Abstract
Smallpox was eradicated more than 30 years ago, but heightened concerns over bioterrorism have brought smallpox and smallpox vaccination back to the forefront. The previously licensed smallpox vaccine in the United States, Dryvax® (Wyeth Laboratories, Inc.), was highly effective, but the supply was insufficient to vaccinate the entire current US population. Additionally, Dryvax® had a questionable safety profile since it consisted of a pool of vaccinia virus strains with varying degrees of virulence, and was grown on the skin of calves, an outdated technique that poses an unnecessary risk of contamination. The US government has therefore recently supported development of an improved live vaccinia virus smallpox vaccine. This initiative has resulted in the development of ACAM2000™ (Acambis, Inc.™), a single plaque-purified vaccinia virus derivative of Dryvax®, aseptically propagated in cell culture. Preclinical and clinical trials reported in 2008 demonstrated that ACAM2000™ has comparable immunogenicity to that of Dryvax®, and causes a similar frequency of adverse events. Furthermore, like Dryvax®, ACAM2000™ vaccination has been shown by careful cardiac screening to result in an unexpectedly high rate of myocarditis and pericarditis. ACAM2000™ received US Food and Drug Administration (FDA) approval in August 2007, and replaced Dryvax® for all smallpox vaccinations in February 2008. Currently, over 200 million doses of ACAM2000™ have been produced for the US Strategic National Stockpile. This review of ACAM2000™ addresses the production, characterization, clinical trials, and adverse events associated with this new smallpox vaccine.
Keywords: smallpox, vaccinia, variola, vaccine, efficacy, safety
Introduction
After a devastating battle spanning centuries, smallpox was eliminated globally in 1980 as a result of the successful eradication program by the World Health Organization (WHO). The live vaccinia virus vaccine, Dryvax® (New York City Board of Health [NYCBH] strain) was one of the vaccines used during the worldwide vaccination campaign. Dryvax® was prepared by harvesting live virus from lesions on the skin of infected cows; thus, sterility of the vaccine was always questionable.1 Adverse events ranging from mild systemic symptoms such as fever, myalgia, and headaches, to serious adverse events including generalized vaccinia, eczema, encephalitis, and even fatality, were seen after Dryvax® vaccinations.2 Therefore, following eradication, smallpox vaccination ended for the public in 1972 and for the military in 1989 in the US.1 The last time Dryvax® was manufactured was in 1978 by Wyeth Laboratories (Marietha, PA), and there were only 15 million doses of the smallpox vaccine left after the suspension of all smallpox vaccinations.
In the 1990s, the US became more sensitive to the possible threat of an accidental or intentional release of smallpox. A directive issued by President Clinton in 1995 initiated counterterrorism programs in several federal agencies, which resulted in a strategy to re-institute smallpox vaccination should it become necessary.3 Vaccination with live vaccinia virus was instrumental to the success of the eradication campaign and, for that reason, a live vaccinia vaccine approach was chosen. Among the vaccinia strains used for worldwide eradication in the 20th century, Dryvax®, consisting of the NYCBH strain, was thought to be the safest, having the fewest adverse events.4 Because of advances in vaccine production technology, a cell culture-based mode of propagation could replace growth on the skin of cows. Growth in culture allows for many improvements in the quality of the vaccine product, such as eliminating possible contaminants and reducing lot-to-lot variation. Nevertheless, Dryvax® was a mixed population of vaccinia strains, and growth of this pool in cell culture might have unintentionally provided selective pressure promoting virulent strains. Furthermore, vaccination with a mixed population could allow more virulent strains to propagate within the host, leading to an increase in the incidence of complications. To this end, Acambis, Inc. (Cambridge, MA) was contracted by the US government to develop and produce a new vaccine from a single purified isolate of vaccinia virus. This article reviews the development, safety profile, immunogenicity, and protective efficacy of ACAM2000™. In addition, the serious adverse events caused by ACAM2000™ will be discussed.
Development and safety of ACAM2000™
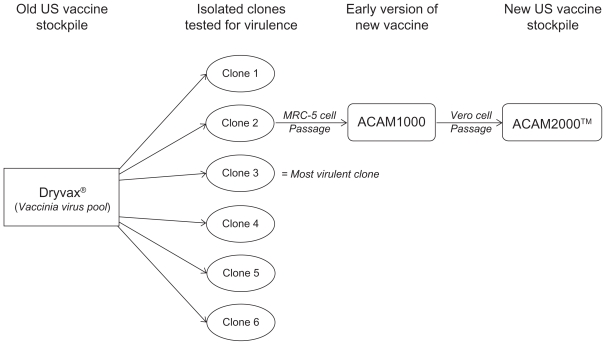
Acambis investigators isolated six individual clones by plaque purification from a pool of 30 vials (3000 doses) of Dryvax®, NYCBH. These clones, designated CL1 through CL6, were tested for virulence in comparison with Dryvax®. The virulence tests included the diameter of erythema and lesions on day eight after scarification of rabbit skin.5 The rabbit scarification model mimics the vaccine “take” observed following human vaccination with Dryvax®, in that a lesion forms at the site of scarification because of local replication of vaccinia virus.6 A second virulence test measured survival time and replication in brain tissue after intracerebral injection of suckling mice.5 The intracerebral suckling mouse model is useful in gauging virulence because strains with a higher incidence of post-vaccinia encephalitis have greater neurovirulence in mice.7–9 Among the six Dryvax®-derived clones, there was significant variation in virulence. Several of the clones were significantly more virulent than Dryvax® in one or more of the assays, with CL3 being the most virulent clone. CL2 had a profile most similar to Dryvax® overall with respect to lesion size and neurovirulence, and was therefore chosen as the new vaccine strain, named ACAM1000 (see Figure 1).5
Figure 1.
Dryvax® consists of a mixed pool of vaccinia virus NYCBH strain. Six vaccinia virus clones were isolated and purified. These were all tested and Clone 2 (CL2) was found to have a virulence profile most closely resembling that of Dryvax®. This clone was propagated on MRC-5 cells and purified to produce the vaccine, named ACAM1000™. Additional expansion was carried out on Vero cells to produce more than 200 million vaccine doses, and the vaccine was renamed ACAM2000™.
Abbreviation: NYCBH, New York City Board of Health.
The goal of the Acambis vaccine initiative was to produce a vaccine that would meet or exceed the safety profile of Dryvax® without sacrificing efficacy. As a test of these criteria, preclinical and clinical safety and efficacy studies compared vaccination of ACAM1000 with Dryvax®. The virulence of ACAM1000 and Dryvax® were evaluated by monitoring survival following intracerebral injection of suckling mice. Mice inoculated with ACAM1000 had a mortality rate that was up to 50% lower than that of Dryvax® in repeated experiments.10 In a comparison of intrathalamic injection of rhesus macaques with 1.25 × 107 pfu of ACAM1000 or 4.9 × 107 pfu of Dryvax®, 50% of the rhesus macaques in the Dryvax® group died, whereas there were no deaths in the ACAM1000 group, with the investigators suggesting that ACAM1000 is less virulent than Dryvax®.10 The difference in virulence could be because of the fourfold larger amount injected into the primates in the Dryvax® group; however, two additional primates were given intrathalamic injection of 1 × 108 pfu of ACAM1000 and did not fall ill. Therefore, ACAM1000 appears to be at least as safe, if not safer, than Dryvax® in animal models.
Efficacy of the vaccinia clones ACAM1000 and CL3 (a virulent clone derived from Dryvax®) was compared with that of Dryvax® using a mouse intranasal challenge model.10 Each vaccine showed comparable survival time after intranasal challenge of mice with vaccinia virus strain WR (Western Reserve) and similar protection against weight loss following challenge with cowpox virus. The major weakness of the vaccinia virus WR model is that the same virus, albeit a different strain, was used as a challenge virus. Consequently, this challenge model does not demonstrate the ability of the live vaccinia virus vaccines to cross protect against different viruses within the Orthopoxvirus family. This issue is addressed, however, by the use of cowpox virus for challenge, which protected against weight loss and death.10 ACAM1000 or CL3 vaccination of mice induced either comparable or increased levels of both neutralizing antibodies and T-cell responses compared with Dryvax®, indicating that ACAM1000 induced acceptable immune responses.10 These results also demonstrate that virulence factors present in CL3, but absent from ACAM1000, do not result in a measurable difference in immunogenicity or efficacy in the mouse model.
In light of the apparent similarity in safety and efficacy of ACAM1000 and Dryvax® in animal models, two Phase I clinical trials were set up with 30 individuals receiving the Dryvax® vaccine and a total of 100 receiving ACAM1000.6,10 A pilot vaccine lot of 750,000 doses of ACAM1000 propagated on MRC-5 cells, a human embryonic lung fibroblast cell line, were produced before this trial. The vaccine was administered by scarification with 15 strokes of a bifurcated needle through a droplet of solution containing 1 × 108 pfu/mL of virus. The results of this study showed that ACAM1000 was similar to Dryvax® in that it elicited a 100% take-rate, 100% seroconversion, and a comparable T-cell response.10
Considering the success of the ACAM1000 vaccine in preclinical and clinical tests, as well as the growing concern of terrorist threats subsequent to the attacks in September of 2001, the US Government increased their contract with Acambis to 209 million vaccine doses. To produce this large quantity with as few passages of ACAM1000 as possible, Acambis partnered with Baxter BioScience (Deerfield, IL) to utilize their large-scale production technology. The new strategy entailed inoculating Vero (African green monkey kidney epithelial) cells with a master seed stock of ACAM1000 (passage 7) and growing them on microcarrier beads in serum-free medium in large 1200-L bioreactors for three passages (see Figure 1).6 The resulting virus stock, renamed ACAM2000™ (passage 10), was partially purified of cell debris by large-pore depth filtration, and cellular genomic material was digested. The lots were tested for bacterial, fungal, and viral contamination and for both human and bovine pathogens.
Double-stranded DNA viruses, such as vaccinia virus, typically have very low rates of mutation from one passage to the next. It is therefore not surprising that the fidelity of the nucleotide sequence was maintained, with passage 10 of ACAM2000™ having an identical genetic sequence to passage 7 of ACAM1000.6 In contrast, the vaccinia strain, VACV-DUKE, was isolated from a patient with progressive vaccinia following Dryvax® vaccination, and was found to have genomic sequence differences from ACAM2000™.11 Unlike ACAM2000™, VACV-DUKE contains a full-length copy of the interferon (IFN)-α/β receptor, which is also present in the virulent clone 3 (CL3). Deletion of this receptor from vaccinia virus WR causes attenuation as measured by intranasal inoculation of mice.12 Additionally, 625 mutational differences were found between CL3 and ACAM2000™.13 These studies also revealed that four virulence factors present in CL3 were absent in ACAM2000™: INF-α/β binding protein, tumor necrosis factor (TNF) receptor, an ankyrin repeat ortholog, and an elongated thymidylate kinase. Therefore, Dryvax® contains a sub-population that produces specific virulence factors, evidence suggesting that ACAM2000™ will be safer than Dryvax®. A possible caveat however is that ACAM2000™ could be less effective than Dryvax® because the missing virulence factors are not presented to the host. Nonetheless, ACAM2000™ retains 100% identity in four open reading frames (ORFs), ORF-99, -161, -167, and -198, known to be important for conferring protective immunity in Copenhagen and WR strains.14–17
Efficacy of ACAM2000™ in animal models
To advance ACAM2000™ to human trials, first a similarity to ACAM1000 and Dryvax® with regard to pathogenicity, induction of immunity, and efficacy had to be demonstrated in animal models. ACAM1000 and ACAM2000™ were both less virulent than Dryvax® in three- to four-day-old suckling mice administered an intracerebral (IC) inoculation with regard to both survival and LD50 analysis.6 ACAM1000 and ACAM2000™ had survival rates of approximately 65%– 70% compared with 10%–20% in the Dryvax®-vaccinated group. Moreover, the lesion size and erythema resulting from either ACAM1000 or ACAM2000™ scarification of rabbit skin was less than or equal to that of Dryvax®.6 Protective efficacy of ACAM1000, ACAM2000™, and Dryvax® vaccination was compared after vaccinia virus WR challenge of BALB/C mice, and the three vaccines provided comparable protection with regard to survival time and amount of vaccine required to achieve 50% survival in a group (see Table 1). Of note, scarification of BALB/C mice with ACAM1000 and ACAM2000™ induced more abundant neutralizing antibodies than Dryvax®, and all three groups had equivalent T-cell responses following scarification of BALB/C mice.6
Table 1.
ACAM2000 efficacy studies in animal models
| Animal species | Vaccinations | Vaccination dose | Challenge virus and dose | Challenge route | Outcome | Reference |
|---|---|---|---|---|---|---|
| BALB/C mice | ACAM2000™ ACAM1000 Dryvax® |
For all 104,105, 106 or 107 pfu/mL | Vaccinia virus-WR strain 100 × LD50 | IN | All vaccines provided equivalent protection | Monath et al6 |
| Cynomolgus macaques | ACAM2000™ Dryvax® |
4.4 × 108 pfu/mL 1.5 × l08 pfu/mL |
Monkeypox virus/Zaire79 3.8 × l07 pfu/mL |
IV | Dryvax® and ACAM2000™ Provided equivalent protection |
Marriott et al22 |
Because of the eradication of smallpox, the true efficacy of vaccine candidates cannot be measured. In 2002, the FDA established the Animal Rule, such that it can rely on the results of animal studies where human trials are not possible or ethical. The most rigorous and clinically relevant model for testing the efficacy of smallpox vaccine candidates is by monkeypox virus challenge of cynomolgus macaques.18–21 Vaccination of cynomolgus macaques with ACAM2000™ or Dryvax® resulted in a 100% take-rate and comparable sizes of vaccination site lesions.22 Additionally, Dryvax® and ACAM2000™ vaccination induced seroconversion in 100% of the cynomolgus macaques and the levels of neutralizing antibodies were comparable between groups. Both vaccines completely protected against death and fever, and almost fully protected against development of rash and presence of virus in throat swabs after intravenous challenge with an otherwise uniformly lethal dose of monkeypox virus (see Table 1). There were more incidents of breakthrough lesions near the inoculation site in the Dryvax® group (three of eight) than in the ACAM2000™ group (one of eight). Additionally, there was breakthrough oral shedding in the Dryvax® vaccinated group (three of eight). However, nearly three times more vaccine was given to the ACAM2000™ group, thus relative efficacy cannot fairly be compared, especially considering that fivefold differences in ACAM2000™ vaccine doses produce markedly different levels of take and antibody response in humans.23 Overall, this study showed that ACAM2000™ provided protection comparable with Dryvax® in a rigorous challenge model, but that neither vaccine offered sterilizing immunity in 100% of the subjects.
ACAM2000™ clinical trials
One hundred vaccinia naïve human subjects were inoculated with 7.7 × 107 pfu/mL of ACAM2000™ in a Phase I trial, with exclusion and inclusion criteria matching that of the previous ACAM1000 and Dryvax® trials (ie, 18–29 years of age, vaccinia-naïve, and with no contraindications to vaccination). ACAM2000™ vaccination resulted in a 99% take-rate, and lesion sizes and induction of neutralizing antibodies comparable with that of ACAM1000.6 In another Phase I trial, 30 vaccinia-naïve subjects per group were inoculated with 1 × 108 pfu/mL of ACAM2000™, ACAM1000, or Dryvax®.24 All subjects had a successful skin reaction or “take” and the majority had positive antibody (96.7% of ACAM2000™ and Dryvax® groups, and 90% of ACAM1000 subjects seroconverted) and T-cell responses. ACAM1000 and ACAM2000™ have equivalent safety and efficacy profiles, therefore a request was made by the US Government in 2003 for future studies to be done with ACAM2000™ alone.
Two Phase II trials were carried out to compare ACAM2000™ and Dryvax®, one in vaccinia-naïve subjects and one in previously vaccinated “experienced” subjects. To determine the lowest effective dose, defined as having a greater than 90% take-rate, these trials compared Dryvax® at the standard dose, to four different doses of ACAM2000™.23 The dose of Dryvax® was 1.6 × 108 pfu/mL and the starting dose of ACAM2000™ was 2.3-fold lower at 6.8 × 107 pfu/mL. Additional groups were given 1:5, 1:10, or 1:20 dilutions of ACAM2000™. All naïve individuals vaccinated with Dryvax® or the highest dose of ACAM2000™ experienced a take, and 96% and 94% seroconverted, respectively, with comparable levels of neutralizing antibody. In contrast, the naïve groups receiving 1:5, 1:10, or 1:20 dilutions of ACAM2000™ had take-rates of 86%, 80%, and 59%, respectively; below the threshold set for efficacy. Therefore, vaccination with 6.8 × 107 pfu/mL of ACAM2000™ is as effective as a 1.6 × 108 pfu/mL dose of Dryvax® in vaccinia-naïve subjects. However, in contrast with Dryvax®, which causes a cutaneous reaction in over 97% of vaccinees even when diluted up to 10-fold (approximately 1 × 107 pfu/mL), a fivefold dilution (1.4 × 107 pfu/mL) of ACAM2000™ failed to offer the requisite 90% take-rate in the vaccinia-naïve group.25 The Phase II clinical trial in vaccinia-experienced subjects showed that the group receiving the highest dose of ACAM2000™ (6.8 × 107 pfu/mL) had an 88% take-rate, compared with 100% in the Dryvax® group (1.6 × 108 pfu/mL). Therefore, ACAM2000™ was not as effective for revaccination as Dryvax®, possibly because the dose of ACAM2000™ was more than two-fold less than that of Dryvax®. Unfortunately, the doses of ACAM2000™ and Dryvax® were not the same in a number of preclinical and clinical studies, which complicates the results because two- or more-fold differences could result in significantly different outcomes.
Two Phase III trials were set up comparing ACAM2000™ (with doses ranging from 1.3–2.2 × 108 pfu/mL) to Dryvax® (1.5 × 108 pfu/mL); one trial for vaccinia-naïve subjects and the other trial for vaccinia-experienced subjects.5,24 In vaccinia-naïve subjects, the take-rates were 96% and 99% for ACAM2000™ and Dryvax®, respectively, indicating that both formulations were effective, although the antibody titers of ACAM2000™ vaccinated subjects were inferior to those receiving Dryvax®. In the Phase III trial for vaccinia-experienced subjects, the ACAM2000™ and Dryvax® groups had 84% and 98% take-rates, respectively, with both vaccines inducing neutralizing antibodies, albeit at higher titers for Dryvax®. Thus, Phase III trials confirmed the results of Phase II trials in that ACAM2000™ is as effective as Dryvax® for vaccination of vaccinia-naïve individuals, but that Dryvax® is superior to ACAM2000™ for revaccination. This phenomenon is most likely because of the decreased level of virulence of ACAM2000™ compared with Dryvax®, such that previously vaccinated individuals were more likely to mount an immune response sufficient to prevent viral replication of ACAM2000™ than that of Dryvax®.
Immunogenicity of ACAM2000™
With regard to induction of protective immune response, both humoral and cellular immune responses play important roles in protecting against poxvirus challenge in animal models and in clinical settings.26 Historically, antibody responses neutralizing virus at dilutions greater than 1:32 were shown to be crucial for protection against smallpox.27 The importance of antibody in protection is highlighted by the observation that vaccinia immune globulin can ameliorate complications from vaccination and can protect against smallpox infection.28 Additionally, in the rhesus macaque monkeypox infection model, immune antibodies are required for protection against monkeypox and vaccine-induced antibodies alone are sufficient for protection against challenge.18,19 ACAM2000™ vaccination resulted in a less robust antibody response than Dryvax® in one Phase I clinical trial (vaccinia-naïve), one of the two Phase II trials (vaccinia-experienced) and both Phase III trials (vaccinia-naïve and vaccinia-experienced). 6,24,29 While ACAM2000™ inoculation generally resulted in a four-fold increase in neutralizing antibodies, the titer was approximately 40% less than that of Dryvax®-vaccinated individuals. Only one study, a Phase II clinical trial in vaccinia-naïve subjects, demonstrated a comparable antibody response between ACAM2000™ and Dryvax® groups.23 However, because most subjects vaccinated with ACAM2000™ developed the requisite four-fold increase in neutralizing antibodies, this would likely offer significant protection in the event of an exposure.
The importance of the cellular immune response in controlling infection of poxviruses is made evident by individuals whose defective cellular immune response predisposes them to generalized vaccinia upon vaccination.30 Generally, Dryvax® vaccination stimulates CD4+ and CD8+ T-cell responses that are stable for decades.31,32 In a Phase I clinical trial, ACAM2000™ and Dryvax® vaccination both induced positive responses in at least one assay for cell-mediated immunity in 100% and 93% of subjects, respectively.24
An issue that has not yet been addressed is that these studies were all done in adults (18 years and older), and the safety and efficacy of ACAM2000™ relative to Dryvax® is unknown in children. Additionally, studies of ACAM2000™ efficacy for post-exposure vaccination in animal models have not yet been reported in the literature. Administration of vaccinia virus within four days of exposure may offer protection based on historical clinical data from smallpox outbreaks.33 Nonetheless, Acambis received FDA approval for ACAM2000™ in 2007 and signed a 10-year contract with the US government in April 2008 for continued production for the US Strategic National Stockpile.
Adverse events with ACAM2000™
Vaccination against smallpox using live vaccinia virus has historically caused a number of different adverse events.2,34,35 The adverse reactions range in severity and typically involve skin, eye, cardiac tissue, or in extremely rare cases, the nervous system. Cutaneous reactions include uticaria, rash, autoinoculation, eczema vaccinatum, generalized vaccinia, and progressive vaccinia (previously termed “vaccinia necrosum”). Ocular vaccinia is a common manifestation of auto-inoculation. Post-vaccinial encephalitis is a rare but potentially fatal complication. The rates of complications vary by age, with serious adverse events (SAEs) generally occurring at a greater rate among the very young, particularly those less than 12 months old, than in older children and adults.
Whereas cardiac events had been reported in the literature before 2003, they were largely unrecognized during the worldwide eradication campaign and were thought to occur very rarely. Only six cases of cardiac complications after smallpox vaccination with the NYCBH strain of vaccinia had been reported in the US before 2003.36 In the past decade, cardiac complications following live vaccinia vaccination have been detected more often. This increase in detection is because of the availability of more sophisticated diagnostic techniques. Cardiac complications resulting from live vaccinia vaccination range in severity from mild to fatal and include myocarditis, pericarditis, arrhythmias, and dilated cardiomyopathy (DCM). Myocarditis is an inflammation of the heart muscle without blockage of the coronary arteries, and pericarditis is an inflammation of the fibrous sack surrounding the heart muscle. DCM is characterized by an enlarged and weakened heart muscle. Myocarditis and pericarditis, also collectively referred to as myopericarditis, can cause palpitations, shortness of breath, fever, sweats, or chest pain and can be diagnosed by an abnormal electrocardiogram (ECG), imaging studies ( echocardiogram), histopathology, or elevated cardiac enzymes. These inflammatory processes can be caused by a number of viral infections and autoimmune disorders, and have sequelae ranging from self-limiting asymptomatic disease to DCM, resulting in fulminant congestive heart failure and possibly death. Interestingly, myocarditis is blamed for causing up to 20% of all cases of sudden death among military recruits.37
To enhance preparedness in the event of an intentional release of smallpox, a 2002 presidential initiative recommended vaccination of enlisted military members, and voluntary participation for civilian health care workers with potential to be first responders. Military and civilian populations were vaccinated with Dryvax® under the guidance of the Department of Defense (DOD) and the Department of Health and Human Services (HHS), respectively.38 By June of 2004, 39,566 civilians had been vaccinated, and by September 2006, more than 1.1 million soldiers were vaccinated. The occurrence of adverse events in both civilian and military populations was carefully monitored. 39,40 Compared with the historical rates of SAEs in the US reported before the 1970s, the rates of events such as generalized vaccinia and autoinoculation were comparable. Cases of progressive vaccinia, eczema vaccinatum, and fetal vaccinia were completely avoided by careful screening of potential vaccinees, and attempting to limit vaccination to those without immunodeficiencies, eczema, or pregnancy. Among 730,580 DOD vaccinees, three cases of post-vaccinial encephalitis and 43 cases of mild generalized vaccinia occurred.41 Complications from vaccination were much less frequent in previously vaccinated individuals than those that were vaccinia-naïve.
While the frequencies of most SAEs were anticipated based on historical findings, a surprisingly large number of cardiac complications were reported in both civilian and military cohorts in the 2003 vaccination campaign. One study that compared US soldiers who received live vaccinia vaccination with unvaccinated soldiers from South Korea showed a similar number of hospitalizations and cases of chest pain between the two groups, suggesting that the high rate of cardiac events was no greater than the baseline of a population.39 However, the occurrence of the vast majority of cardiac adverse events within 30 days of vaccination, and clustering within 7–12 days post-vaccination, suggests a direct link between vaccination with live vaccinia virus and incidence of cardiac complications.40 Of 730,580 US armed forces personnel vaccinated with Dryvax®, 86 cases of myopericarditis with moderate or severe clinical presentation occurred in otherwise healthy vaccinees.42 The single fatal case of myocarditis was in a female. An earlier report calculated a rate of myopericarditis 7.5-fold higher than the expected background rate among 347,516 primary vaccinees (56 cases, at a rate of 161 per million).43 Of 37,901 HHS vaccinees, 21 civilians were diagnosed with myopericarditis (at a rate of 554 per million), all of which were mild cases that resolved without further complications.36 Additionally, four DOD and three HHS cases of DCM occurred among previously healthy subjects, with two requiring heart transplants. 40
Ten patients among HHS vaccinees experienced ischemic cardiac events (ICEs), which are characterized as cardiac damage by a mechanism of constriction or blockage of blood flow. Seven of these patients had pre-existing cardiac risk factors, and two cases resulted in death.44 Of DOD vaccinees, 16 cases of ICE occurred with three fatalities. The rates of cardiac events were so high within the first two months of the HHS vaccination program, the CDC formed a cardiac team to specifically monitor cardiac-related SAEs. From the time this correlation was recognized, patients were pre-screened and those with at least three cardiac risk factors were deferred from vaccination. Possibly because of this screening, no additional ICEs were reported in the final 6638 HHS vaccinees.
Serious and nonserious adverse events following vaccination of 2983 people with ACAM2000™ (1307 naïve and 1676 experienced) were compared with that of Dryvax®.5 With regard to overall common adverse events such as flu-like symptoms, lymph node pain, and reaction at the vaccination site, 99% of ACAM2000™ vaccinees had at least one adverse event compared with 100% of those receiving Dryvax®. In general, the individuals vaccinated with ACAM2000™ had a slightly lower, but statistically significant, incidence of several specific adverse events (including lymph node pain, injection site pain and pruritus). Additionally, the rates of flu-like symptoms among ACAM2000™-vaccinated subjects were lower for experienced vaccinees compared with the vaccinia-naïve group (55% versus 76%).5
With the higher than expected rate of cardiac complications in the HHS and DOD vaccination programs, the ACAM2000™ vaccine trials closely monitored patients by performing ECGs and serum tests for troponin I enzyme levels on all subjects in two Phase III clinical trials and in one Phase I clinical trial. The result was astounding in that people vaccinated with either ACAM2000™ or Dryvax® had ECG and enzyme levels fitting a diagnosis of either myocarditis or pericarditis at a rate of more than 10 times that seen in the recent DOD and HHS vaccinations. In vaccinia-naïve subjects, myopericarditis occurred at a rate of 5730 per million (seven cases in 1307 subjects) in ACAM2000™ vaccinees, and 1038 per million (three cases in 363 subjects) in Dryvax® vaccines respectively.5 No cases of myopericarditis occurred among 1819 vaccinia-experienced subjects vaccinated with either vaccine.40 The rates of myopericarditis for ACAM2000™ and Dryvax® were not statistically different in these trials.5 Thus, ACAM2000™ does not offer a significant reduction in cardiac adverse events compared with Dryvax®. A second important conclusion from these trials is that vaccination with either strain of NYCBH-derived live vaccinia virus results in a much higher rate of cardiac complications than previously thought, despite limiting enrollment into the studies to subjects with no known cardiac risk factors. The increased detection of adverse cardiac events can be attributed to the use of ECG and enzyme tests, as well as surveying for cardiac symptoms on post-vaccination questionnaires. Surveillance studies of adverse events after smallpox vaccination in the US in 1968 did not include questions related to possible cardiac events.45 Interestingly, many of the cases of myocarditis and pericarditis detected by the advanced screening were sub-clinical or asymptomatic.
In a Phase I clinical trial, 18.9% (17 of 90) of subjects vaccinated with Dryvax®, ACAM1000 or ACAM2000™ had a biologic false positive (BFP) syphilis test.46 The association of smallpox vaccination with a BFP syphilis test result has long been known, and has been reported to occur in as few as 4% or as many as 45% of vaccine recipients.47,48 A BFP syphilis test result also occurs in many individuals with autoimmune disorders such as lupus, or infections such as HIV or parvovirus B19.49 Interestingly, patients with lupus or parvovirus B19 infections are predisposed to myocarditis and pericarditis as well as other cardiac complications.50,51 The BFP syphilis test result indicates an individual has developed autoimmune antiphospholipid antibodies. Only a subset of ACAM2000™ or Dryvax® vaccinees with a BFP syphilis test result had myocarditis or pericarditis, and not all vaccinees with cardiac complications had a BFP syphilis test result. Thus, a direct correlation between antiphospholipid antibodies and myocarditis or pericarditis was not found among vaccinees; however, it is possible that the study was too small for the results to reach statistical significance. A causative role of antiphospholipid antibody responses and myocarditis and pericarditis has been reported for lupus.52 The possible association of vaccination, antiphospholipid antibodies, and cardiac adverse events could reveal a mechanism by which live vaccinia vaccination causes cardiac damage and warrants further study. Of particular concern is that acute infectious myocarditis has been reported to result in lasting cardiac damage.53
The antiviral compound ST-246 inhibits viral replication in ACAM2000™-vaccinated mice.54 Another antiviral compound, cidofovir, reduced Dryvax® vaccination side effects in cynomolgus macaques, but also compromised the protection against monkeypox challenge.55 Thus, co-administration of antiviral drugs may help reduce the frequency and severity of SAEs following vaccination with ACAM2000™ or other live vaccinia strains, but may also interfere with protective efficacy. Alternatively, a vaccine with a stronger safety profile, possibly attenuated vaccinia vaccines such as modified vaccinia Ankara (MVA), LC16m8, or subunit vaccine formulations, could be used as a first vaccination, followed by vaccination with ACAM2000™.
Conclusions
Vaccination with Dryvax® ended in 1972 for the public and in 1976 for health care workers, while the US military continued vaccination until 1989. The US vaccination policy changed in 2002, requiring vaccination for all military personnel and for “smallpox response teams” composed of civilian public health care staff. The remaining doses of Dryvax® were destroyed in 2008, with the national stockpile now consisting of over 200 million doses of ACAM2000™. Currently ACAM2000™ is used for all DOD personnel. Although ACAM2000™ has safety and efficacy data similar to that of Dryvax® in preclinical and clinical studies, there are still concerns related to SAEs, such as cardiac complications. In case of necessary global vaccination after unintentional or intentional release of variola virus, as many as one in 145 vaccinees could be expected to develop myopericarditis, the seriousness of which is not entirely understood.40 In this event, meticulous screening, education of vaccinees, and co-administration of antiviral drugs, should greatly reduce the frequency and severity of SAEs. However, there is still a need for a safer smallpox vaccine for the general population and an alternative approach is especially needed for the large number of immunocompromised individuals, infants, and others with contraindications, for whom ACAM2000™ cannot be given.
Footnotes
Disclosures
The authors report no conflict of interest in this work.
Disclaimer
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the US Army or the Department of Defense.
References
- 1.Fenner F World Health Organization. Smallpox and its eradication. Geneva: World Health Organization; 1988. [Google Scholar]
- 2.Schwartz B, Lebwohl M. Complications of the smallpox vaccine. Int J Dermatol. 2005;44(4):289–292. doi: 10.1111/j.1365-4632.2004.02568.x. [DOI] [PubMed] [Google Scholar]
- 3.Henderson DA. The inside story of eradicating a worldwide killer. Amherst: Prometheus Books; 2009. Smallpox: The death of a disease. [Google Scholar]
- 4.Kretzschmar M, Wallinga J, Teunis P, Xing S, Mikolajczyk R. Frequency of adverse events after vaccination with different vaccinia strains. PLoS Med. 2006;3(8):e272. doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acambis I FDA C. ACAM2000™ smallpox vaccine: Vaccines and Related Biological Products Advisory Committee (VRBPAC) 2007. [Google Scholar]
- 6.Monath TP, Caldwell JR, Mundt W, et al. ACAM2000™ clonal Vero cell culture vaccinia virus (New York City Board of Health strain) – a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8(Suppl 2):S31–S44. doi: 10.1016/j.ijid.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marennikova SSCK, Maltseva NN, Shelukhina EM, Fedorov VV. Proceedings of: the symposium on smallpox. Yugoslavia Academy of Sciences and Arts; Zagreb, Yugoslavia. 2–3 September 1969. [Google Scholar]
- 8.Marennikova SSCK, Maltseva NN, Shelukhina EM, Fedorov VV. International symposium on smallpox vaccine 1972. Bilthoven, The Netherlands: Karger, Basel; Oct, 1973. [Google Scholar]
- 9.Handley L, Buller RM, Frey SE, Bellone C, Parker S. The new ACAM2000™ vaccine and other therapies to control orthopoxvirus outbreaks and bioterror attacks. Expert Rev Vaccines. 2009;8(7):841–850. doi: 10.1586/erv.09.55. [DOI] [PubMed] [Google Scholar]
- 10.Weltzin R, Liu J, Pugachev KV, et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat Med. 2003;9(9):1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Chen N, Feng Z, et al. Genomic sequence and analysis of a vaccinia virus isolate from a patient with a smallpox vaccine-related complication. Virol J. 2006;3:88. doi: 10.1186/1743-422X-3-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Symons JA, Alcami A, Smith GL. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81(4):551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 13.Osborne JD, Da Silva M, Frace AM, et al. Genomic differences of Vaccinia virus clones from Dryvax® smallpox vaccine: The Dryvax®- like ACAM2000™ and the mouse neurovirulent Clone-3. Vaccine. 2007;25(52):8807–8832. doi: 10.1016/j.vaccine.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Aldaz-Carroll L, Ortiz AM, et al. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine. 2007;25(7):1214–1224. doi: 10.1016/j.vaccine.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z, Earl P, Americo J, et al. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc Natl Acad Sci U S A. 2006;103(6):1882–1887. doi: 10.1073/pnas.0510598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25(10):1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornburg NJ, Ray CA, Collier ML, Liao HX, Pickup DJ, Johnston RE. Vaccination with Venezuelan equine encephalitis replicons encoding cowpox virus structural proteins protects mice from intranasal cowpox virus challenge. Virology. 2007;362(2):441–452. doi: 10.1016/j.virol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11(7):740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 19.Edghill-Smith Y, Bray M, Whitehouse CA, et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191(3):372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 20.Nigam P, Earl PL, Americo JL, et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366(1):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saijo M, Ami Y, Suzaki Y, et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80(11):5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marriott KA, Parkinson CV, Morefield SI, Davenport R, Nichols R, Monath TP. Clonal vaccinia virus grown in cell culture fully protects monkeys from lethal monkeypox challenge. Vaccine. 2008;26(4):581–588. doi: 10.1016/j.vaccine.2007.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Artenstein AW, Johnson C, Marbury TC, et al. A novel, cell culture- derived smallpox vaccine in vaccinia-naïve adults. Vaccine. 2005;23(25):3301–3309. doi: 10.1016/j.vaccine.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 24.Frey SE, Newman FK, Kennedy JS, et al. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000™ and Dryvax® in healthy vaccinia-naïve adults. Vaccine. 2009;27(10):1637–1644. doi: 10.1016/j.vaccine.2008.11.079. [DOI] [PubMed] [Google Scholar]
- 25.Frey SE, Couch RB, Tacket CO, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346(17):1265–1274. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy RB, Ovsyannikova IG, Jacobson RM, Poland GA. The immunology of smallpox vaccines. Curr Opin Immunol. 2009;21(3):314–320. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack TM, Noble J, Jr, Thomas DB. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21(2):214–218. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins RJ, Lane JM. Clinical efficacy of intramuscular vaccinia immune globulin: A literature review. Clin Infect Dis. 2004;39(6):819–826. doi: 10.1086/422999. [DOI] [PubMed] [Google Scholar]
- 29.Acambis I. Package insert. ACAM2000™ smallpox (vaccinia) vaccine, live. 2008 [Google Scholar]
- 30.Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968: Results of ten statewide surveys. J Infect Dis. 1970;122(4):303–309. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- 31.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 32.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 33.Mortimer PP. Can postexposure vaccination against smallpox succeed? Clin Infect Dis. 2003;36(5):622–629. doi: 10.1086/374054. [DOI] [PubMed] [Google Scholar]
- 34.Wollenberg A, Engler R. Smallpox, vaccination and adverse reactions to smallpox vaccine. Curr Opin Allergy Clin Immunol. 2004;4(4):271–275. doi: 10.1097/01.all.0000136758.66442.28. [DOI] [PubMed] [Google Scholar]
- 35.Aragon TJ, Ulrich S, Fernyak S, Rutherford GW. Risks of serious complications and death from smallpox vaccination: A systematic review of the United States experience, 1963–1968. BMC Public Health. 2003;3:26. doi: 10.1186/1471-2458-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgan J, Roper MH, Sperling L, et al. Myocarditis, pericarditis, and dilated cardiomyopathy after smallpox vaccination among civilians in the United States, January–October 2003. Clin Infect Dis. 2008;46(Suppl 3):S242–S250. doi: 10.1086/524747. [DOI] [PubMed] [Google Scholar]
- 37.Eckart RE, Scoville SL, Campbell CL, et al. Sudden death in young adults: A 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141(11):829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 38.Webpage TMVAMH. [Accessed on 4 December 2009]. Available at www.vaccines.mil.
- 39.Eckart RE, Shry EA, Atwood JE, et al. Smallpox vaccination and ischemic coronary events in healthy adults. Vaccine. 2007;25(50):8359–8364. doi: 10.1016/j.vaccine.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 40.Neff J, Modlin J, Birkhead GS, et al. Monitoring the safety of a smallpox vaccination program in the United States: Report of the Joint Smallpox Vaccine Safety Working Group of the advisory committee on immunization practices and the Armed Forces Epidemiological Board. Clin Infect Dis. 2008;46(Suppl 3):S258–S270. doi: 10.1086/524749. [DOI] [PubMed] [Google Scholar]
- 41.Van Dam CN, Syed S, Eron JJ, et al. Severe postvaccinia encephalitis with acute disseminated encephalomyelitis: Recovery with early intravenous immunoglobulin, high-dose steroids, and vaccinia immunoglobulin. Clin Infect Dis. 2009;48(4):e47–e49. doi: 10.1086/596553. [DOI] [PubMed] [Google Scholar]
- 42.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: A review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23(17–18):2078–2081. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Arness MK, Eckart RE, Love SS, et al. Myopericarditis following smallpox vaccination. Am J Epidemiol. 2004;160(7):642–651. doi: 10.1093/aje/kwh269. [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow DL, Roper MH, Morgan J, et al. Ischemic cardiac events during the Department of Health and Human Services Smallpox Vaccination Program, 2003. Clin Infect Dis. 2008;46(Suppl 3):S234–S241. doi: 10.1086/524745. [DOI] [PubMed] [Google Scholar]
- 45.Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med. 1969;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- 46.Monath TP, Frey SE. Possible autoimmune reactions following smallpox vaccination: The biologic false positive test for syphilis. Vaccine. 2009;27(10):1645–1650. doi: 10.1016/j.vaccine.2008.10.084. [DOI] [PubMed] [Google Scholar]
- 47.Lynch FW, Kimball AC, Kernan PD. Serologic tests for syphilis following smallpox vaccination and including Reiter protein complement fixation technic. J Invest Dermatol. 1960;34:219–222. doi: 10.1038/jid.1960.33. [DOI] [PubMed] [Google Scholar]
- 48.Grossman LJ, Peery TM. Biologically false-positive serologic tests for syphilis due to smallpox vaccination. Am J Clin Pathol. 1969;51(3):375–378. doi: 10.1093/ajcp/51.3.375. [DOI] [PubMed] [Google Scholar]
- 49.Harris EN, Pierangeli SS, Gharavi AE. Diagnosis of the antiphospholipid syndrome: A proposal for use of laboratory tests. Lupus. 1998;7(Suppl 2):S144–S148. doi: 10.1177/096120339800700232. [DOI] [PubMed] [Google Scholar]
- 50.Jain D, Halushka MK. Cardiac pathology of systemic lupus erythematosus. J Clin Pathol. 2009;62(7):584–592. doi: 10.1136/jcp.2009.064311. [DOI] [PubMed] [Google Scholar]
- 51.von Landenberg P, Lehmann HW, Modrow S. Human parvovirus B19 infection and antiphospholipid antibodies. Autoimmun Rev. 2007;6(5):278–285. doi: 10.1016/j.autrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Leung WH, Wong KL, Lau CP, Wong CK, Liu HW. Association between antiphospholipid antibodies and cardiac abnormalities in patients with systemic lupus erythematosus. Am J Med. 1990;89(4):411–419. doi: 10.1007/BF01453668. [DOI] [PubMed] [Google Scholar]
- 53.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- 54.Berhanu A, King DS, Mosier S, et al. ST-246 inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestation. Antimicrob Agents Chemother. 2009;53(12):4999–5009. doi: 10.1128/AAC.00678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei H, Huang D, Fortman J, Wang R, Shao L, Chen ZW. Coadministration of cidofovir and smallpox vaccine reduced vaccination side effects but interfered with vaccine-elicited immune responses and immunity to monkeypox. J Virol. 2009;83(2):1115–1125. doi: 10.1128/JVI.00984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]