Abstract
Neurons of the amygdala respond to a variety of stressors. The basolateral amygdala (BLA) receives dense norepinephrine (NE) innervation from the locus coeruleus, and stressful and conditioned stimuli cause increases in NE levels within the BLA. Furthermore, chronic stress exposure leads to sensitization of the stress response. The actions of NE in different structures involved in the stress circuit have been shown to play a role in this sensitization response. Here, we examine how chronic cold stress alters NE modulation of spontaneous and evoked activity in the BLA. In controls, NE inhibited spontaneous firing in the majority of BLA neurons, with some neurons showing excitation at lower doses and inhibition at higher doses of NE. NE also decreased the responsiveness of these neurons to electrical stimulation of the entorhinal and sensory association cortices. After chronic cold exposure, NE caused increases in spontaneous activity in a larger proportion of BLA neurons than in controls, and now produced a facilitation of responses evoked by stimulation of entorhinal and sensory association cortical inputs. These studies show that chronic cold exposure leads to an increase in the excitatory effects of NE on BLA neuronal activity, and suggest a mechanism by which organisms may display an enhancement of hormonal, autonomic, and behavioral responses to acute stressful stimuli after chronic stress exposure.
Keywords: amygdala, norepinephrine, stress, locus coeruleus, sensitization, cold
The amygdala is activated by a large variety of acute stressors (Rosen et al. 1998, Akirav et al. 2001, Dayas et al. 2001), and plays a facilitatory role in components of the stress response (Feldman et al. 1995, Van de Kar and Blair 1999, Herman et al. 2003). The basolateral complex of the amygdala (BLA) receives dense noradrenergic (NE) projections from the locus coeruleus (LC, Asan et al. 1998), which causes an alpha adrenergic inhibition or a beta adrenergic excitation of BLA neurons (Buffalari and Grace, 2007). The LC has an excitatory influence on stress-related circuits as well (Plotsky et al. 1987, Al-Damluji 1988, Ziegler et al. 1999, Sved et al. 2002). Levels of NE increase in the BLA during stress exposure (Galvez et al. 1996, Hatfield et al. 1999, Williams et al. 1998), and neurons of the BLA respond to stressful stimuli that also activate LC neurons (Rasmussen et al. 1986, Aston-Jones et al. 1991, Correll et al. 2005; Buffalari and Grace, 2007). In addition to their roles in acute stress responses, the LC and BLA may play roles in chronic stress.
Chronic or repeated exposure to stressful stimuli leads to a sensitization of many components of the stress response. Prior exposure to chronic cold enhances activation of LC neurons to footshock (Mana and Grace 1997) and corticotrophin-releasing hormone (Jedema et al. 2001). Stress-induced NE efflux in target regions is enhanced after chronic cold or repeated immobilization (Nisenbaum et al. 1991, Pacak et al. 1992, Gresch et al. 1994, Jedema et al. 1999), which may cause alterations in LC targets. Stress-induced release of NE in the bed nucleus (Cecchi et al. 2002), paraventricular nucleus of the hypothalamus (Ma and Morilak 2005a) and amygdala (Ma and Morilak 2005b) facilitates activation of the HPA axis to acute immobilization stress. Therefore, in addition to alterations in the NE system that occur with chronic stress, alterations in NE postsynaptic actions play a role in the behavioral effects of chronic stress.
Chronic stress is also known to alter BLA activity. Chronic immobilization increases spine density on BLA pyramidal neurons (Mitra et al. 2005), and stress enhances plasticity in BLA circuits (Vouimba et al. 2004, 2006). Furthermore, chronic cold exposure alters responses of BLA neurons to footshock (Correll et al. 2005). This study investigates how exposure to chronic cold alters NE modulation of BLA neurons. The effects of NE on BLA spontaneous and afferent-evoked activity was examined. Entorhinal (EC) or sensory association cortices (Te3) were stimulated. Chronic cold exposure was used as a model of chronic stress, as cold-exposed animals reliably exhibit sensitization of the NE system (Nisenbaum et al. 1991, Pacak et al. 1992, Gresch et al. 1994, Jedema et al. 1999). To investigate the time course of the development of alterations in LC targets during chronic cold exposure, two experimental groups were used: rats exposed to 7 days (which is subthreshold for stress-induced sensitization; (Finlay et al. 1997)) or 14 days of cold. These studies examine the role of the amygdala as a contributor to the sensitization of the stress response.
Materials and Methods
Surgical Preparation
All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the USPHS, and all experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (250–400g) were housed upon entrance to the colony and kept on a 12:12 light/dark cycle, maintained at constant temperature, and given food and water ad libitum. After 5 days of housing in pairs, rats were separated, with one cage mate singly-housed and remaining in the control housing room until removal for experimental use. To conserve the use of animals, data from a portion of the control rats (12/16) were derived from a previous study (Buffalari and Grace 2007). Additional data from separate control animals (n=4/16) were found to be identical to that of the previous study and therefore are reported together. The other cage mate was shaved and placed in the cold room and also singly-housed for 7 days or 14 days. Upon removal, each cold-exposed animal was singly-housed in the control room for 24 hours before testing.
Rats were anesthetized with 8% chloral hydrate (400mg/kg, i.p.) and implanted with jugular catheters for administration of supplemental anesthetic. Rats were then placed in a stereotaxic apparatus, with supplemental doses of chloral hydrate (i.v.) to maintain a constant level of anesthesia. Temperature was monitored with a rectal thermometer probe and maintained at 37°C. An incision was made along the scalp and the underlying skull was exposed. Burr holes were drilled in the skull and the dura under the burr holes was removed. The locations (from bregma) of the BLA (−5.0L, −3.0C), Te3 (−6.1L, −6.8C, −4.7V), and the EC (−5.0L, −6.8C, −8.2V) were calculated using a stereotaxic atlas (Paxinos and Watson 1997) for implantation of stimulating electrodes.
Electrophysiology
Multibarrel microiontophoretic electrodes (five barrels; Activational Systems, Warren, MI) were constructed using a vertical microelectrode puller, broken back under microscopic control, and filled with 2% Pontamine sky blue in 2M NaCl to yield electrodes with a central recording barrel impedance of 4–8MOhms. These electrodes were lowered through the BLA in successive vertical tracks. Neurons within the BLA were isolated; those with a signal-to-noise ratio greater than 3:1 were used for data analysis. Spontaneous activity was recorded for a minimum of 5 minutes before further manipulations.
Electrical Stimulation
Bipolar, concentric stimulating electrodes were lowered into either the Te3 or the EC at the conclusion of surgical preparation. Recordings did not begin until a minimum of 45 minutes after lowering of the stimulating electrodes. Neurons responsive to either Te3 or EC stimulation were identified using a search-stimulate protocol. Single-pulse stimuli (300–900 μA, 0.25ms, 0.5Hz) were delivered to the Te3 or EC while the recording electrode was lowered through the BLA. Responsive neurons were identified and isolated. Neurons were characterized as having presumed orthodromic monosynaptic, orthodromic polysynaptic, or antidromic responses to EC stimulation based on the following criteria: Responses were operationally defined as orthodromic if they had an onset latency of <20ms, showed a failure to show substantial changes in latency in response to increases in current intensity, their onset latency remained fairly consistent with approximately 1–5ms of variability in evoked spike latency, and evoked spikes follow paired pulse simulation at 50Hz but not 400Hz. Antidromic responses were characterized by their constant onset latency with virtually no variability, and their ability to followed paired pulses at 400Hz. Monosynaptic responses were differentiated from polysynaptic spikes by an examination latency onset and variability, as well as polysynaptic failure to follow paired pulses at 50Hz.
Microiontophoresis
The central barrel of the 5-barrel microelectrode was filled with 2% Pontamine sky blue in 2 M NaCl for electrophysiological recordings. One of the outer barrels was filled with 3 M NaCl for automaticcurrent balancing, and the remaining barrels were filled with 200uM NE dissolved in 0.1M NaCl. NE was retained with (−) current and ejectedwith (+) iontophoretic current (E104B;Fintronics). Retaining currents rangedbetween 12–18 nA while drug ejection currents ranged between 5–40nA. Repeated, increasing doses from 5–40nA were used with at least 2 minutes of spontaneous activity measured between each period of drug ejection.
Neurons were tested for response to NE in a dose-response fashion (5–40nA). Those neurons that responded to lower doses of NE (5–10nA) did so in a moderate, variable manner, often showing nonsignificant changes in firing rate. Significant and consistent responses emerged in BLA neurons exposed to higher doses of NE (40nA). Therefore, this dose was used for analysis of NE effects on spontaneous activity. However, evoked activity was significantly altered by lower doses of NE, therefore the effects of NE on evoked activity focused on the 10nA dose; effects are also reported for higher doses where tested.
Histology
At the conclusion of electrophysiological experiments, Pontamine sky blue dye was ejected by passing constant current through the recording electrode (10nA, 15 minutes) to mark the location of the recording site. Anodal current was passed through the stimulating electrode to create a small lesion to identify its location. Rats were killed by an overdose of anesthetic, followed by decapitation and brain removal. Brains were fixed in 10% formalin for a minimum of 24 hours, and then cryoprotected with 25% sucrose solution in 0.1M phosphate buffer. Subsequently, coronal slices were cut on a cryostat into 40μm sections, mounted on glass slides, and stained with cresyl violet. Recording sites were identified by the presence of the Pontamine sky blue dye spot, and the location of stimulating electrodes was identified by the presence of a small lesion at the end of the electrode track.
Chronic Cold Exposure
Rats were randomly assigned to cold-exposed and control groups. After five days of acclimation to the housing room during which all animals were pair-housed, cage mates were assigned as matched pairs to either the control group or a cold-exposed group (7 or 14 days). Rats that were to undergo chronic cold exposure were shaved caudally from their forelimbs and housed singly in hanging wire mesh cages in a cold room at 5°C for 7 or 14 days (Jedema et al. 1999, Jedema et al. 2001, Mana and Grace 1997). Controls were kept in the control housing room Both control and cold exposed rats were housed singly during the exposure phase prior to experimental use. Food and water were available ad libitum. Any animals showing signs of cold-induced injury or tissue damage were immediately removed from the protocol. In order to maintain consistency with protocols demonstrating sensitization of aspects of the stress response (Nisenbaum et al. 1991, Gresch et al. 1992, Jedema et al. 1999, Jedema and Grace 2001), chronic cold-exposed animals were removed from the cold room and housed singly in the colony room for 20–24 hours before recordings.
Data Analysis
Firing rate is expressed as spikes/second. An effect was labeled as a significant excitatory or inhibitory effect if, during NE iontophoresis, the average firing rate changed by 25% or more from baseline. This was based on conservative estimates of change necessary to yield significance in a t-test, given the observed firing rate variability. Pre- and post-NE iontophoresis firing rates were compared using paired t-tests. Effects of NE iontophoresis on responses evoked by afferent stimulation were evaluated in the following manner: Current applied to EC or Te3 was adjusted to achieve between 50–60% probability of evoked spike responses. Fifty single pulses at this current intensity were applied and the number of stimuli resulting in evoked spikes was measured. This was compared to evoked spike firing during NE iontophoresis. These spike probabilities were compared using paired t-tests. Changes in evoked responses were expressed as percent increase or decrease in the probability of evoking spike discharge in BLA neurons during NE iontophoresis. A minimum of 3 minutes of baseline spontaneous firing was recorded before delivery of a second stimulation period of 50 sweeps concomitant with NE iontophoresis. The proportions of neurons inhibited or excited in control rats, rats exposed to 7 days of chronic cold, and rats exposed to 14 days of chronic cold were compared using Chi Square tests. The magnitude of percent inhibition by NE on evoked activity in cold-exposed rats was compared to the NE-induced inhibition of evoked activity in control rats using t-tests. The effects of 7 or 14 days of chronic cold on baseline firing rates were compared using a one way ANOVA. Interactions between cold stress groups and neuronal location with respect to baseline firing rates were examined using a two-way ANOVA.
Neurons examined for spontaneous activity displayed a wide range of firing rates and likely included both projection neurons and interneurons. In order to more confidently identify neurons as projection cells (as firing rate alone is not an entirely reliable predictor of projection neuron vs interneuron, Likhtik et al 2006), a subclass of neurons was confirmed as projection cells if they could be antidromically activated by EC stimulation. Histological verification also revealed subclasses of neurons in the lateral and basolateral nuclei of the BLA. These neurons were examined separately for their response to NE iontophoresis; pre- and post-NE firing rates were compared using paired t-tests.. Proportions of neurons in the lateral nucleus displaying excitatory versus inhibitory responses were compared to proportions of neurons in the basolateral nucleus displaying excitatory versus inhibitory responses using Chi Square analyses.
Results
Spontaneous Activity
A total of 140 neurons were examined from 35 rats (n=16 control rats, n=7 rats cold-exposed for 7 days, n=12 rats cold-exposed for 14 days). In control rats, 47 neurons were examined for NE-induced changes in spontaneous activity (8 of those were antidromically activated and confirmed as projection neurons), and 15 were examined for responses to Te3 (n=8) or EC (n=7) stimulation before or during NE iontophoresis. In rats exposed to 7 days of cold stress, 15 neurons were examined for NE-induced changes in spontaneous activity (6 of those were antidromically activated and confirmed as projection neurons), and 15 were examined for responses to Te3 (n=7) or EC (n=8) stimulation before or after NE iontophoresis. In rats exposed to 14 days of cold stress, 32 neurons were examined for NE-induced changes in spontaneous activity (7 of those were antidromically activated and confirmed as projection neurons), and 16 were examined for responses to Te3 (n=7) or EC (n=9) stimulation before or after NE iontophoresis.
Chronic cold does not alter spontaneous activity of BLA neurons after 7 or 14 days
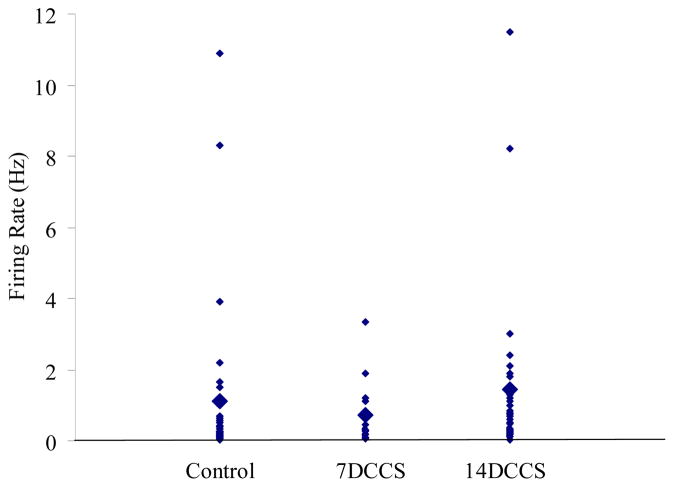
There were no significant differences in spontaneous firing rates of neurons of the BLA in control rats (avg FR= 1.3±0.45Hz), rats exposed to 7 days of cold (avg FR= 0.75±0.11Hz), or rats exposed to 14 days of cold (avg FR= 1.4±0.13Hz, Figure 1). Although there was a trend for decreased spontaneous firing rates after 7 days and increased spontaneous firing rates after 14 days, these were not significant (p=0.35, one way ANOVA).
Figure 1.
Chronic cold exposure does not affect spontaneous firing rates of neurons within the BLA. Spontaneous firing rates of neurons from control rats, rats exposed to 7 days of cold, and rats exposed to 14 days of cold. Large diamonds represent group averages, small diamonds represent individual neurons.
The spontaneous firing rates of neurons in control rats, 7 day cold rats and 14 day cold rats did not differ when examined based on neuronal location. That is, there was no significant interaction between experimental group and neuron location (two-way ANOVA, p>0.1). The average firing rate across all groups and all locations was 1.2±0.17 Hz.
The average firing rate of neurons confirmed by antidromic activation to be projection neurons (n=21, all groups) was 0.18±0.09Hz, which was significantly lower than the firing rates of spontaneously active neurons (p=0.01, t-test). Spontaneously active neurons displayed a wide range of firing rates and likely included both projection neurons and interneurons. The spontaneous activity of projection neurons was no different (p=0.7, one way ANOVA) in control rats (avg FR= 0.22±0.04Hz, n=8), rats exposed to 7 days of cold (avg FR= 0.13±0.04Hz, n=6), or rats exposed to 14 days of cold (avg FR= 0.17±0.03Hz, n=7). As the majority of projection neurons were located in the basolateral nucleus (n=6 in controls, n=4 in 7 day cold rats, n=5 in 14 day cold rats), projection neurons were not further divided based on neuronal location for analysis of interaction between experimental group and neuron location.
Chronic cold alters NE modulation of spontaneous activity after 14 but not 7 days
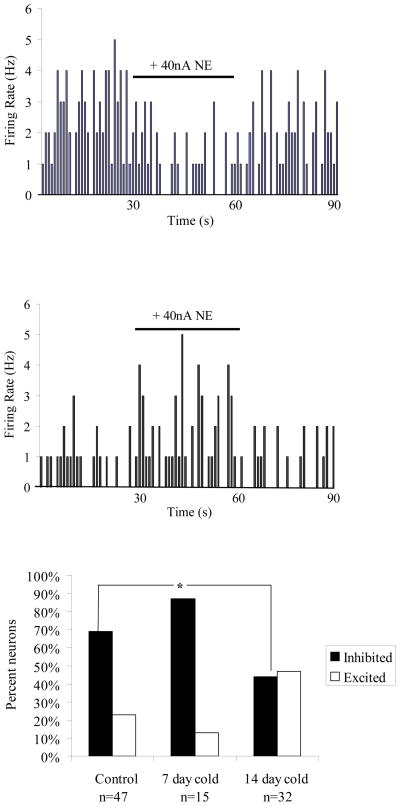
Iontophoresis of NE (200uM, 40nA) caused a significant decrease in spontaneous activity (by 30±5.1%, p=0.001, t-test) of the majority of neurons (70%; n= 33/47) of the BLA in control rats (Figure 2A). A smaller proportion of neurons (21%; n=10/47) showed a significant increase in spontaneous activity (by 107±9.1%, p=0.005, t-test). Neurons from rats exposed to 7 days of chronic cold stress displayed responses to NE that were not significantly different from those observed in control rats (p=0.35, Chi Square, Figure 2C). However, in rats exposed to 14 days of chronic cold stress, significantly more neurons displayed excitatory responses to NE than in control rats, and significantly fewer neurons displayed inhibitory responses to NE than in control rats (p=0.02, Chi Square, Figure 2B example neuron, Figure 2C group data). No differences in the magnitude of NE-induced inhibition or excitation were observed (data not shown).
Figure 2.
Noradrenergic modulation of spontaneous activity in BLA neurons is altered after fourteen days of chronic cold exposure, but not seven. A) Example of a neuron from a control rat showing an inhibition in spontaneous activity during microiontophoresis of NE. B) Example of a neuron from a 14 day cold-exposed rat displaying an excitation of spontaneous activity during microiontophoresis of NE. C) Neurons from control rats and rats exposed to seven days of cold showed primarily inhibitory responses to NE, however in rats exposed to 14 days of cold, more neurons display excitatory responses to NE.
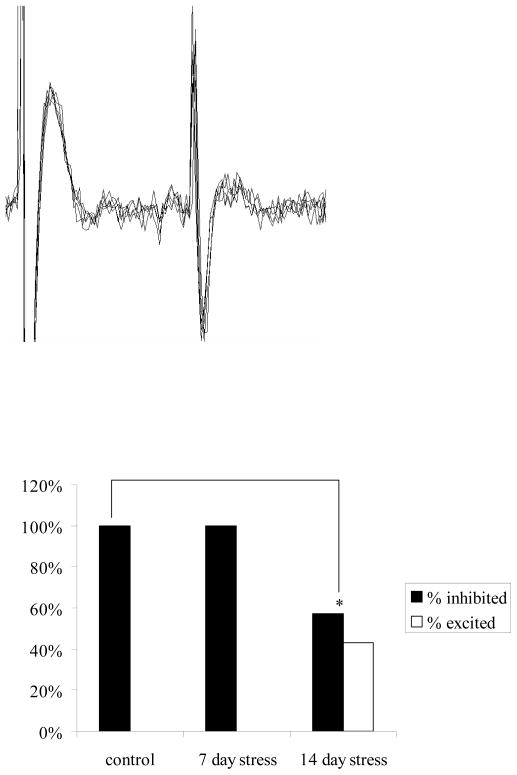
A subset of neurons were antidromically activated and confirmed as projection neurons in control, 7 day cold exposed, and 14 day cold exposed rats (Figure 3A). In controls, NE significantly inhibited all projection neurons (n=8/8, p=0.002, t-test, Figure 3B). In rats exposed to 14 days of cold stress significantly different proportions of responses were seen (p=0.05, Chi Square). A portion of neurons were still inhibited by NE (57%; n=4/7, p=0.01, t-test); however, other projection neurons displayed excitatory responses to NE iontophoresis (43%; n=3/7, p=0.03, Figure 3B). There were no significant differences between control rats and rats exposed to 7 days of cold stress (p=0.48, Chi Square), in that all projection neurons were inhibited in rats exposed to 7 days of cold stress (n=6/6, p=0.02, t-test, Figure 3B).
Figure 3.
Noradrenergic modulation of spontaneous activity in BLA projection neurons is altered after fourteen days of chronic cold exposure, but not seven. A) Electrophysiological trace of a BLA neuron displaying an antidromic spike in response to entorhinal cortical stimulation. Note the constant latency of the onset of the response. B) All BLA neurons confirmed as projection cells via antidromic activation from control and seven day cold-exposed rats were inhibited by microiontophoresis of NE. However, while a subset of neurons from rats exposed to 14 days of cold also displayed an inhibition, others displayed excitatory responses to NE iontophoresis.
Chronic cold alters NE modulation of evoked activity after 7 and 14 days
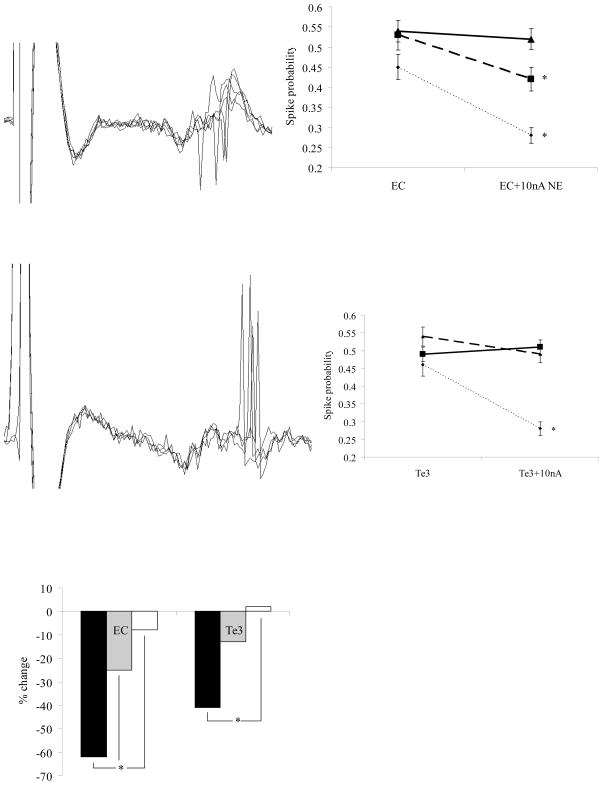
Electrical stimulation of EC caused orthodromic, excitatory responses in neurons of the BLA in both control and stressed rats (Figure 4A). Stress exposure did not alter baseline responses to EC stimulation (latency, spike probability, # neurons per track, data not shown). In control rats iontophoresis of NE (10nA), significantly decreased the spike probability of neurons in response to EC stimulation (n=7/7, p=0.005, t-test, Figure 4B). In rats exposed to 14 days of cold, iontophoresis of NE (10nA) resulted in NE-induced effects on EC-evoked activity that were significantly different from controls. In 14 day cold rats, NE decreased the spike probability of 56% of neurons in response to EC stimulation (n=5/9, p=0.05); however, in 44% of neurons NE increased the spike probability (n=4/9, p=0.03, Figure 4F). These alterations in the proportions of neurons showing inhibitory versus excitatory effects were significantly different (p=0.04, Chi Square). In addition, after 14 days of cold exposure, the magnitude of the overall NE-induced change was significantly decreased. In control rats, NE decreased spike probability by an average of 62±8%, which was significantly different from baseline EC-evoked activity (p=0.005 t-test, Figure 4E). After 14 days of cold, this dropped to an average decrease of 8±3%, which was not significantly different from baseline EC-evoked activity (p=0.65, t-test), and was significantly less than the inhibition seen in controls (p=0.001, t-test, Figure 4E). After 7 days of cold stress, NE decreased spike probability in the majority of neurons (n=6/8), and therefore the proportions of neurons displaying excitation/inhibition were not significantly different between controls and 7 day cold exposed rats (p=0.01, Chi Square, Figure 4F). However, whereas NE still inhibited EC-evoked activity in 7 day cold rats (by 25±4%, p=0.02, t-test), the degree of inhibition was significantly less than in control rats (p=0.03, t-test), and not in all neurons (n=1/8 neurons increased spike probability by 14%, n=1/8 neurons no change in evoked activity, Figure 4F).
Figure 4.
Noradrenergic modulation of evoked activity in BLA neurons is altered after seven and fourteen days of chronic cold exposure. A) Electrophysiological trace of a BLA neuron displaying an excitatory, orthodromic spike in response to entorhinal cortical stimulation. B) In control rats (dotted line), BLA activity evoked by stimulation of entorhinal cortex was significantly decreased during microiontophoresis of NE. In rats exposed to 7 or 14 days of cold, the inhibitory actions of NE were lessened (7 days, dashed line) and abolished (14 days, solid line). C) Electrophysiological trace of a BLA neuron displaying an excitatory, orthodromic spike in response to sensory association cortical stimulation. D) In control rats (dotted line), BLA activity evoked by stimulation of sensory association cortex was significantly decreased during microiontophoresis of NE. In rats exposed to 7 or 14 days of cold, the inhibitory actions of NE on evoked activity were lessened (14 days, solid line) and abolished (7 days, dashed line). E) Chronic cold exposure decreased the magnitude of NE-induced inhibition of Te3 and EC-evoked activity after 14 days of exposure, but not 7 days. F) Chronic cold exposure decreased the proportion of neurons that showed NE-induced inhibition of Te3 and EC-evoked activity after seven (gray bars) or 14 (white bars) of activity.
Electrical stimulation of Te3 caused orthodromic, excitatory responses in neurons of the BLA in both control and stressed rats (Figure 4C). Stress exposure did not alter the nature of baseline responses to Te3 stimulation (latency, spike probability, # neurons per track, data not shown). In control rats, NE iontophoresis significantly decreased the spike probability of neurons in response to Te3 stimulation (n=8/8, p=0.002, t-test, Figure 4D). In rats exposed to 14 days of cold, iontophoresis of NE (10nA) resulted in significant differences in the proportions of BLA neurons displaying inhibitory/excitatory effects of NE on Te3-evoked activity (p=0.001, Chi Square). In 14 day cold exposed rats, iontophoresis of NE (10nA) significantly increased the spike probability in 71% of neurons in response to Te3 stimulation (n=5/7, p=0.02), whereas in 29% of neurons NE decreased the spike probability (n=2/7, p=0.01, Figure 4D,F). Cold exposure also decreased the magnitude of NE-induced inhibition of Te3-evoked activity of BLA neurons (p=0.006, t-test, Figure 4E). In control rats, NE significantly decreased spike probability by an average of 41±7% from baseline. After 14 days of cold exposure, this changed to an average increase of 2±8% (not significant from baseline, p=0.65, t-test), which was significantly different than the NE-induced inhibition seen in controls (p=0.006, t-test). After 7 days of cold exposure, NE decreased spike probability in 57% of neurons (n=4/7), but not to a significant degree (by 13±6%, p=0.08, t-test). There were significant differences in the proportions of neurons displaying inhibitory/excitatory effects on evoked activity to NE when 7 day cold rats were compared to controls (p=0.04, Chi Square, Figure 4F). There were no differences in the magnitude of NE-induced decrease of Te3-evoked activity in rats exposed to 7 days of cold versus controls (p=0.06, t-test), although a trend was noted. One neuron did display increased spike probability (by 38%, n=1/7), with others displaying no significant change (n=2/7, Figure 4F).
Discussion
This study demonstrates that chronic stress induces alterations in the responses of BLA neurons to NE. Following chronic cold exposure, a significantly higher proportion of BLA neurons exhibited excitatory responses to NE iontophoresis, but only after 14 days of exposure. Chronic cold exposure also decreases the inhibitory effects of NE on BLA neuronal activity evoked by stimulation of EC and Te3. Stress causes a facilitation of some afferent-evoked activity after 7 days exposure. This facilitatory effect of NE on evoked activity was more pronounced after 14 days cold exposure. Thus, chronic stress-induced alterations in the NE system (Nisenbaum et al. 1991, Pacak et al. 1992, Gresch et al. 1994, Mana and Grace et al. 1997, Jedema et al. 1999, Jedema and Grace 2001) also impact the electrophysiological activity in NE target regions. This characterizes the BLA as an important site influencing sensitization of the stress response, and extends previous work examining other regions that may underlie pathological changes that occur as a result of stress exposure.
Effects of stress on BLA neurons and their responses to NE
Repeated administration of the same stressor leads to habituation of the stress response to that stressor. Neurons of the BLA display a decreased response to footshock with repeated presentations (Shors 1999). However, after repeated or chronic stress, portions of the stress response display sensitization, or a potentiated response, to novel stressors. This phenomenon is also displayed by neurons of the BLA. In control rats, footshock causes both excitatory and inhibitory responses in BLA spontaneous activity. However, after exposure to chronic cold stress, BLA neurons display only excitatory responses to this stimulus (Correll et al. 2005). Our present work suggests a mechanism by which these effects may be occurring. During footshock, NE is increased in the BLA (Galvez et al. 1996, Williams et al. 1998, Hatfield et al. 1999). In control rats, neurons of the BLA display largely inhibitory responses to NE, with few neurons displaying excitatory responses. After chronic cold exposure, NE causes more excitatory responses in neurons of the BLA, with few neurons displaying inhibition. This increase in excitatory responses to NE may explain the increase in excitatory responses of BLA neurons to footshock following chronic cold exposure. The finding that this only happens with lengths of stress exposure that lead to sensitization (i.e., 14 days) suggests that this is the behaviorally relevant alteration underlying the sensitized response. However, we did see changes in NE modulation of evoked activity after 7 days, despite evidence examining NE efflux showing the sensitization of the NE system does not occur at 7 days of exposure (Finlay et al. 1997). We suggest that alterations may be occurring at the receptor level on presynaptic glutamatergic terminals before they occur on postsynaptic BLA neurons. Alternatively, those studies examining sensitized NE efflux used microdialysis, which examines extracellular levels of NE. Smaller, more subtle changes that occur at the level of the synapse after 7 days may not be detectable with this technique.
Several potential mechanisms are suggested for the alteration in the effects of NE on BLA neuronal activity by chronic stress. First, after chronic stress, stress-induced NE efflux in terminal regions is enhanced (Nisenbaum et al. 1991, Pacak et al. 1992, Gresch et al. 1994, Jedema et al. 1999), which may lead to differential effects on BLA neuronal activity. Our data argue against this possibility. Dose-dependent effects of NE demonstrate increasing levels of inhibition with increasing doses of NE in the majority of BLA neurons. Furthermore, in a subset of neurons, excitatory responses were seen at low doses of NE, with inhibitory responses emerging at higher doses.
A second mechanism by which the neuronal actions of NE could be modified is via an alteration in the levels of NE receptors in the BLA. Chronic social stress decreases alpha-2 receptor binding in the LC, suggesting a loss of NE-induced feedback inhibition on LC neurons (Flugge et al. 1996, Meyer et al. 2000). Furthermore, (Jedema, submitted) chronic stress decreases apha-2-mediated inhibition of neurons in the LC via an increase in RGS-7. Chronic cold exposure increases sensitivity of alpha-2 receptors in the hippocampus (Nisenbaum et al.. 1993). The LC and the BLA may act in a coordinated manner to facilitate the stress response, whereas the hippocampus and prefrontal cortex play an inhibitory role (Herman et al. 1995, 1998). While no one has examined the effect of stress or NE on neurons in these regions specifically, people have examined the effects of stress on plasticity. Stress decreases plasticity in the prefrontal cortex (Maroun et al 2006) and hippocampus, and causes dendritic atrophy in the hippocampus as well, (Wantanabe et al. 1992, Magarinos et al. 1996), while increasing plasticity and causing dendritic hypertrophy in the BLA (Vouimba et al. 2004, Vyas et al. 2002, Mitra et al. 2005). Alpha-2 receptor sensitization in the hippocampus decrease the inhibitory role of the hippocampus on stress reactivity, but desensitization enhances the facilitatory LC and BLA influence on the stress response. Such a scenario corresponds well with a sensitized response to stress following chronic exposure.
Changes in receptor affinity, G proteins mediating responses, or second messenger systems may all also play a role in alterations of NE actions on neuronal activity. Repeated tail pinch or immobilization causes receptors to be less efficacious in producing intracellular cAMP responses (Bellavia and Gallara 1998) possibly secondary to changes in RSG proteins (Jedema et al., 2008). However, one of these studies (Bellavia and Gallara 1998) also demonstrates subsensitive beta-receptors, and another found hypersensitive alpha-2 receptors (Garcia-Vallejo et al. 1998) after chronic variable stress. This evidence complicates interpretation of the current results.
Previous studies have used firing rate and spike duration together to differentiate presumed projection neurons (low) from putative interneurons (high, Rosenkranz and Grace 2001). However, considered alone, firing rate does not appear to be adequate to identify neuronal subtypes (Likhtik et al 2006; Rosenkranz and Grace, 2001). Furthermore, these studies suggested that neurons that can be reliably identified as interneurons make up a very small population of the neurons of the BLA. Given the large variation in firing rates, we used antidromic activation to precisely confirm neurons as projection neurons. While these neurons were inhibited in control and 7 day cold-exposed animals, a portion of them were excited in 14 day cold-exposed animals. Of course, this study cannot distinguish between a direct excitatory effect on projection neurons vs one mediated by decreased NE-induced inhibition of GABAergic interneurons that synapse onto BLA pyramidal neurons
Effects of stress on evoked responses
Prior to chronic stress, NE was found to inhibit BLA responses to EC and Te3 stimulation. However, after chronic stress exposure, some of these inputs are potentiated in the presence of NE. We anticipated NE may differentially affect sensory input (Te3) vs. higher cortical input (EC). However, this was not the case. During conditioning to aversive stimuli, it is possible that one or both of these inputs undergoes learning-related alterations. However, in the absence of conditioning, such changes are not relevant in control rats. Chronic stress-induced alterations may lead to nonspecific potentiation of inputs. Indeed, chronic stress has been shown to increase dendritic spines within the BLA (Mitra et al. 2005), and enhance synaptic plasticity in BLA circuits, phenomena often associated with conditioning (Radley et al. 2006). If, after chronic stress exposure, inputs that were previously inhibited are potentiated, this may manifest itself behaviorally by producing rats that are hyperresponsive to stressful stimuli, as has been reported (Nisenbaum et al. 1991, Pacak et al. 1992, Gresch et al. 1994, Jedema et al. 1999). Furthermore, these rats may be poorer learners if mechanisms of plasticity have been disrupted. Rats exposed to chronic restraint or psychosocial stress demonstrate impairments in spatial and object memory (Conrad et al. 2003, Park et al. 2001)
Stress is associated with a reduction in GABAergic transmission in the amygdala. BLA pyramidal neurons are under tight inhibitory control by GABAergic synapses (Rainnie et al. 1991, Washburn and Moises 1992, Woodson et al. 2000; Rosenkranz & Grace 2002). However, immobilization and cold lead to decreased levels of GAD and GABA in the brain (Otero Losado 1988), and swim stress decreases GABA concentrations as well (Briones-Aranda et al. 2005). Restraint stress decreases GABAergic inhibitory control of the stress response by the amygdala (Marineja et al. 2002). GABAergic blockade in the BLA leads to anxiety responses (Sanders and Shekhar 1991, Sajdyk and Shekhar 1997), an effect also seen after restraint stress (Rodriguez-Manzanares et al. 2005). These data are consistent with disruption of NE-GABAergic circuits in the BLA by chronic stress.
NE afferents form asymmetrical synapses onto GABAergic interneurons of the BLA (Li et al. 2001). This could contribute to the inhibitory effects seen in control rats; i.e. NE-induced excitation of GABAergic interneurons that synapse onto glutamatergic pyramidal cells within the BLA (Muller et al. 2006). Stress disrupts normal NE-induced facilitation of GABAergic transmission in the BLA seen in control rats (Braga et al. 2004). Similar processes may be induced following cold exposure. Decreased GABAergic transmission could result from a desensitization or decrease in NE receptors present on these GABAergic interneurons. This would decrease GABA neuron excitation by NE, leading to less inhibition in BLA pyramidal neurons, an effect demonstrated in these current data.
The present studies cannot rule out whether the changes in evoked responses occurred due to changes in amygdala neurons versus an alteration in the afferent neurons in the entorhinal cortex or sensory association cortex. However, the data presented here strongly favor an action of chronic cold that is mediated by a change in BLA neurons. First, cold exposure did not affect baseline responses of BLA neurons to afferent stimulation: the current amplitude required to evoked an orthodromic BLA neuron spike, the number of responsive neurons per track, and the average current amplitude required to reach 50% spike probability were not different between control and cold-exposed animals. Furthermore, these cold-exposed changes were limited to NE modulation, and NE was iontophoresed directly onto BLA neurons, which support an intra-amygdala mechanism. Finally, the finding that the change in the response of the BLA neuron to stimulation of either a primary sensory afferent (Te3) or a limbic afferent (entorhinal cortex) was essentially identical strongly supports a chronic cold-induced alteration within the BLA neurons themselves, rather than a common effect on two very different afferent structures. Nonetheless, it is certainly possible that alterations of glutamatergic afferent terminals targeting BLA neurons may have also contributed to the chronic cold-induced changes observed.
It should be noted that the present studies do not definitively distinguish between effects caused by 14 days of cold exposure versus effects caused by 7 days of cold exposure that may have a delayed onset (revealed in the present study at 14 days). However, previous studies examining NE efflux in response to a stressor after 7 or 14 days of cold exposure show conclusively that acute stress-evoked NE efflux, as measured with microdialysis, was enhanced in animals exposed to 14 but not 7 days of cold exposure even when the animals exposed to 7 days of cold were not evaluated until 14 days following a return to a normal environment (FINLAY REF).. Therefore, t it is unlikely that the current results can be explained by effects that occur with 7 days of exposure that have a delayed onset, and that 14 days of cold exposure are required to induce the changes observed.
Implications
Extensive exposure to stress is often associated with the development and/or enhancement of pathology and disease. Indeed, post-traumatic stress disorder has been modeled as a severe pathological form of the sensitization of the stress response (Rau et al. 2006). Previous studies have suggested a potential involvement of the BLA in the circuit mediating the sensitization of the stress response. The importance of NE in the PVN, BNST, and medial amygdala has been established (Ma and Morilak 2005a,b). The present studies lend further support for an important role of the BLA in sensitization, and provide an electrophysiological mechanism by which NE could act in target regions to mediate such a condition. Identifying the structures and circuits that may undergo pathological changes during abnormal levels of stress, and the mechanisms underlying such changes, will promote the understanding of how such disorders develop, and aid in the design of potential treatments for such conditions.
Acknowledgments
We would like to thank Christy Smolak and Nicole MacMurdo for their expert histological processing of tissue and technical assistance, Brian Lowry for the development of the data acquisition and analysis software, and Dr. Hank P. Jedema and Dr. Amiel Rosenkranz for help in thoughtful discussion and input regarding these data.
Footnotes
The authors have no known conflicts of interest.
References
- Akirav I, Sandi C, Richter-Levin G. Differential activation of hippocampus and amygdala following spatial learning under stress. European Journal of Neuroscience. 2001;14:719–725. doi: 10.1046/j.0953-816x.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- Al-Damluji S. Adrenergic mechanisms in the control of corticotrophin secretion. Journal of Endocrinology. 1988;119(1):5–14. doi: 10.1677/joe.0.1190005. [DOI] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Advances in Anatomy, Embryology, and Cell Biology. 1998;142:L1–118. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Progress in Brain Research. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Bellavia SL, Gallaria RV. Modification of the beta- and alpha-2-adrenergic sensitivity of rat submandibular glands by environmental stimuli and stress. Archives of Oral Biology. 1998;43:933–939. doi: 10.1016/s0003-9969(98)00084-3. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha-1A adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology. 2004;29:45–58. doi: 10.1038/sj.npp.1300297. [DOI] [PubMed] [Google Scholar]
- Briones-Aranda A, Rocha L, Picazo O. Alterations in GABAergic function following forced swimming stress. Pharmacology, Biochemistry, and Behavior. 2005;80(3):463–70. doi: 10.1016/j.pbb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic Modulation of Basolateral Amygdala Neuronal Activity: Opposing Influences of Alpha-2 and Beta Receptor Activation. The Journal of Neuroscience. 2007;27(45):12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112(1):13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Grote KA, Hobbs RJ, Ferayorni A. Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiology of Learning and Memory. 2003;79(1):32–40. doi: 10.1016/s1074-7427(02)00018-7. [DOI] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biological Psychiatry. 2005;58(5):382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. European Journal of Neuroscience. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Weidenfeld J. Limbic pathways and hypothalamic neurotransmission mediating adrenocortical responses to neutral stimuli. Neuroscience and Biobehavioral Reviews. 1995;19(2):235–240. doi: 10.1016/0149-7634(94)00062-6. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Jedema HP, Rabinovic AD, Mana MJ, Zigmond MJ, Sved AF. Impact of corticotropin-releasing hormone on extracellular norepinephrine in prefrontal cortex after chronic cold stress. Journal of Neurochemistry. 1997;69(1):144–50. doi: 10.1046/j.1471-4159.1997.69010144.x. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Garcia-Vallejo P, Gomez FM, Infante C, Ginestal E, Giralt MT. Chronic variable stress induces supersensitivity of central alpha-2-adrenoceptors which modulate the jaw-opening reflex in the rat. Brain Research. 1998;801(1–2):72–77. doi: 10.1016/s0006-8993(98)00547-2. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress-induced sensitization of dopamine and norepinephrine efflux in the medial prefrontal cortex of the rat. The Journal of Neurochemistry. 1994;63(2):575–583. doi: 10.1046/j.1471-4159.1994.63020575.x. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Research. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to the inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. Journal of Neuroendocrinology. 1995;7(6):475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Colgas CM, Carlson SL. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience. 1998;86(2):449–459. doi: 10.1016/s0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Renda A, Bodie B. Norepinephrine and gamma-amino-butyric acid: Interaction in limbic stress circuits: effects of reboxetine on GABAergic neurons. Biological Psychiatry. 2003;53:166–174. doi: 10.1016/s0006-3223(02)01449-x. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Sved AF, Zigmond MJ, Finlay JM. Sensitization of norepinephrine release in medial prefrontal cortex: effect of different chronic stress protocols. Brain Research. 1999;830:211–217. doi: 10.1016/s0006-8993(99)01369-4. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Finlay JM, Sved AF, Grace AA. Chronic cold exposure potentiates CRH-evoked increases in electrophysiological activity of locus coeruleus neurons. Biological Psychiatry. 2001;49:351–359. doi: 10.1016/s0006-3223(00)01057-x. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gonzales-Burgos G, Sved AF, Tobe B, Grace AA. Chronic cold exposure increases RGS7 expression and decreases alpha-2 autoreceptor mediated inhibition of noradrenergic locus coeruleus neurons. European Journal of Neuroscience. 2008 doi: 10.1111/j.1460-9568.2008.06208.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Nishijo H, Wang Q, Uwano T, Tamura R, Ohtani O, Ono T. Light and electron microscopic study of cholinergic and noradrenergic elements in the basolateral nucleus of the rat amygdala: evidence for interactions between the two systems. The Journal of Comparative Neurology. 2001;439:411–425. doi: 10.1002/cne.1359. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitizes the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. The Journal of Neuroendocrinology. 2005;17(11):761–769. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Norepinephrine release in medial amygdala facilitates activation of the hypothalamic-pituitary-adrenal axis in response to acute immobilization stress. The Journal of Neuroendocrinology. 2005;17(1):22–28. doi: 10.1111/j.1365-2826.2005.01279.x. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. The Journal of Neuroscience. 1996;16(10):3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mana MJ, Grace AA. Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience. 1997;81(4):1055–1064. doi: 10.1016/s0306-4522(97)00225-x. [DOI] [PubMed] [Google Scholar]
- Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. European Journal of Neuroscience. 2006;24(10):2917–22. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- Martineja ID, Rodriguez Manzanares PA, Lacerra C, Molina VA. Gabaergic modulation of the stress response in frontal cortex and amygdala. Synapse. 2002;45:86–94. doi: 10.1002/syn.10085. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patters of spine formation in the basolateral amygdala. Proceedings of the National Academy of Science of the United States of America. 2005;102(6):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Coupled networks of parvalbumin-immunoreactive interneurons in the rat basolateral amygdala. The Journal of Neuroscience. 2005;25(32):7366–7376. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. The Journal of Comparative Neurology. 2006;494(4):635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. The Journal of Neuroscience. 1991;11(5):1478–1484. doi: 10.1523/JNEUROSCI.11-05-01478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum LK, Abercrombie ED. Presynaptic alterations associated with enhancement of evoked release and synthesis of norepinephrine in hippocampus of chronically cold-stressed rats. Brain Research. 1993;608:280–287. doi: 10.1016/0006-8993(93)91469-9. [DOI] [PubMed] [Google Scholar]
- Otero Losada ME. Changes in central GABAergic function following acute and repeated stress. British Journal of Pharmacology. 1988;93(3):483–90. doi: 10.1111/j.1476-5381.1988.tb10302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacak K, Armando I, Fukuhara K, Kvetnansky R, Palkovits M, Kopin IJ, Goldstein DS. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: an in vivo microdialysis study. Brain Research. 1992;589(1):91–6. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Park CR, Campbell AM, Diamond DM. Chronic psychosocial stress impairs learning and memory and increases sensitivity to yohimbine in adult rats. Biological Psychiatry. 2001;50:994–1004. doi: 10.1016/s0006-3223(01)01255-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1997. [Google Scholar]
- Plotsky PM, Otto S, Sutton S. Neurotransmitter modulation of corticotrophin releasing factor secretion into the hypophysial-portal circulation. Life Sciences. 1987;41(10):1311–1317. doi: 10.1016/0024-3205(87)90211-6. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Johnson LR, Janssen WG, Martino J, Lamprecht R, Hof PR, LeDoux JE, Morrison JH. Associative Pavlovian conditioning leads to an increase in spinophilin-immunoreactive dendritic spines in the lateral amygdala. European Journal of Neuroscience. 2006;24(3):876–84. doi: 10.1111/j.1460-9568.2006.04962.x. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. Journal of Neurophysiology. 1991a;66(3):999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Research. 1986;371(2):324–34. doi: 10.1016/0006-8993(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neuroscience and Biobehavioral Reviews. 2006;29(8):1207–23. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. The Journal of Neuroscience. 2005;25(38):8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of prelimbic and infralimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. The Journal of Neuroscience. 2002;22(1):324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by gamma-aminobutyric acid-a receptor blockade in the basolateral amygdala of rats. Journal of Pharmacology and Experimental Therapeutics. 1997;283:969–977. [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Blockade of GABAA receptors in the region of the anterior basolateral amygdala of rats elicits increases in heart rate and blood pressure. Brain Research. 1991;567:101–110. doi: 10.1016/0006-8993(91)91441-3. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress and re-exposure to the stressful context suppress spontaneous unit activity in the basolateral amygdala via NMDA receptor activation. Neuroreport. 1999;10(13):2811–2815. doi: 10.1097/00001756-199909090-00021. [DOI] [PubMed] [Google Scholar]
- Sved AF, Cano G, Passerin AM, Rabin B. The locus coeruleus, Barrington’s nucleus, and neural circuits of stress. Physiology and Behavior. 2002;77:737–742. doi: 10.1016/s0031-9384(02)00927-7. [DOI] [PubMed] [Google Scholar]
- Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Frontiers in Neuroendocrinology. 1999;20(1):1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, Diamond D, Richter-Levin G. Effects of inescapable stress on LTP in the amygdala vs. the dentate gyrus of freely behaving rats. European Journal of Neuroscience. 2004;19(7):1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress. 2006;9(1):29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992;2(4):431–5. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. The Journal of Neuroscience. 1992;12(10):4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behavioral Neuroscience. 1998;112(6):1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- Woodson W, Farb CR, Ledoux JE. Afferents from the auditory thalamus synapse on inhibitory interneurons in the lateral nucleus of the amygdala. Synapse. 2000;38(2):124–37. doi: 10.1002/1098-2396(200011)38:2<124::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. The Journal of Neuroendocrinology. 1999;11(5):361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]