individuals may possess dissimilar genetic predispositions at birth for certain traits and behaviors. Because genetic sequences are formed at conception and remain largely unchanged during lifetime, genetic effects are not confounded by social contextual and other environmental influences during one’s lifetime. Genetic sequences, however, are not immune to social contextual influences before conception. For example, assortative mating likely plays a significant role in DNA sequence arrangement.
There are a number of specific areas in which knowledge of genetic dispositions may advance sociology. First, measures of genetic predispositions may increase a sociological model’s predictive power. Adding genetic information may enrich sociological models and deepen the general understanding of human outcomes under study. Second, genetic measures can isolate purer effects of social contexts from genetic confounders. Many effects of social contexts yielded by conventionally sociological models may be overestimated because of genetic confounding. For example, conventionally estimated effects of social origin (measured by parental education, parental occupation, and parental income) on children’s educational attainment may not be “purely” environmental. Because parents and children share 50% of genetic material, the transmission from parents to children are both social and genetic. This is the so-called gene-environment correlation. In this case, parental genetic effects and parental environmental effects are correlated and entangled. “Purer” effects of social origin can be estimated to the extent that relevant genetic measures are included in the analysis that are correlated with measures of parental measures.
The crucial question is not whether genes are predictive of a human outcome, but whether the genes are correlated with social contexts. If the two are uncorrelated, genetic information improves prediction, but does not confound the estimates of social contextual effects. The current difficulty with this strategy is that many of the relevant genetic measures are unknown. The control for the confounding will not be adequate if only isolated genetic variants are taken into account.
Third, for the reason just described, gene-environment interaction analysis will likely remain a more fruitful vehicle for some time to come for social scientists whose primary interest is to understand effects of social contexts. Gene-environment interaction refers to the principle that an environment may influence how sensitive we are to the effects of a genotype and vice versa (Hunter 2005; Kendler 2001; Plomin et al 1977). A biological example of this comes from individuals with the Δ-32 deletion in the CCR5 gene; these individuals have lower rates of HIV infection and slower disease progression than those without the deletion when exposed to similar environmental conditions (Smith 1997). This example illustrates how environmental exposure is mitigated by genotype.
A social-science example comes from Caspi and colleagues (2002) who found that a functional polymorphism in the monoamine oxidase A gene modifies the effect of maltreatment on violent behavior. Only maltreated children with a genotype generating low levels of MAOA expression tended to develop the violent behavior problem. Maltreated children with a genotype that produces high levels of MAOA activity were less affected. The main effect of MAOA is not observed. This case shows how the manifestation of a genotype effect depends on a social context.
Ignoring information on genetic dispositions may lower our ability to discern the effect of a social context. Individuals with different genetic dispositions may respond to the same social context differently. In such a case, a social theory that assumes a uniform social influence on all individuals would not be able to measure up against empirical data. For example, suppose that there are two alleles (A and B) at one genetic locus: 10% of the sample have A and the other 90% have B. Suppose that only individuals with A are responsive to an environmental influence. Then if the genetic variation is ignored, the environmental effect, which is an average over A individuals and much more numerous B individuals, will likely remain hidden from the analysis. Our findings on gene-environment interaction between the DAT1 gene and cognitive ability (to be described) provide a case in point.
Finally, sociologists may be interested in genetic influences themselves on social behaviors. For many human outcomes (e.g., obesity and hypertension), sociological analysis is understandably more interested in the influences of social contexts than the other causes of the outcomes. This preference may change in studies whose purpose is to understand social behaviors (e.g., popularity, social isolation, and serial divorcees). In such cases, the influences of social contexts may not be the only the focal point of sociological analysis. Genetic causes may be of additional interest in sociological analysis because of the interest in the outcomes.
The Dopamine Transporter Gene and Pattern of Sexual Partnering
Both animal and human studies have demonstrated a genetic basis for sexual behavior. A study based on 1,600 female twin pairs reported an estimated heritability of 0.40 for the self-reported number of sexual partners (Cherkas et al 2004). Another study based on pairs of full siblings, half siblings, cousins and a small number of same-sex twins from the National Longitudinal Study of Youth identified a genetic contribution (heritability) to age at first sex in the all-ethnicity sample (0.37), the white sample (0.51), and the male sample (0.54) (Rodgers et al 1999). Evidence for age at first sex is also reported from the National Longitudinal Study of Adolescent Health; the evidence is based on both a biometrical analysis of twins and an association with the variants of the DRD4 gene (Guo and Tong 2006).
The neurotransmitter3 dopamine has been shown to facilitate male sexual activity in all investigated species including rodents and humans (Dominguez and Hull 2005). Melis and Argiolas (1995)’ findings suggest a major role for dopaminergic receptors in both the preparatory and consummatory phase of male sexual behavior; but their role in female sexual behavior is less conclusive. Hull et al (2002) discussed three mechanisms through which male sexual behavior is affected by dopamine among rats: Dopamine increases male sexual arousal and courtship behavior, enhances the motor acts of mounting behavior, and facilitates genital response to stimulation.
The soluble carrier family 6 dopamine transporter member three gene (DAT, locus symbol: SLC6A3) codes for a dopamine transporter protein (DAT), which limits the level and duration of dopamine receptor activation (Bannon and Whitty 1995). For decades, major investigations have targeted on the role of dopaminergic neurotransmission in complex behaviors involving addition and reward (Bressan and Crippa 2005). Though the specific functions that dopamine plays are not entirely understood, evidence has been cumulating for an important role of dopamine in the regulation of the additive and rewarding behaviors. A number of animal studies demonstrate that natural rewarding stimuli such as food, drink, and sex increase the in-vivo4 release of dopamine in the nucleus accumbens (Kalivas 2002). A mouse model that had knocked out5 the dopamine transporter gene established the central importance of dopamine transporter in controlling synaptic dopamine levels as well as its role as an obligatory target for the behavioral and biochemical action of amphetamine and cocaine (Giros et al 1996). DAT1 is thus an important component for the maintenance of normal dopaminergic neurotransmission.
Vanderbergh et al. (1992) identified a polymorphic 40-bp variable number of tandem repeats (VNTR6) in the DAT1 gene which is most commonly observed repeat 9 (DAT1*9R) to 10 times (DAT1*10R). Biochemical functional analysis shows that the DAT1* 9 allele is associated with lower levels of DAT in comparison to the 10 allele (Fuke et al 2005; Fuke et al 2001; VanNess et al 2005). A number of studies have demonstrated an association between the 10R allele and attention deficit hyperactivity disorder (ADHD) (Cook et al 1995; Cornish et al 2005; Daly et al 1999; Gill et al 1997; Waldman et al 1998). The DAT1*9R allele was reported to be associated with both a lower score in novelty seeking and a greater success in smoking cessation (Cornish et al 2005; Sabol et al 1999).
Gender and Pattern of Sexual Partnering
In this study, we focus on male pattern of sexual partnering and we justify the focus theoretically and empirically. Males and females differ substantially with respect to pattern of sexual partnering. Males of most mammalian species show a strong desire towards variety in sexual partners. In the laboratory, this phenomenon has been referred to as the ‘Coolidge Effect’ (Bermant 1976; Bermant et al 1968; Wilson et al 1963). The Coolidge effect was first observed among rats. The same effect is more striking in farm animals such as sheep and cattle (Wilson 1992). Studies based on self-reports among human subjects found that partner variety is of greater interest to male adolescents (Small et al 1993), male college students (Clark and Hatfield 1989), and male adults (Buss and Schmitt 1993; Carroll et al 1985; McBurney et al 2005; Oliver and Hyde 1993; Simpson and Gangestad 1991) than their female counterparts.
Two broad theoretical approaches have been developed to explain the gender differences in number of sexual partners or, more generally, the gender differences in reproductive strategies: the evolutionary theory and the social control of human sexuality. The evolutionary theory argues that the differences in the relative costs and benefits of sexual behaviors between the two genders over the evolutionary process have produced the gender differences in mating predispositions (Geary 1998; Hrdy 1981; Maynard 1977; Symons 1979).
Social theories have emphasized social regulation of human sexuality as a source for gender differences in sexual behavior (Delamater 1981; DeLamater 1989). Social theories argue that the gender differences are at least partially due to double sexual standards because men traditionally enjoy more power than women in virtually all the major institutions: politics, religion, economics, and family. Our focus on males is further justified by the empirical findings that the DAT1 gene is not associated with number of sexual partners among females in our sample (Table 2). This initial contingency table analysis is confirmed by regression analysis (data not shown).
Table 2.
Mean number of sexual partners (sample size) by genotype, gender and age group
| Age group | 9R/9R | 9R/10R | 10R/10R | |
|---|---|---|---|---|
| All Males | 18–23 | 2.42 (33) | 4.92 (243) | 5.29 (415) |
| 23–26 | 4.08 (25) | 6.85 (158) | 7.07 (289) | |
| All Females | 18–23 | 4.12 (42) | 4.44 (263) | 3.56 (481) |
| 23–26 | 4.60 (20) | 5.80 (164) | 4.60 (317) | |
| White Males | 18–23 | 2.24(21) | 5.19(224) | 4.91(164) |
| 23–26 | 3.78(18) | 6.21(145) | 7.05(109) |
METHODS AND DATA
Data Source
The data source for our analysis is the National Longitudinal Study of Adolescent Health (Add Health), a nationally representative sample of more than 20,000 adolescents in grades 7–12 in 1994–5 in the United States (Harris et al 2003). Add Health is longitudinal; the respondents have since been followed by two additional in-home interviews in 1995–6 (Wave II) and 2002 (Wave III). Add Health is school-based and the adolescents were from 134 schools. The school sample was stratified by region, ethnic mix, size, urbanicity (urban/suburban/rural), and school type (public/private/parochial).
Data were collected from adolescents themselves, their parents, siblings, friends, peers, fellow students, and school administrators. Existing national data sources about neighborhoods and communities have been incorporated. In Wave III in 2002, DNA samples were collected from a subset of about 2,500 siblings. Our study uses the approximate 680 white male participants whose analysis information is available. Our gene-environment interaction analysis focuses on white males because of the small sizes of the other ethnic groups, especially the small size of the DAT1*9R/9R category. Only ten individuals in the African American sample are in this subcategory. The white male sample consists of 39 9R/9Rs.
Measures
Number of Sexual Partners
At Wave III, the respondents aged 18–26 were asked the question: “With how many partners have you ever had vaginal intercourse, even if only once?” As with any survey of sensitive private information, reporting accuracy is a concern. To protect confidentiality, reduce non-responses, and increase reporting accuracy, this section of the interview was self-administered by audio-CASI (Computer Assisted Self Interview). The sensitive question was read to respondents by means of audio headphones. Respondents were given instructions by the computer on how to complete their answers. The technique has been shown to reduce the disparity between men and women in the reported number of sex partners (Tourangeau and Smith 1996).
As in most surveys that ask about number of sexual partners (Smith 1992), the number of female partners reported by males exceeds the number of male partners reported by females in Add Health with an overall male-female ratio of 1.3 to 1. Because a heterosexual intercourse involves a male and a female, in a closed population the total number of copulations for males must equal those for females; the total number of sexual partners for males must also equal those for females. There are a number of explanations that may potentially account for the higher reported male-to-female ratio. The male and female respondents in our Add Health sibling sample may refer to different sets of sexual partners. A small number of females may have a large number of partners and these females are less likely to respond to social survey. Finally, the males might have over-reported.
DNA Preparation and Genotyping
At Wave III in 2002, in collaboration with the Institute for Behavioral Genetics in Boulder, Colorado, Add Health collected, extracted, and quantified DNA samples from the sibling sub-sample. Genomic DNA was isolated from buccal cells7 using a modification of published methods (Freeman et al 1997; Lench et al 1988; Meulenbelt et al 1995). All of the methods employed Applied Biosystems instruments and reagents. Microsatellite and VNTR polymorphisms were done using fluorescent primers that were analyzed on an ABI capillary electrophoresis instrument. The additional details on DNA collection and genotyping are provided at Add Health website (Smolen and Hewitt, http://www.cpc.unc.edu/projects/addhealth/).
A 40-bp variable number tandem repeat (VNTR) polymorphism in the 3′ untranslated region of the DAT1 gene has been genotyped with a modified method of Vandenbergh et al (1992). This VNTR ranges from 3 to 11 copies with the 9-repeat (9R or 440 bp) and 10-repeat (10R or 480 bp) polymorphisms being the two most common alleles (Doucettestamm et al 1995). In the Add Health sibling sample, the 9R and 10R account for about 21% and 76% of all alleles, respectively. Our analysis used only individuals with genotypes8 of one 10R, two 10Rs, and two 9Rs. The individuals with other genotypes (about 2%) are excluded from the analysis. In our analysis sample, 94.2% and 5.8% of the white respondents possess Any10R and 9R/9R genotypes, respectively (Table 1). A χ2 test for the polymorphism reveals no deviation from the Hardy-Weinberg equilibrium.
Table 1.
Descriptive statistics: Mean or proportion and standard deviation, white males
| Mean | S.D | |
|---|---|---|
| Allele Proportion | ||
| 10R (480) | 0.741 | 0.438 |
| Genotype Proportion | ||
| Any10R (480) | 0.942 | 0.234 |
| Individual and family traits | ||
| Age (18–20) | 19.73 | 39.79 |
| Age (21–23) | 58.61 | 49.25 |
| Age (24–26) | 21.66 | 41.19 |
| 2 biological parents | 0.682 | 0.466 |
| High school | 0.279 | 0.449 |
| < high school | 0.034 | 0.182 |
| Some college | 0.211 | 0.408 |
| ≥ college | 0.445 | 0.497 |
| Missing on education | 0.031 | 0.174 |
| Single | 0.580 | 0.494 |
| Cohabited and married | 0.077 | 0.267 |
| Married, not cohabited | 0.071 | 0.257 |
| Cohabited, not married | 0.267 | 0.443 |
| Church Attendance | 0.147 | 0.354 |
| Peabody vocabulary test | 0.00 | 1.00 |
| Physical maturity | 0.545 | 0.498 |
| Contextual characteristics | ||
| Poverty: < 11.6 % | 0.636 | 0.481 |
| Poverty: 11.6%–23.9% | 0.208 | 0.406 |
| Poverty: ≥ 23.9 % | 0.091 | 0.287 |
| Missing on poverty | 0.065 | 0.247 |
| Church adherents/capita | 54.4 | 14.3 |
| % had sex by 16 | 41.0 | 11.2 |
| No. of Persons | 674 | |
Social Contextual and Individual Measures
Family structure is a variable of four categories: two-biological-parent families, single-parent families, step-parent families, and other types of families including families with adopted and foster children. In our final models, we use a two-category variable of the presence or absence of two biological parents. Parental education also has four categories: less than high school, high school graduation, some college, and at least a college degree. Add Health measures the level of education from both the mother and the father of a respondent. We use the higher of the two when both are available.
Our analysis includes three measures at the contextual level. Poverty at the neighborhood level is measured by the proportion of families living below the official poverty line in a Census block group, which is the smallest geographic area for which the Census Bureau publishes sample data. In 1990, block groups averaged 452 housing units or 1,100 individuals. The block groups are divided into low-poverty, median-poverty, and high-poverty categories. The two cutoff points for the three categories are 11.6% (the median proportion of families in a block group living below the poverty line) and 23.9% (the 75th percentile of the proportion of families in a block group living below the poverty line). Church adherents per capita represents the percent of church adherents in the county, and % had sex by 16 stands for the percent of students having had sexual intercourse by age 16 in the school.
Our measure of marital and cohabitation experience includes categories of being single, having been cohabited and married, having been only married, and having been only in a cohabiting relationship. Church attendance measures attendance frequency with categories of never, less than monthly, less than weekly, and weekly or more. To reduce the number of parameters that need to be estimated in the interaction analysis, we use a two-category variable of attending religious service weekly or more and attending religious service less than weekly or never.
The Add Health Peabody Picture Vocabulary Test (PVT) is a slightly shortened version of the standard Peabody Picture Vocabulary Test (Lubin et al 1984; Rice and Brown 1967), which is usually considered a verbal IQ test. In our main regression analysis, PVT is standardized into a Z score: subtracting every PVT score from the sample mean and then dividing the result by its standard deviation. Physical maturity is a dummy variable constructed from the Wave-I question of whether the adolescent’s physical development is more advanced when compared to other boys of same age. Table 1 provides the mean or proportion and its associated deviation of these variables.
Statistical Models
We carried out the analysis of gene-environment interactions via the GEE Poisson regression model. Equation (1) describes the basic regression model for the interaction analysis
| (1) |
where E(Yij | xij ) is the expected number of sexual partners for individual i in sibling cluster j given the individual’s characteristics xij; ANY10Rij is one of the two genotypes in the DAT1 VNTR polymorphism under investigation for the individual; SCij is a column vector denoting social contextual measures; β1 and β2 (a row vector) represent the effects of ANY10Rij and SCij, respectively; and [ANY10R*sc]ij is a product of ANY10R and a single social contextual measure. The exposure for number of sexual partners was explicitly adjusted by the age categorical variable. The dependence among the siblings is addressed by the generalized estimating equations (GEE) (Liang and Zeger 1986), which has long been established in the statistical literature as a standard approach for addressing correlated data. The GEE models were implemented using SAS.
RESULTS
Mean Number of Sexual Partners by Genotype, Gender, and Age
Table 2 shows the mean number of sexual partners by genotype and age group for all males, all females, and white males. Among the males, the 10R allele is associated with higher number of sexual partners. In the younger male group of 18–22, possessing one or two copies of 10R approximately doubles the number of partners. In the older male group of 23–26, possessing one or two copies of 10R increases the number of partners from about 4 to 7. As expected, age is positively related to the number of reported partners; but there does not seem be an interaction between age and the effect of the DAT1 variants; that is, the effect of DAT1 does not seem to vary across the life stages of adolescence and young adulthood.
Among females, the 10R allele is not associated with higher number of sexual partners. This is the case in both age groups, suggesting an interaction between DAT1 and gender. The same relationship between the DAT1 variants and number of sexual partners observed among all males are also observed among white males. In the rest of the article, we will only describe results from the white male sample.
Regression Results
The three models of sexual partners in Table 3 establish the main effect of the Any10R genotype. The first GEE model controls for age group and addresses the sibling structure of the data so that the statistical significance of a genotype effect can be tested. In the first model, those with the DAT1*Any10R genotype report 93% [exp(0.66)=1.93] more sexual partners than those with the DAT1*9R/9R genotype.
Table 3.
GEE Poisson models of number of sexual partners: Main effects of dopamine transporter and multilevels of social contexts – white males
| DAT1+Age | Social Contexts No DAT1 | Social Contexts + DAT1 | |
|---|---|---|---|
| Beta (se) | Beta (se) | Beta (se) | |
| Intercept | 1.29(0.28)*** | 1.344(0.374)*** | 0.904(0.438)* |
| Dopamine transporter | |||
| Any10R | 0.66(0.26)* | -- | 0.468(0.227)* |
| Individual and family traits | |||
| Age (24–26) | -- | -- | -- |
| Age (18–20) | −0.60(0.17)*** | −0.549(0.181)** | −0.537(0.181)** |
| Age (21–23) | −0.21(0.15) | −0.194(0.153) | −0.187(0.151) |
| 2 biological parents | −0.048(0.12) | −0.063(0.122) | |
| High school | -- | -- | -- |
| < high school | 0.01(0.308) | 0.03(0.309) | |
| Some college | 0.091(0.171) | 0.11(0.17) | |
| >=college | 0.129(0.148) | 0.132(0.148) | |
| Missing on education | 0.331(0.313) | 0.312(0.313) | |
| Single | -- | -- | |
| Cohabited and married | 0.351(0.19) | 0.339(0.189) | |
| Married, not cohabited | −0.185(0.201) | −0.185(0.202) | |
| Cohabited, not married | 0.439(0.135)** | 0.44(0.134)** | |
| Church Attendance | −0.901(0.193)*** | −0.896(0.192)*** | |
| Peabody vocabulary test | −0.049(0.057) | −0.044(0.057) | |
| Physical maturity | 0.279(0.113)* | 0.288(0.113)* | |
| Contextual characteristics | |||
| Poverty: < 11.6 % | -- | -- | |
| Poverty: 11.6%–23.9% | −0.239(0.139) | −0.238(0.139) | |
| Poverty: >=23.9 | 0.092(0.206) | 0.08(0.206) | |
| Poverty: Missing on poverty | 0.041(0.19) | 0.036(0.189) | |
| Church per capita | −0.4(0.428) | −0.358(0.428) | |
| % had sex before age 16 | 1.214(0.524)* | 1.128(0.534)* | |
| Number of persons | 674 | 674 | 674 |
0.05;
0.01;
0.001.
The second and third models in Table 3 include a set of social contextual factors as well as individual controls. In the third model, those with the DAT1*Any10R genotype report 60% more sexual partners than those with the DAT1*9R/9R genotype. Compared with the basic first GEE model, the genotype effect obtained after adding social contextual and individual factors is reduced from 93% more to 60% more, but the level of significance has remained about the same. The second model does not include the genotype Any10R. However, its coefficients and standard errors are rather similar to those in the third model, suggesting moderate correlations between Any10R and the included social contextual and individual factors.
In the full model, church attendance is strongly and negatively related to number of sexual partners. Those who attend religious services at least weekly report about 60% [1−exp(−0.90)] fewer sexual partners than those who never attend religious services or attend religious services less than weekly. Marital status and cohabitation experience are also significantly related to number of sexual partners. Those who have only cohabitated report highest number of sexual partners (58% higher than those who are single) followed by those who have cohabited and married, those who are single, and those who have only been married. Physical maturity at Wave I is associated with a larger number of partners. Only one contextual measure is significantly predicting number of sexual partners. One percent increase in the proportion of the students having had sex by age 16 in school raises the number of partners by about 2.1%.
Because the reference category of DAT1*9R/9R has only about 6% of the white sample, it is more appropriate to refer to 9R/9R as the protective genotype than to refer to Any10R as the risky genotype. Given that about 94% of the sample falls into the Any10R category, the average number of sexual partners for Any10R should be close to the sample mean. Re-estimating the protective effect by coding 9R/9R as one and Any10R as zero (the reference category) yields the coefficient of −0.468, which is exactly the same in value, but has an opposite sign. The t-ratio remains the same as well. Thus, according to the full model in Table 3, the 9R/9Rs report only about 63% as many sexual partners as the Any10Rs.
Table 4 explores the interactive effects of the dopamine transporter gene and a number of social contextual and individual ‘non-genetic’ characteristics. The first three of the four models in Table 4 each add a single interaction term to the full main-effect model in Table 3. In the last model, all three interaction terms are added simultaneously. The first model examines the interaction between Any10R and the proportion having sex by 16 in school; the second, the interaction between Any10R and standardized PVT Z-score; and the third the interaction between Any10R and physical maturity at Wave I. All three interaction terms as well as their corresponding main effects are significant in the first three models. PVT Z-score is not significant in the main-effect model in Table 4, but its main effect and interaction effect are both statistically significant.
Table 4.
GEE Poisson models of number of sexual partners: Interactions between socioeconomic-cultural factors and dopamine transporter – White males
| Interacting With | % had sex by 16 | Peabody vocabulary test | Physical maturity | All three |
|---|---|---|---|---|
| Beta (se) | Beta (se) | Beta (se) | Beta (se) | |
| Intercept | −0.868(0.789) | 0.971(0.418)* | 0.437(0.406) | −0.348(0.629) |
| Dopamine transporter | ||||
| Any10R | 2.318(0.724)** | 0.43(0.18)* | 0.954(0.208)*** | 1.795(0.595)** |
| Individual and family traits | ||||
| Age (24–26) | -- | -- | -- | -- |
| Age (18–20) | −0.537(0.18)** | −0.54(0.179)** | −0.529(0.181)** | −0.535(0.18)** |
| Age (21–23) | −0.177(0.152) | −0.183(0.15) | −0.183(0.152) | −0.176(0.151) |
| 2 biological parents | −0.061(0.122) | −0.065(0.123) | −0.061(0.121) | −0.063(0.122) |
| High school | -- | -- | -- | -- |
| < high school | 0.034(0.307) | 0.026(0.308) | 0.03(0.308) | 0.027(0.307) |
| Some college | 0.108(0.169) | 0.103(0.17) | 0.109(0.17) | 0.102(0.17) |
| >=college | 0.129(0.148) | 0.129(0.149) | 0.129(0.148) | 0.127(0.148) |
| Missing on education | 0.311(0.312) | 0.315(0.313) | 0.311(0.312) | 0.313(0.312) |
| Single | -- | -- | -- | -- |
| Cohabited and married | 0.347(0.188) | 0.34(0.188) | 0.341(0.189) | 0.346(0.188) |
| Married, not Cohabited | −0.176(0.2) | −0.172(0.2) | −0.175(0.202) | −0.161(0.201) |
| Cohabited, not married | 0.447(0.133)*** | 0.436(0.132)*** | 0.442(0.134)*** | 0.442(0.132)*** |
| Church attendance | −0.894(0.192)*** | −0.9(0.192)*** | −0.904(0.192)*** | −0.902(0.193)*** |
| Peabody vocabulary test | −0.04(0.057) | −0.57(0.236)* | −0.044(0.057) | −0.396(0.241) |
| Physical maturity | 0.28(0.114)* | 0.287(0.114)* | 0.876(0.262)*** | 0.586(0.19)** |
| Contextual Characteristics | ||||
| Poverty: < 11.6 % | -- | -- | -- | -- |
| Poverty: 11.6%–23.9% | −0.229(0.138) | −0.224(0.139) | −0.238(0.138) | −0.224(0.139) |
| Poverty: >=23.9 | 0.101(0.206) | 0.093(0.206) | 0.083(0.206) | 0.104(0.206) |
| Poverty: Missing | 0.045(0.189) | 0.046(0.189) | 0.034(0.189) | 0.047(0.188) |
| Church adherents/capita | −0.36(0.429) | −0.353(0.429) | −0.354(0.428) | −0.351(0.43) |
| % Had sex by 16 | 5.404(1.662)** | 1.055(0.527)* | 1.092(0.536)* | 3.613(1.369)** |
| Any10R × | ||||
| % had sex by 16 | −4.471(1.744)* | −2.673(1.49) | ||
| Peabody vocabulary test | 0.538(0.243)* | 0.363(0.252) | ||
| Physical maturity | −0.603(0.292)* | −0.311(0.225) | ||
| Number of persons | 674 | 674 | 674 | 674 |
0.05;
0.01;
0.001.
When all three interaction terms are estimated in the same model, only the interaction term involving proportion having sex by 16 is marginally significant. The lack of statistical power is probably a major factor in the non-significance of the multiple interaction terms in the last model in Table 4. In the section of Discussion and Conclusion, only the models with a single interaction term are interpreted.
Profiling Individuals with 9R/9R and Any10R
In this section, we provide a profile for individuals with the 9R/9R genotype and the Any10R genotype. Our data have established a protective effect of 9R/9R relative to Any10R with respect to number of sexual partners. But, are the 9R/9Rs systematically different from the Any10Rs in family SES, academic performance, cognitive ability, religiosity, physical maturity, and popularity among peers? Is the 9R/9R genotype also protective of other risky health behaviors in addition to pattern of sexual partnering?
These questions are explored preliminarily in Table 5, which reports the mean or proportion of individual characteristics, parental characteristics, and health behaviors for both 9R/9R and Any10R. All measures of characteristics and behaviors are from Add Health Wave I or the earliest Wave at which the data are available. The results based on other Waves are similar and not presented. To perform statistical significance tests, we regress each characteristic on Any10R controlling for age when appropriate. The regression model is linear, logit, or poisson depending on the distribution of a particular characteristic. The GEE regression framework is used to handle the sibling clustering in the Add Health sample (Liang and Zeger 1986). Table 5 reports the regression coefficient for Any10R and the associated t-ratio.
Table 5.
Comparing the 9R/9R and Any10R genotype, white males (N=674)
| Mean | |||||
|---|---|---|---|---|---|
| 9R/9R | Any10R | β | t-ratio | Model | |
| Individual Characteristics | |||||
| Age w1 | 15.77 | 15.65 | 0.009 | .0.03 | linear |
| Physical maturity w1 | 0.62 | 0.54 | −0.34 | −1.00 | logit |
| GPA w1 | 3.08 | 2.75 | −0.39 | −2.98** | linear |
| Cognitive ability (PVT) | 108.5 | 104.8 | −3.33 | −1.96* | linear |
| Church attendance | 0.40 | 0.35 | −0.20 | −0.73 | logit |
| Popularity (received) | 5.48 | 5.34 | 0.001 | 0.00 | linear |
| Popularity (sent) | 4.74 | 4.81 | 0.003 | 0.00 | linear |
| Sensitive to other people’s feelings w2 | 2.07 | 1.98 | −0.047 | −0.69 | linear |
| Parental characteristics | |||||
| Presence of two biological parents | 0.65 | 0.68 | 0.001 | 0.001 | logit |
| % Of parents having a college degree | 0.77 | 0.66 | −0.31 | −1.19 | Logit |
| Risky Health Behaviors | |||||
| Number of sexual partners | 2.94 | 5.66 | 0.69 | 2.59** | poisson |
| Days binge drinking | 0.41 | 0.81 | 0.71 | 1.99* | poisson |
| Number of cigarettes/day w1 | 0.62 | 2.74 | 2.18 | 4.56*** | poisson |
| How often wear seatbelt w1 | 3.37 | 2.92 | −0.46 | −2.97** | linear |
| Delinquency summary scale | 1.22 | 2.00 | 0.87 | 1.99* | linear |
| Delinquency Items | |||||
| Burglarize a building-w1 | 0.025 | 0.067 | 0.038 | 1.33 | linear |
| Use or threaten with a weapon-w1 | 0.025 | 0.070 | 0.045 | 1.53 | linear |
| Sell marijuana or other drugs | 0.10 | 0.14 | 0.032 | 0.38 | linear |
| Steal worth < $50-w1 | 0.25 | 0.38 | 0.18 | 1.17 | linear |
| Take part in a group fight-w1 | 0.15 | 0.25 | 0.11 | 1.67 | linear |
| Deliberately damage property of others | 0.25 | 0.36 | 0.13 | 1.38 | linear |
| Seriously injure someone-w1 | 0.25 | 0.29 | 0.054 | 0.52 | linear |
| Carry weapon to school-w1 | 0.05 | 0.081 | 0.033 | 0.83 | linear |
| Self injured and treated by a doctor or nurse | 0.05 | 0.15 | 0.10 | 2.61** | linear |
| Pulled a knife/gun on someone-w1 | 0.00 | 0.055 | 0.049 | 4.78*** | linear |
| Shot/stabbed someone-w1 | 0.00 | 0.02 | 0.02 | 3.20** | linear |
| Carry weapon to school-w1 | 0.075 | 0.12 | 0.045 | 0.76 | linear |
0.05;
0.01;
0.001.
The similarities and differences between individuals with 9R/9R and individuals with Any10R are systematic. The 9R/9Rs do not differ significantly from the Any10Rs in age, physical maturity, church attendance, number of friend nominations received (popularity received), number of friend nominations sent (popularity sent), and how sensitive to other people’s feelings. The two groups are not different in family socioeconomic status as measured by the presence of two biological parents and whether at least one parent has a college degree. The 9R/9Rs, however, have a higher cognitive ability (108.5 vs. 104.8) and a higher GPA (3.08 vs. 2.75) than the Any10Rs.
It turns out that the 9R/9Rs not only have fewer sexual partners, but also have fewer binge drinking days (about 50% fewer), smoke far fewer cigarettes per day, are more likely to wear seatbelt when driving or riding a car, and score higher on the delinquency summary scale than the Any10Rs. All of these differences in risky health behaviors are statistically significant. To unpack the delinquency summary scale, we regress each of the 12 items in a GEE regression on Any10R controlling for age. Although only three of the 12 items are significant, all of the differences are in the expected directions.
DISCUSSION AND CONCLUSION
Our empirical analysis has yielded a substantial main effect of the VNTR polymorphism in the DAT1 gene on number of sexual partners among white male adolescents and young adults. This main effect is present in the contingency table analysis (Table 2), the initial GEE regression model that controls for age and sibling clustering (Table 3), and the full GEE regression model that controls for a set of additional social contextual and individual characteristics (Table 3). However, DAT1 should not be viewed as a sex gene. More appropriately, we consider the effect of the 9R/9R genotype a protective effect relative to the Any10R genotype, to which 94% of the sample belong.
The full model in Table 3 points to the importance of multilevels of social contexts. Marriage and frequent church attendance on the part of the individual both reduce the number of sexual partners. Proportion of the students in school having had sex by 16 is significantly and positively associated with number of sexual partners.
Profiling the 9R/9R and Any10R individuals has uncovered intriguing similarities and differences between the two groups. The two groups seem to have similar family background. The two groups do not differ in age, physical maturity, religiosity, and popularity. While the 9R/9Rs have a slightly higher GPA and a slightly higher cognitive ability score, the 9R/9Rs are less likely to engage in risky health behaviors than the Any10Rs including having multiple sexual partners, binge drinking, smoking, not wearing a seatbelt when riding or driving a car, and other delinquent and criminal acts. These findings provide further support for the protective effect of 9R/9R and suggest that the protective effect may go beyond pattern of sexual partnering. Are individuals with 9R/9R “straight arrows” who are behaviorally more conservative than the population average?
Interpreting gene-environment interactions is not always straightforward. When a genotype interacts with a social context measure, it means that the genotype effect and the social context effect depend on each other. The interaction can be viewed from two angles. One reveals how a social-context effect varies by genotype, that is, a social-context effect may be weaker or stronger for one genotype than another. The other angle examines how a genotype effect differs across social-contextual categories. Both may be interesting to sociologists.
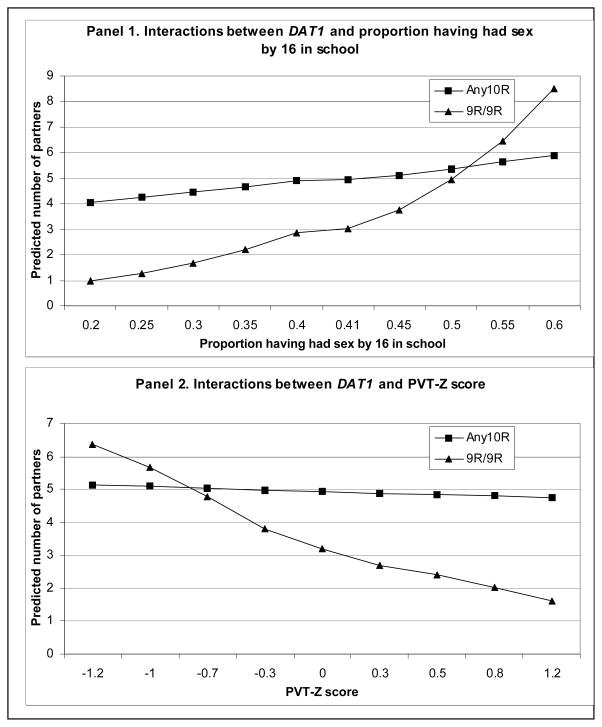
Figure 1 is created to assist the interpretation of gene-environment interactions for the regression results presented in Table 4. Panel 1 describes the interaction between the genotype effect and the effect of the prevailing sexual norm in school. It plots the predicted number of sexual partners against proportion having had sex by 16 in school for two genotypes (Any10R and 9R/9R). The values of the other variables in the regression equation are set at the sample means.
Figure 1.
Interpreting gene-environment interactions for number of sexual partners – based on results in Table 4
The main-effect model in Table 3 indicates a protective effect of 9R/9R relative to the large majority in the Any10R genotype. Panel 1 suggests that the protective effect depends on the proportion having had sex by 16 in school. The effect diminishes as the proportion increases. The protective effect disappears completely as the proportion approaches 0.50; 0.41 is the average proportion in the sample (Table 1).
The interpretation could also focus on the effect of prevailing sexual practice in school. Panel 1 confirms the positive relationship between number of sexual partners and the proportion having sex. The relationship seems to hold for both genotypes; however, the effect of the prevailing sexual practice in school is much larger on the 9R/9R genotype than the Any10R genotype. Roughly, when the proportion having had sex in school increases from 0.3 to 0.5, the predicted number of partners for 9R/9R increases from 1.5 to 5 while the predicted number of partners for Any10R only increases from 4.5 to 5.5.
Although the social normative explanation is supported, its significance is difficult to evaluate. Several authors (Furstenberg et al 1987; Lauritsen 1994) point out that the apparent normative differences may merely reflect the differences in the underlying social structure. To control for differences in social structural conditions, we have included in all models family structure and parental education at the individual level, and prevalence of poverty and number of church adherents per capita at the contextual level. A normative explanation persists after these social structural measures are controlled for.
Four decades of data in multiple national studies show continuing long-term trends in the United States towards increased acceptance of premarital sex and unmarried cohabitation (Thornton and Young-DeMarco 2001). Udry (1996) reasons that in contemporary industrial societies, individual behavior is increasingly more influenced by biological differences reflecting inherent behavioral predispositions and less influenced by societal pressures to conform. Our empirical data provide evidence for this reasoning, but also point to a more complicated picture. The relative importance of biology and environment may be a function of genotype. The Any10Rs that account for 94% of the sample are only slightly affected by social pressure while the behavior of the 9R/9Rs varies dramatically as social pressure shifts (Panel 1 of Figure 1).
Panel 2 in Figure 1 describes the interaction between genotype and cognitive ability. The protective effect of 9R/9R is, again, conditional on another factor - cognitive ability. The protective effect of 9R/9R only holds at the medium or high levels of cognitive ability. At low levels of cognitive ability, the protective effect of 9R/9R is not observed.
It is interesting to note that while cognitive ability is not significantly related to number of partners in the main-effect model in Table 3, both the main effect of cognitive ability and its interaction with Any10R turn significant in the interaction model in Table 4. This apparent paradox can be explained by the interaction described in Panel 2, which shows that the protective effect of high cognitive ability only holds among those with the 9R/9R genotype. The protective effect is not present among the Any10Rs. In this case, the gene-environment interaction analysis has unveiled an underlying story that is invisible when the relationship between cognitive ability and number of partners is examined without considering genotype.
The gene-environment interaction involving cognitive ability seems to be a case of low intelligence trumping genetics. The behaviorally conservative 9R/9R genotype may be generally more averse to risks and more concerned about the consequences of unwanted pregnancy and STDs than the Any10R genotype. But those 9R/9Rs with lower cognitive ability may miscalculate and especially underestimate the risks involved, thus reducing or eliminating the protective genotype effect. However, if cognitive ability is subject to important genetic influences, the DAT1 by cognitive ability interaction would be partially a gene-gene interaction.
Study participants who consider themselves more physically mature report a higher number of partners; however, the proportional increase for the 9R/9R genotype is larger (1.4 times more) than the Any10R genotype (0.35 times more).
The gene-environment interaction results beg the more general question of why social contextual and individual effects tend to be larger for the 9R/9R genotype than the Any10R genotype. This pattern is observed in the interaction analysis involving both prevailing sexual practice in school and cognitive ability. Are social contextual effects generally larger for the more behaviorally ‘conservative’ genotype? This can be tested explicitly for other genes and other risky behaviors. If the hypothesis is supported empirically, the next question is: Are individuals less averse to risks more likely to confirm to social pressure and more easily shaped by other individual characteristics than the average crowd?
We hope that our most significant and lasting contribution will be the introduction of the approach of genetics-informed sociology – a contribution that goes beyond the case of male sexual behavior. The approach advocates capitalizing on genetic information in a study of sociological influences. Individuals with dissimilar genetic predispositions may be more sensitive or less sensitive to the same social influence. Understanding a social influence may depend upon the understanding of genetic heritage.
There are a number of specific ways a sociological analysis may benefit from genetic information. Caspi and colleagues (2002) show that the riskier genotype in the MAOA gene is not sufficient to lead to antisocial behavior. It is the combination of both the riskier genotype and abuse in childhood that gives rise to antisocial behavior.
The results from Table 3 have lent support for the influences of social contexts. When a gene-environment interaction is allowed, however, the story becomes more complicated. The interaction that involves the proportion having sex suggests that the protective effect of 9R/9R tends to be lost in schools in which higher proportions of students start having sex early. The interaction that includes cognitive ability exhibits a protective effect of 9R/9R that tends to be present at higher levels of cognitive ability. The protective effect of cognitive ability is only visible in the interaction analysis in which the DAT1 variant is allowed to interact with the effect of cognitive ability. Ignoring genetic information, the protective effect of cognitive ability would have gone undetected.
Genetics-informed sociological analysis has ramifications for theory building. For certain human traits and behaviors, the “one size” of a single social theory may not fit all. Explaining a trait or behavior may require a theory that accommodates the complex interplay between socioeconomic-cultural influences and individual genetic predispositions.
False-positive findings are a huge concern in the genetics community. Evidence that links genetic variants and human phenotypes typically needs to be replicated multiple times to be considered particularly convincing. Thus far, geneticists have been primarily interested in the main effects of genes. Sociologists are “structurally” more interested in gene-environment interactions than main effects. Interaction results are considerably more difficult to estimate than main effects. A low statistical power is almost always an issue. Even when the sample is reasonably large, a key category could be small. Thus, the first waves of genetic results based on sociological data must be viewed with caution. The experience in the genetics community shows that multiple replication is an essential step for reducing false positive findings. The results presented in this article are based on about 1/6 of the Add Health sample. We are planning a replication of our current results when the DNA data and the related genotyping for the entire Add Health sample (n ≈15,000) become available.
Acknowledgments
This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). No direct support was received from grant P01-HD31921 for this analysis.
Footnotes
Different forms (different DNA sequence) of a gene are called alleles which may be found at a given location on members of a homologous set of chromosomes. Structural variations between alleles may lead to different phenotypes for a given trait.
The International HapMap Project is a multi-country endeavor to identify and catalog genetic similarities and differences in human beings. The Project is a collaboration among scientists and funding agencies from Japan, the United Kingdom, Canada, China, Nigeria, and the United States.
Neurotransmitters are chemicals that allow the movement of information from one neuron across the gap between it and the adjacent neuron.
In vitro, within glass in Latin, is an experimental method where the experiment is performed in a test tube, or outside a living organism or cell.
The gene knockout is created by selectively disabling a specific target gene in a particular type of cells called embryonic stem cells. The technique has been used to make several thousand different knockout mice. Knockout mice have become one of the most useful scientific tools in helping to understand the human genome and its roles in disease.
Variable number of tandem repeats, a chromosomal locus at which a particular repetitive sequence is present in different numbers in different individuals of a population. Most of our DNA sequence is identical to DNA sequence of others. However, there are inherited regions of DNA that can vary from one individual to another. Variations in DNA sequence among individuals are termed “polymorphisms”. Sequences with a high degree of polymorphism are very useful for DNA analysis which often attempts to link human outcomes to variations in DNA sequence. VNTR, STR, and SNPs are classes of DNA polymorphisms.
Buccal cells are the cells from the inner lining of the mouth. These cells are routinely shed and replaced by new cells. As the old cells die, they accumulate in the saliva in the mouth and can easily be collected by a simple procedure using mouthwash.
In general, the genotype is the specific genetic makeup (the specific genome) of an individual. Here we refer to an individual’s genotype with regard to a particular gene of interest and it refers to what combination of alleles the individual carries.
Contributor Information
Guang Guo, Department of Sociology and Carolina Center for Genome Sciences, the University of North Carolina, Chapel Hill.
Yuying Tong, Department of Sociology, the Chinese University of Hong Kong.
Tianji Cai, Department of Sociology, the University of North Carolina, Chapel Hill.
THE REFERENCES
- Bannon MJ, Whitty CJ. NEUROKININ RECEPTOR GENE-EXPRESSION IN SUBSTANTIA-NIGRA -LOCALIZATION, REGULATION, AND POTENTIAL PHYSIOLOGICAL SIGNIFICANCE. Canadian Journal of Physiology and Pharmacology. 1995;73:866–870. doi: 10.1139/y95-119. [DOI] [PubMed] [Google Scholar]
- Bermant G. Sexual behavior: hard times with the Coolidge effect. In: Siegel M, editor. Pyschological research: inside story. New York: Harper & Row; 1976. [Google Scholar]
- Bermant G, Lott DF, Anderson L. TEMPORAL CHARACTERISTICS OF COOLIDGE EFFECT IN MALE RAT COPULATORY BEHAVIOR. Journal of Comparative and Physiological Psychology. 1968;65:447. doi: 10.1037/h0025841. [DOI] [PubMed] [Google Scholar]
- Billy JO, Brewster KL, Grady WR. CONTEXTUAL EFFECTS ON THE SEXUAL-BEHAVIOR OF ADOLESCENT WOMEN. Journal of Marriage and the Family. 1994;56:387–404. [Google Scholar]
- Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nature Genetics. 2003;33:228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour - review of data from preclinical research. Acta Psychiatrica Scandinavica. 2005;111:14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Brewster KL. RACE DIFFERENCES IN SEXUAL-ACTIVITY AMONG ADOLESCENT WOMEN - THE ROLE OF NEIGHBORHOOD CHARACTERISTICS. American Sociological Review. 1994;59:408–424. [Google Scholar]
- Browning CR, Leventhal T, Brooks-Gunn J. Sexual initiation in early adolescence: The nexus of parental and community control. American Sociological Review. 2005;70:758–778. [Google Scholar]
- Browning CR, Olinger-Wilbon M. Neighborhood structure, social organization, and number of short-term sexual partnerships. Journal of Marriage and the Family. 2003;65:730–745. [Google Scholar]
- Buss DM, Schmitt DP. SEXUAL STRATEGIES THEORY - AN EVOLUTIONARY PERSPECTIVE ON HUMAN MATING. Psychological Review. 1993;100:204–232. doi: 10.1037/0033-295x.100.2.204. [DOI] [PubMed] [Google Scholar]
- Capaldi DM, Crosby L, Stoolmiller M. Predicting the timing of first sexual intercourse for at-risk adolescent males. Child Development. 1996;67:344–359. [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Volk KD, Hyde JS. DIFFERENCES BETWEEN MALES AND FEMALES IN MOTIVES FOR ENGAGING IN SEXUAL INTERCOURSE. Archives of Sexual Behavior. 1985;14:131–139. doi: 10.1007/BF01541658. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cates W, Stone KM. FAMILY-PLANNING, SEXUALLY-TRANSMITTED DISEASES AND CONTRACEPTIVE CHOICE - A LITERATURE UPDATE.1. Family Planning Perspectives. 1992;24:75–84. [PubMed] [Google Scholar]
- CDC. CDC Press Release. Mar 11, 2008. Nationally Representative CDC Study Finds 1 in 4 Teenage Girls Has a Sexually Transmitted Disease. [Google Scholar]
- Cherkas LF, Oelsner EC, Mak YT, Valdes A, Spector TD. Genetic influences on female infidelity and number of sexual partners in humans: A linkage and association study of the role of the vasopressin receptor gene (AVPR1A) Twin Research. 2004;7:649–658. doi: 10.1375/1369052042663922. [DOI] [PubMed] [Google Scholar]
- Clark R, Hatfield E. Gender differences in receptivity to sexual offers. Jounral of Psychology and Human Sexuality. 1989;2:39–55. [Google Scholar]
- Collins FS, Morgan M, Patrinos A. The human genome project: Lessons from large-scale biology. Science. 2003;300:286–290. doi: 10.1126/science.1084564. [DOI] [PubMed] [Google Scholar]
- Cook EH, Stein MA, Krasowski MD, Cox NJ, Olkon DM, Kieffer JE, et al. ASSOCIATION OF ATTENTION-DEFICIT DISORDER AND THE DOPAMINE TRANSPORTER GENE. American Journal of Human Genetics. 1995;56:993–998. [PMC free article] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Molecular Psychiatry. 2005;10:686–698. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Molecular Psychiatry. 1999;4:192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- Delamater J. THE SOCIAL-CONTROL OF SEXUALITY. Annual Review of Sociology. 1981;7:263–290. doi: 10.1146/annurev.so.07.080181.001403. [DOI] [PubMed] [Google Scholar]
- DeLamater J. The social control of human sexuality. In: Mckinney K, Specher S, editors. Human Sexuality: The Societal and Interpersonal Context. Norwood, New Jersey: Ablex Publishing Corporation; 1989. [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiology & Behavior. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Doucettestamm LA, Blakely DJ, Tian J, Mockus S, Mao J. POPULATION GENETIC-STUDY OF THE HUMAN DOPAMINE TRANSPORTER GENE (DAT1) Genetic Epidemiology. 1995;12:303–308. doi: 10.1002/gepi.1370120307. [DOI] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plomin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behavior Genetics. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Fuke S, Sasagawa N, Ishiura S. Identification and characterization of the Hesr1/Hey1 as a candidate trans-acting factor on gene expression through the 3′ non-coding polymorphic region of the human dopamine transporter (DAT1) gene. Journal of Biochemistry. 2005;137:205–216. doi: 10.1093/jb/mvi020. [DOI] [PubMed] [Google Scholar]
- Fuke S, Suo S, et al. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1(2):152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Furstenberg FF, Morgan SP, Moore KA, Peterson JL. RACE DIFFERENCES IN THE TIMING OF ADOLESCENT INTERCOURSE. American Sociological Review. 1987;52:511–518. [Google Scholar]
- Gagnon J, Simon W. Sexual conduct: The social origins of human sexuality. Chicago: Aldine; 1973. [Google Scholar]
- Geary D. Male, female, the evolution of human sex differences. Washington DC: American Psychological Association; 1998. [Google Scholar]
- Gill M, Daly G, Heron S, Hawi Z, Fitzgerald M. Confirmation of association between attention deficit hyperactivity disorder and a dopamine transporter polymorphism. Molecular Psychiatry. 1997;2:311–313. doi: 10.1038/sj.mp.4000290. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gottfredson MR, Hirschi T. A general theory of crime. Stanford, CA: Stanford University Press; 1990. [Google Scholar]
- Guo G, Tong YY. Age at first sexual intercourse, genes, and social context: Evidence from twins and the dopamine D4 receptor gene. Demography. 2006;43:747–769. doi: 10.1353/dem.2006.0029. [DOI] [PubMed] [Google Scholar]
- Halpern CT, Joyner K, Udry JR, Suchindran C. Smart teens don’t have sex (or kiss much either) Journal of Adolescent Health. 2000;26:213–225. doi: 10.1016/s1054-139x(99)00061-0. [DOI] [PubMed] [Google Scholar]
- Harris KM, Florey F, Tabor J, Bearman PS, Jones J, Udry JR. The National Longitudinal Study of Adolescent Health: Research design. 2003 Available online at: http://www.cpc.unc.edu/projects/addhealth/design. 2005.
- Hatcher JP, Hagan JJ. The effects of dopamine D-3/D-2 receptor agonists on intracranial self stimulation in the rat. Psychopharmacology. 1998;140:405–410. doi: 10.1007/s002130050782. [DOI] [PubMed] [Google Scholar]
- Hernandez LM, Blazer DG. Genes, behavior, and the social environment: Moving beyond the nature/nurture debate. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- Hetherington EM. DIVORCE - CHILDS PERSPECTIVE. American Psychologist. 1979;34:851–858. doi: 10.1037//0003-066x.34.10.851. [DOI] [PubMed] [Google Scholar]
- Hirschi T. Causes of Delinquency. Los Angeles, CA: University of California Press; 1969. [Google Scholar]
- Hrdy S. The woman that never evolved. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- Hull E, Meisel R, Sachs BD. Male sexual behavior in Hormones, brain, and behavior. San Diego: Acacemic Press/Elsevior; 2002. [Google Scholar]
- Hunter DJ. Gene-environment interactions in human diseases. Nature Reviews Genetics. 2005;6:287–298. doi: 10.1038/nrg1578. [DOI] [PubMed] [Google Scholar]
- Ioannidis J, Ntzani E, Trikalinos T, Contopoulos-Ioannidis D. Replication validity of genetic association studies. Nat Genet. 2001;29:306–309. doi: 10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG. Genetic associations in large versus small studies: an empirical assessment. Lancet. 2003;361:567. doi: 10.1016/S0140-6736(03)12516-0. [DOI] [PubMed] [Google Scholar]
- IOM. The Hidden Epidemic: Confronting Sexually Transmitted Diseases. Washington: National Academic Press; 1997. [PubMed] [Google Scholar]
- Kalivas P. Neurocircuitry of addiction. In: Davis KLCD, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology-the fifth generation of progress. Lippincott Williams and Wilkins; 2002. [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness - An update. Archives of General Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kost K, Forrest JD. AMERICAN WOMENS SEXUAL-BEHAVIOR AND EXPOSURE TO RISK OF SEXUALLY-TRANSMITTED DISEASES. Family Planning Perspectives. 1992;24:244–254. [PubMed] [Google Scholar]
- Lauritsen JL. EXPLAINING RACE AND GENDER DIFFERENCES IN ADOLESCENT SEXUAL-BEHAVIOR. Social Forces. 1994;72:859–884. [Google Scholar]
- Lench N, Stanier P, Williamson R. SIMPLE NON-INVASIVE METHOD TO OBTAIN DNA FOR GENE ANALYSIS. Lancet. 1988;1:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Leventhal T, Brooks-Gunn J. The neighborhoods they live in: The effects of neighborhood residence on child and adolescent outcomes. Psychological Bulletin. 2000;126:309–337. doi: 10.1037/0033-2909.126.2.309. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. LONGITUDINAL DATA-ANALYSIS USING GENERALIZED LINEAR-MODELS. Biometrika. 1986;73:13–22. [Google Scholar]
- Lubin B, Larsen RM, Matarazzo JD. PATTERNS OF PSYCHOLOGICAL TEST USAGE IN THE UNITED-STATES - 1935–1982. American Psychologist. 1984;39:451–454. [Google Scholar]
- Maynard S. The evolution of sex. New York: Cambridge University Press; 1977. [Google Scholar]
- McBurney DH, Zapp DJ, Streeter SA. Preferred number of sexual partners: tails of distributions and tales of mating systems. Evolution and Human Behavior. 2005;26:271–278. [Google Scholar]
- Melis MRaAA. Dopamine and sexual behavior. Neurosci biobehav Rev. 1995:19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJM, Boomsma DI, Slagboom PE. HIGH-YIELD NONINVASIVE HUMAN GENOMIC DNA ISOLATION METHOD FOR GENETIC-STUDIES IN GEOGRAPHICALLY DISPERSED FAMILIES AND POPULATIONS. American Journal of Human Genetics. 1995;57:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Moody J. The Importance of Relationship Timing for Diffusion. Social Forces. 2002;1:25–56. [Google Scholar]
- Moore K, Simms Margaret, Betsey Charles. Choice and Circumstance: Racial Differences in Adolescent Sexuality and Fertility. NJ: Transcation Books; 1986. [Google Scholar]
- Oliver MB, Hyde JS. GENDER DIFFERENCES IN SEXUALITY - A METAANALYSIS. Psychological Bulletin. 1993;114:29–51. doi: 10.1037/0033-2909.114.1.29. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Breakthrough of the year - Human genetic variation. Science. 2007;318:1842–1843. doi: 10.1126/science.318.5858.1842. [DOI] [PubMed] [Google Scholar]
- Plomin R, Defries JC, Loehlin JC. GENOTYPE-ENVIRONMENT INTERACTION AND CORRELATION IN ANALYSIS OF HUMAN-BEHAVIOR. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Rice JA, Brown LF. VALIDITY OF PEABODY PICTURE VOCABULARY TEST IN A SAMPLE OF LOW IQ CHILDREN. American Journal of Mental Deficiency. 1967;71:602. [PubMed] [Google Scholar]
- Risch NJ. Searching for genetic determinants in the new millennium. Nature. 2000;405:847–856. doi: 10.1038/35015718. [DOI] [PubMed] [Google Scholar]
- Rodgers JL, Rowe DC, Buster M. Nature, nurture and first sexual intercourse in the USA: Fitting behavioural genetic models to NLSY kinship data. Journal of Biosocial Science. 1999;31:29–41. doi: 10.1017/s0021932099000292. [DOI] [PubMed] [Google Scholar]
- Sabol SZ, Nelson ML, Fisher C, Gunzerath L, Brody CL, Hu S, et al. A genetic association for cigarette smoking behavior. Health Psychology. 1999;18:7–13. doi: 10.1037//0278-6133.18.1.7. [DOI] [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JA, Gangestad SW. INDIVIDUAL-DIFFERENCES IN SOCIOSEXUALITY - EVIDENCE FOR CONVERGENT AND DISCRIMINANT VALIDITY. Journal of Personality and Social Psychology. 1991;60:870–883. doi: 10.1037//0022-3514.60.6.870. [DOI] [PubMed] [Google Scholar]
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Small SA, Silverberg SB, Kerns D. ADOLESCENTS PERCEPTIONS OF THE COSTS AND BENEFITS OF ENGAGING IN HEALTH-COMPROMISING BEHAVIORS. Journal of Youth and Adolescence. 1993;22:73–87. [Google Scholar]
- Smith MW, Dean Michael, Carrington Mary, Winkler Cheryl, Huttley Gavin A, Lomb Deborah A, Goedert James J, O’Brien Thomas R, Jacobson Lisa P, Kaslow Richard, Buchbinder Susan, Vittinghoff Eric, Vlahov David, Hoots Keith, Hilgartner Margaret W. Contrasting Genetic Influence of CCR2 and CCR5 Variants on HIV-1 Infection and Disease Progression. Science 15. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- Smith TW. DISCREPANCIES BETWEEN MEN AND WOMEN IN REPORTING NUMBER OF SEXUAL PARTNERS - A SUMMARY FROM 4 COUNTRIES. Social Biology. 1992;39:203–211. doi: 10.1080/19485565.1992.9988817. [DOI] [PubMed] [Google Scholar]
- South SJ, Baumer EP. Deciphering community and race effects on adolescent premarital childbearing. Social Forces. 2000;78:1379–1407. [Google Scholar]
- Stark R. Religion as context: Hellfire and delinquency one more time. Sociology of Religion. 1996;57:163–173. [Google Scholar]
- Stark R, Bainbridge WS. Religion, Deviance, and Social Control. New York: Routledge; 1996. [Google Scholar]
- Stark R, Kent L, Doyle DP. RELIGION AND DELINQUENCY - THE ECOLOGY OF A LOST RELATIONSHIP. Journal of Research in Crime and Delinquency. 1982;19:4–24. [Google Scholar]
- Steinthorsdottir V, Thorleifsson G, Reynisdottir I, Benediktsson R, Jonsdottir T, Walters GB, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nature Genetics. 2007;39:770–775. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- Symons D. The evolution of human sexuality. New York: Oxford University Press; 1979. [Google Scholar]
- The_International_HapMap_Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A. INFLUENCE OF THE MARITAL HISTORY OF PARENTS ON THE MARITAL AND COHABITATIONAL EXPERIENCES OF CHILDREN. American Journal of Sociology. 1991;96:868–894. [Google Scholar]
- Thornton A, Young-DeMarco L. Four decades of trends in attitudes toward family issues in the United States: The 1960s through the 1990s. Journal of Marriage and the Family. 2001;63:1009–1037. [Google Scholar]
- Tourangeau R, Smith TW. Asking sensitive questions - The impact of data collection mode, question format, and question context. Public Opinion Quarterly. 1996;60:275–304. [Google Scholar]
- Udry JR. BIOLOGICAL PREDISPOSITIONS AND SOCIAL-CONTROL IN ADOLESCENT SEXUAL-BEHAVIOR. American Sociological Review. 1988;53:709–722. [Google Scholar]
- Udry JR. Biosocial models of low-fertility societies. Population and Development Review. 1996;22:325–336. [Google Scholar]
- Udry JR, Billy JOG. INITIATION OF COITUS IN EARLY ADOLESCENCE. American Sociological Review. 1987;52:841–855. [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. HUMAN DOPAMINE TRANSPORTER GENE (DAT1) MAPS TO CHROMOSOME-5P15.3 AND DISPLAYS A VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DATI regulates in vitro dopamine transporter density. Bmc Genetics. 2005:6. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, et al. Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: Heterogeneity owing to diagnostic subtype and severity. American Journal of Human Genetics. 1998;63:1767–1776. doi: 10.1086/302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JM, Williams DR. Religion and Adolescent Health-Compromising Behavior. In: Schulenberg Maggs JL, Hurrelmann K, editors. Health Risks and Developmental Transitions During Adolescence. New York: Cambridge University Press; 1997. pp. 444–468. [Google Scholar]
- Weinstock H, Berman S, Cates W. Sexually transmitted diseases among American youth: Incidence and prevalence estimates, 2000. Perspectives on Sexual and Reproductive Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- Wilson G. The Great Sex Divide. Washington DC: Scott-Townsend; 1992. [Google Scholar]
- Wilson JR, Kuehn RE, Beach FA. MODIFICATION IN SEXUAL BEHAVIOR OF MALE RATS PRODUCED BY CHANGING STIMULUS FEMALE. Journal of Comparative and Physiological Psychology. 1963;56:636. doi: 10.1037/h0042469. [DOI] [PubMed] [Google Scholar]
- Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]