Abstract
Ultrafiltration and a series of chromatographic steps were used to isolate and purify polysaccharides from Tremella aurantialba fruit bodies. Three crude fractions (TAP50w, TAP10–50w, and TAP1–10w), five semi-purified fractions (TAPA–TAPE), and one purified fraction (TAPA1) were obtained. A sulfated derivative of TAPA1 (TAPA1-s) was prepared by chemical modification. The immunostimulating activity of the polysaccharide fractions in vitro was determined using the mouse spleen lymphocyte proliferation assay. Of the three crude fractions tested, cell proliferation rates were increased most by TAP50w. Furthermore, TAPA1-s was markedly more stimulatory than TAPA1, indicating that sulfonation was an effective way to enhance the immunostimulating activity of polysaccharide.
Keywords: Tremella aurantialba, Purification, Polysaccharide, Sulfation, Immunostimulating activity
1. Introduction
Members of the genus Tremella, belonging to the order Tremellales and the family Tremellaceae, have attracted growing interest in the biomedical field, mainly due to their reported pharmacological properties, which include immunoenhancing (Ma and Lin, 1992), anti-tumor (Ukai et al., 1972), anti-hypoglycemic (Kiho et al., 2001; Wang et al., 2002) and anti-diabetic activities (Kiho et al., 2001; Zhang et al., 2004). In China, ‘Tremella polysaccharide’ has been used for cancer prevention and for stimulating the immune system (de Baets et al., 2002). Compared with other Tremella species such as T. mesenterica, T. fuciformis, and T. aurantia (de Baets and Vandamme, 2001), however, relatively little is known about the nature and bioactivity of polysaccharides extracted from fruit bodies of Tremella aurantialba Bandoni and Zang, a highly valued edible and medicinal fungus. In order to determine the development potential of this fungus, and since immunomodulation is a basic function associated with many polysaccharides (Su et al., 2006), we evaluated the immunomodulatory activity of T. aurantialba polysaccharide.
Most traditional methods used to extract and purify fungal polysaccharides are time-consuming and require large volumes of organic solvents. Therefore, in this study we have applied more advanced procedures, including ultrafiltration, anion-exchange chromatography, and gel chromatography, to isolate and purify polysaccharides from T. aurantialba. These procedures avoid excessive loss of polysaccharide material and avert structural changes to the samples that occasionally occur when chemical reagents are used.
We have obtained three crude polysaccharide fractions, five partially-purified polysaccharide fractions, and a single purified polysaccharide fraction, TAPA1. Since sulfated polysaccharide is reported to exhibit enhanced and/or more diverse biological properties compared with non-sulfated polysaccharide (Nie et al., 2006; Peng et al., 2005), a sulfated derivative of TAPA1 (TAPA1-s) was prepared by chemical modification in order to further investigate structure-activity relationships. In vitro immunostimulating activity of each fraction was determined using the mouse spleen lymphocyte proliferation assay.
2. Materials and methods
2.1. Materials
Fruit bodies of T. aurantialba were provided by the Kunming Edible Fungi Institute of General National Supply and Marketing Cooperative of the People’s Republic of China. DEAE-Sepharose™ Fast Flow and High-Resolution Sephacryl S-500 were purchased from Amersham Pharmacia Company (Sweden). The standard monosaccharides and dextrans were from Sigma-Aldrich Company (USA). Phytohemagglutinin (PHA), penicillin, and streptomycin were from Amersco Company (USA). RPMI-1640 medium and fetal bovine serum (FBS) were from Gibco Company (USA). Alamar Blue™ reagent was from Biosource International Company (USA). All other reagents were of analytical reagent grade and from Chinese sources.
2.2. Isolation and purification of polysaccharides
2.2.1. Extraction of crude polysaccharides
Dried T. aurantialba fruit bodies (3 kg) were mechanically chopped into small pieces and immersed in 30 L 95% (v/v) ethanol. After 24 h, the solid residue was collected by filtration and the procedure was repeated twice in order to remove lipid material. The residue was air-dried and then extracted with hot water using a 50-L extractor vessel. Optimum extraction conditions (temperature, solid:water ratio, extraction time, number of extractions) were determined by orthogonal experimentation.
2.2.2. Fractionation of crude polysaccharides by ultrafiltration
Aqueous extracts were combined and fractionated on the basis of molecular weight by ultrafiltration using a Millipore™ ultrafiltration system equipped sequentially with 0.1-m2 membranes of 500, 100, and 10 kDa molecular weight cut-off (Millipore, USA). The end-point of each ultrafiltration cycle was indicated when the electrical conductance value of the feed solution was almost identical with that of distilled water (39.4 µS/cm). The three crude polysaccharide fractions obtained were designated TAP50w, TAP10–50w, and TAP1–10w, respectively.
2.2.3. Purification of polysaccharides by anion-exchange chromatography
TAP50w was dissolved in distilled water (8 mg/ml), centrifuged (400×g, 10 min), and the supernatant was applied to a DEAE-Sepharose™ Fast Flow column (XK 26 mm×100 cm). The column was eluted first with filtered (0.45-µm membrane) distilled water and then with a 0–2.0 mol/L NaCl gradient (2340 ml) at a flow rate of 4 ml/min. Fractions (15 ml) were collected, and polysaccharides were detected using the phenol-sulfuric acid method (Dubois et al., 1956). Fractions corresponding to individual peaks were combined and five samples of semi-purified polysaccharides (TAPA–TAPE) were obtained.
2.2.4. Further purification of fraction TAPA by gel chromatography
Fraction TAPA was further purified by gel permeation chromatography using a High-Resolution Sephacryl S-500 column (XK 16 mm×100 cm) attached to an ÄKTA Explorer chromatography system fitted with a refractive index detector (RID-10 A, Shimadzu, Japan). Aliquots (1 ml) were applied to the column, which was eluted with 0.2 mol/L NaNO3 at a flow rate of 0.5 ml/min.
2.3. Determination of purity and molecular weight
Homogeneity and the molecular mass of the purified sample were estimated as described by Du et al. (2009).
2.4. Preparation of the sulfated derivative TAPA1-s
TAPA1-s was prepared according to the modified method described previously (Inoue et al., 1983; Cui et al., 2008). Briefly, TAPA1 (100 mg) was suspended in anhydrous formamide (10 ml) by stirring at room temperature for 15 min followed by addition of 2 ml sulfating reagents (286 µl chlorosulfonic acid (CSA) and 1.714 ml anhydrous pyridine in a ratio of 1:6 (v/v)). The mixture was maintained at room temperature for 2 h with continuous stirring, and then incubated at 30 °C for 5 h. After rapid cooling to room temperature, the solution was neutralized with 15% (w/v) aqueous NaOH, dialyzed, concentrated, and lyophilized to obtain the sulfated derivative TAPA1-s (Cui et al., 2008).
2.5. Determination of the sulfate content in TAPA1-s
The sulfate content of TAPA1-s was estimated according to the BaCl2-gelatin method described previously (Chaidedgumjorn et al., 2002). The degree of substitution (DS) was calculated from 162×C S/(96−80×C S), where C S (%) is the content of SO4 2− (Cui et al., 2008).
2.6. Proliferation of mice spleen lymphocytes in vitro
2.6.1. Preparation of mice spleen lymphocytes
C57 BL/6 male mice (aged 8–10 weeks) were killed by cervical dislocation, and the spleens removed and washed three times with phosphate buffered saline, cut into small pieces, and then pressed through a stainless steel mesh (100 mesh) to obtain a single spleen cell suspension. After centrifugation (400×g, 6 min), red cells in the spleen cell suspension were lysed with Tris-HCl-NH4Cl solution (pH 7.2) (Zhang et al., 2002). The cell suspension was further diluted with 5 vols of RPMI-1640 medium and, after mixing and centrifugation, the pelleted cells were resuspended in RPMI-1640 medium to a concentration of 2×106 cells/ml.
2.6.2. Spleen cell proliferation assay
Aliquots (180 µl) of the cell suspension and 20 µl of different test agents were added to each well of a 96-well plate. PBS and PHA (6 μg/ml) served as the negative and positive controls, respectively. After incubation at 37 °C in a 5% CO2 atmosphere for 72 h, 20 µl Alamar blue reagent (Biosource, Nivelles, Belgium) was added to each well and the incubation continued for another 6 h. Absorption values at 570 nm (A 570) and 600 nm (A 600) were measured using a micro enzyme-linked immunosorbent assay (ELISA) autoreader. The proliferation rate (r) was calculated according to the following formula described previously (Shen et al., 2008): r (%)=[117216×A 570 (sample)−80856×A 600 (sample)]/[117216×A 570 (control)−80856×A 600 (control)]×100%.
2.7. Statistical analysis
All data are presented as mean±standard deviation (SD) of three determinations. Data were analyzed using STST2 statistical software (developed by Nanjing Agricultural University, China). Statistical analyses were performed using the Student’s t-test.
3. Results and discussion
3.1. Extraction and ultrafiltration of T. aurantialba polysaccharides
T. aurantialba fruit bodies were pre-treated with 95% (v/v) ethanol to remove lipophilic substances and low molecular weight materials that might interfere with the polysaccharide extraction process. Orthogonal experiments revealed that the optimum conditions for hot water extraction were as follows: temperature, 100 °C; number of extractions, 3; solid: liquid ratio, 1:10; extraction time, 3 h. Three crude polysaccharide fractions (TAP50w, TAP10–50w, and TAP1–10w) were obtained by subsequent ultrafiltration (Table 1). TAP50w was obtained in highest yield (3.67%), had a high polysaccharide content (62.2%), and was selected for further study.
Table 1.
Fractionation of T. aurantialba polysaccharides by ultrafiltration
| Fraction | Molecular weight range (kDa) | Yield (%) | Polysaccharide content (%) |
| TAP50w | >500 | 3.67 | 62.2 |
| TAP10–50w | 100–500 | 0.03 | 66.0 |
| TAP1–10w | 10–100 | 0.14 | 20.4 |
3.2. Purification of fraction TAP50w
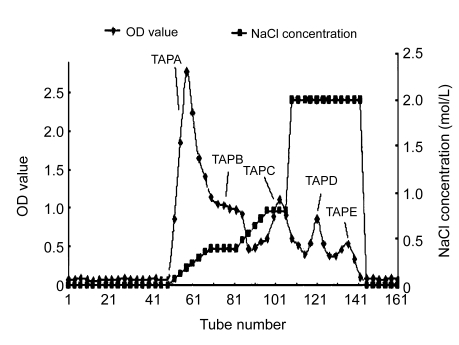
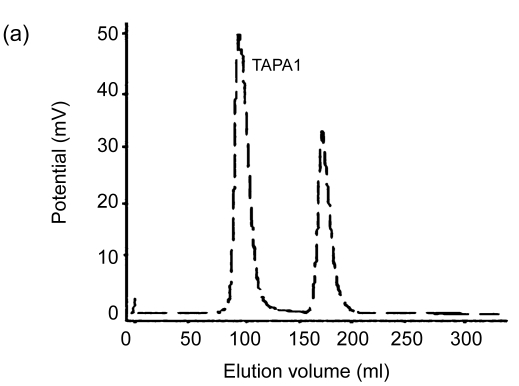
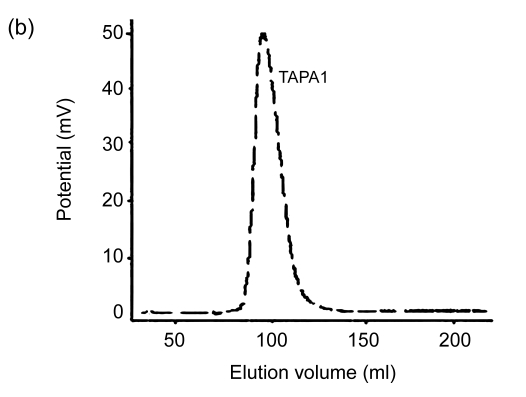
Lyophilized TAP50w fraction yielded five peaks, namely TAPA–TAPE, following chromatography on a DEAE-Sepharose™ Fast Flow anion-exchange column and elution with a NaCl gradient (Fig. 1). Yields of TAPA, TAPB, TAPC, TAPD, and TAPE were 31.6%, 12.1%, 9.8%, 3.3%, and 2.4%, respectively. Further purification of TAPA using Sephacryl S-500 gel chromatography yielded two carbohydrate peaks. The peak eluting first (TAPA1) (Fig. 2) was collected, and appeared as a single symmetrical peak on high performance liquid chromatography (HPLC).
Fig. 1.
Elution curve of TAP50w using DEAE-Sepharose™ Fast Flow anion-exchange chromatography
Fig. 2.
Elution curves of TAPA using High-Resolution Sephacryl S-500 gel chromatography. (a) The first elution curve; (b) The second elution curve
3.3. Properties of polysaccharide fraction TAPA1
Purified polysaccharide fraction, TAPA1, had a estimated molecular weight of 1.35×106 Da by HPLC, and a carbohydrate content of 98.7% by the phenol-sulfuric acid method. Monosaccharide composition analysis, methylation data, and nuclear magnetic resonance (NMR) spectral analysis showed that TAPA1 contained an α-(1→3)-linked mannopyranosyl backbone, partially substituted at position 4 with xylose side chains and at position 2 with side chains composed of either xylose, mannose, and glucuronic acid or of xylose and mannose (Du et al., 2009).
3.4. Properties of the sulfated derivative TAPA1-s
The DS of sulfate groups in TAPA1-s, the sulfated derivative of TAPA1, was 0.05 and the sulfate content was 2.88%. Although some polysaccharide degradation usually occurs during the sulfation reaction (Zhang et al., 2005; Han et al., 2005), the molecular weight of TAPA1-s (1.35×106 Da) was almost identical to TAPA1, indicating that no degradation had occurred. This may be due to the use of formamide as the solvent and the moderate temperature (30 °C) used during sulfation (Cui et al., 2008). No characteristic absorption bands corresponding to sulfate groups appeared, however, in the infrared (IR) spectrum of TAPA1-s (Zhang et al., 2005), and the sulfated position could not be determined by NMR spectrum, possibly due to the low degree of substitution and sulfate content.
3.5. Effect of different T. aurantialba polysaccharide fractions on mouse spleen lymphocyte proliferation in vitro
The crude polysaccharide fractions TAP50w, TAP10–50w, and TAP1–10w all significantly stimulated the proliferation of mouse spleen lymphocytes (MSLs) (P<0.05) in vitro (Table 2). At 50 μg/ml dose levels, the proliferation rates observed in the presence of the three fractions were (299.96±5.4)%, (278.89±9.4)%, and (250.76±1.7)%, respectively. Cell proliferation rates were increased most by increased concentrations of TAP50w, which exhibited the highest overall effect among the three fractions.
Table 2.
Effect of different T. aurantialba polysaccharide fractions on mouse spleen lymphocyte proliferation in vitro*
| Fraction | Proliferation rate (%) |
||
| 50 μg/ml | 200 μg/ml | 500 μg/ml | |
| TAP50w | 299.96±5.4c | 453.90±3.8cd | 474.01±9.0bc |
| TAP10–50w | 278.89±9.4c | 365.28±9.3f | 443.83±4.2d |
| TAP1–10w | 250.76±1.7d | 329.00±7.9g | 324.08±6.3f |
| TAPA | 296.46±2.1c | 438.27±9.3de | 454.15±5.4d |
| TAPB | 426.90±6.3a | 460.50±4.2bc | 423.11±9.0e |
| TAPC | 283.04±7.9c | 293.44±8.2h | 239.61±3.8g |
| TAPD | 213.26±6.1e | 248.68±2.5 | 202.03±6.3h |
| TAPE | 298.46±8.1c | 428.27±3.2e | 459.97±3.8cd |
| TAPA1 | 320.51±9.0b | 468.25±1.3b | 483.15±1.7b |
| TAPA1-s | 339.67±3.6b | 484.10±6.4a | 593.98±5.4a |
Positive control: PHA (6 μg/ml), proliferation rate (565.82±15.5)%; negative control: PBS, proliferation rate (100±4.8)%. Values within each column with different letters (a–h) are significantly different at P<0.05 using the Student’s t-test
Among the five fractions (TAPA–TAPE) obtained following anion-exchange chromatography of TAP50w, both TAPA and TAPE stimulated the proliferation of MSLs in vitro in a dose-dependent manner (Table 2). At 500 μg/ml concentration, both of these two fractions exhibited a similar potency as 6 μg/ml PHA, which served as the positive control. The effects of fractions TAPC and TAPD on cell proliferation were lower and might reflect differences in structural parameters including monosaccharide composition, and configuration of glycosidic bonds and the glycosidic ring. Cells proliferation rates observed in the presence of 50, 200, and 500 μg/ml TAPB were (426.90±6.3)%, (460.50±4.2)%, and (423.11±9.0)%, respectively, suggesting that this fraction contained some inhibitory components.
Fraction TAPA1, obtained from TAPA by gel permeation chromatography, was shown to be a homogenous acidic heteropolysaccharide, and to stimulate MSL proliferation more effectively than either of the two fractions (TAP50w and TAPA) from which it was derived (Table 2). At 500 μg/ml, the proliferation rates of TAPA1, TAPA, and TAP50w were (483.15±1.7)%, (454.15±5.4)%, and (474.01±9.0)%, respectively.
3.6. Effect of the sulfated derivative, TAPA1-s, on mouse spleen lymphocyte proliferation in vitro
Sulfated modification has been found to be an important method of improving the bioactivity of polysaccharides (Wang et al., 2009). Increasing evidence suggests that the bioactivity of sulfated polysaccharides is closely related to structural parameters including molecular weight, DS, position of sulfation, and monosaccharide composition (Duarte et al., 2001; Bohn and BeMiller, 1995). Consequently, further studies on the structure-activity relationships are required in the case of sulfated polysaccharides (Wang et al., 2009). In this study, we also tested the immunostimulatory activity of the sulfated derivative, TAPA1-s, in vitro using the mouse spleen lymphocyte proliferation assay. TAPA1 was used in control experiments. Recorded cell proliferation rates in the presence of 50, 200, and 500 μg/ml TAPA1-s were (339.67±3.6)%, (484.10±6.4)%, and (593.98±5.4)%, respectively (Table 2). All these values were higher compared with the corresponding values for fraction TAPA1, clearly indicating that sulfation was effective in enhancing immunostimulating activity.
4. Conclusions
Membrane separation technology and a series of chromatographic steps were used to isolate and purify polysaccharides from T. aurantialba fruit bodies. Three crude polysaccharide fractions, TAP50w, TAP10–50w, and TAP1–10w, were prepared by ultrafiltration, with the highest yields obtained in the case of TAP50w using a membrane of 500 kDa molecular weight cut-off. Further purification of TAP50w using successive chromatographic steps yielded five semi-purified fractions (TAPA–TAPE) and one purified polysaccharide fraction (TAPA1). A sulfated derivative of TAPA1, TAPA1-s, was prepared by chemical modification in an attempt to improve immune activities. Immunostimulatory activities of all the fractions were estimated in vitro using the mouse spleen lymphocyte proliferation assay, and TAP50w was more stimulatory compared to TAP10–50w and TAP1–10w. TAPA1-s was markedly more stimulatory than TAPA1, indicating that sulfonation was an effective way of enhancing immunostimulating activity. In order to correlate structural features with bioactivity, the structure of TAPA1 has since been investigated in our laboratory using 2D-NMR spectra and methylation analysis, and the results published elsewhere (Du et al., 2009). The structural properties of TAPA1-s, especially the position(s) of sulfate substitution and the relationship between the DS and immunostimulating activity, are the subject of continuing research.
Acknowledgments
We thank Dr. John BUSWELL, Institute of Edible Fungi, Shanghai Academy of Agricultural Sciences, China, for linguistic revision of the manuscript.
Footnotes
Project (No. 2006BAD06B08) supported by the National Key Technology R & D Program of China
References
- 1.Bohn JA, BeMiller JN. (1→3)-β-D-glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydrate Polymers. 1995;28(1):3–14. doi: 10.1016/0144-8617(95)00076-3. [DOI] [Google Scholar]
- 2.Chaidedgumjorn A, Toyoda H, Woo ER, Lee KB, Kim YS, Toida T, Imanari T. Effect of (1→3)- and (1→4)-linkages of fully sulfated polysaccharides on their anticoagulant activity. Carbohydrate Research. 2002;337(10):925–933. doi: 10.1016/S0008-6215(02)00078-2. [DOI] [PubMed] [Google Scholar]
- 3.Cui HX, Liu Q, Tao YZ, Zhang HF, Zhang LN, Ding K. Structure and chain conformation of a (1→6)-α-D-glucan from the root of Pueraria lobata (Willd.) Ohwi and the antioxidant activity of its sulfated derivative. Carbohydrate Polymers. 2008;74(4):771–778. doi: 10.1016/j.carbpol.2008.04.034. [DOI] [Google Scholar]
- 4.de Baets S, Vandamme EJ. Extracellular Tremella polysaccharides: structure, properties and applications. Biotechnology Letters. 2001;23(17):1361–1366. doi: 10.1023/A:1011645724220. [DOI] [Google Scholar]
- 5.de Baets S, du Laing S, François C, Vandamme EJ. Optimization of exopolysaccharide production by Tremella mesenterica NRRL Y-6158 through implementation of fed-batch fermentation. Journal of Industrial Microbiology and Biotechnology. 2002;29(4):181–184. doi: 10.1038/sj.jim.7000276. [DOI] [PubMed] [Google Scholar]
- 6.Du XJ, Zhang JS, Yang Y, Ye LB, Tang QJ, Jia W, Liu YF, Zhou S, Hao RX, Gong CY, et al. Structural elucidation and immuno-stimulating property of an acidic heteropolysaccharide (TAPA1) from Tremella aurantialba . Carbohydrate Research. 2009;344(5):672–678. doi: 10.1016/j.carres.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Duarte MER, Cardoso MA, Noseda MD, Cerezo AS. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum . Carbohydrate Research. 2001;333(4):281–293. doi: 10.1016/S0008-6215(01)00149-5. [DOI] [PubMed] [Google Scholar]
- 8.Dubois M, Gillis KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28(3):350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 9.Han F, Yao W, Yang X, Liu X, Gao X. Experimental study on anticoagulant and antiplatelet aggregation activity of a chemically sulfated marine polysaccharide YCP. International Journal of Biological Macromolecules. 2005;36(4):201–207. doi: 10.1016/j.ijbiomac.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Kawnmoto K, Nakajimo H, Kohno M, Kadoya S, Mizunoet D. Chemical modification and antitumor activity of a D-manno-D-glucan from Microellobosporia grisea . Carbohydrate Research. 1983;115:199–208. doi: 10.1016/0008-6215(83)88148-8. [DOI] [PubMed] [Google Scholar]
- 11.Kiho T, Kochi M, Usui S, Hirano K, Aizawa K, Inakuma T. Antidiabetic effect of an acidic polysaccharide (TAP) from Tremella aurantia and its degradation product (TAP-H) Biological & Pharmaceutical Bulletin. 2001;24(12):1400–1403. doi: 10.1248/bpb.24.1400. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Lin Z. Effect of Tremella polysaccharide on IL-2 production by mouse splenocytes. Acta Pharmaceutica Sinica. 1992;27:1–4. (in Chinese) [PubMed] [Google Scholar]
- 13.Nie X, Shi B, Ding Y, Tao W. Preparation of a chemically sulfated polysaccharide derived from Grifola frondosa and its potential biological activities. International Journal of Biological Macromolecules. 2006;39(4-5):228–233. doi: 10.1016/j.ijbiomac.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Peng Y, Zhang L, Zeng F, Kennedy JF. Structure and antitumor activities of the water-soluble polysaccharides from Ganoderma tsugae mycelium. Carbohydrate Polymers. 2005;59(3):385–392. doi: 10.1016/j.carbpol.2004.10.009. [DOI] [Google Scholar]
- 15.Shen XX, Yang Y, Zhang JS, Tang QJ, Jia W. Isolation, purification and immunomodifying activity of polysaccharide from Phellinus igniarius . Acta Edulis Fungi. 2008;15(2):37–41. (in Chinese) [Google Scholar]
- 16.Su Z, Dai Z, Yang J. Research progress on immunologic mechanism of polysaccharide. Journal of Yunnan Agricultural University. 2006;21(2):205–209. (in Chinese) [Google Scholar]
- 17.Ukai S, Hirose K, Kiho T, Hara C, Irikura T. Antitumor activity on sarcoma 180 of the polysaccharides from Tremella fuciformis Berk. Chemical & Pharmaceutical Bulletin. 1972;20:2293–2294. doi: 10.1248/cpb.20.2293. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Qu W, Chu S, Li M, Tian C. Studies on the preventive and therapeutic effects of the polysaccharide of Tremella aurantialba mycelia on diet-induced hyperlipidemia in mice. Acta Nutrimenta Sinica. 2002;24(4):431–432. (in Chinese) [Google Scholar]
- 19.Wang L, Huang HY, Wei YY, Li XX, Chen ZX. Characterization and anti-tumor activities of sulfated polysaccharide SRBPS2a obtained from defatted rice bran. International Journal of Biological Macromolecules. 2009;45(4):427–431. doi: 10.1016/j.ijbiomac.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JS, Tang QJ, Zimmerman-Kordmann M, Reutter W, Fan H. Activation of B lymphocytes by GLIS, a bioactive proteoglycan from Ganoderma lucidum . Life Sciences. 2002;71(6):623–638. doi: 10.1016/S0024-3205(02)01690-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Chen L, Xu X, Lin Y, Cheung PCK, Kennedy JF. Comparison on chain stiffness of a water-nsoluble (1→3)-α-D-glucan isolated from Poria cocos mycelia and its sulfated derivative. Carbohydrate Polymers. 2005;59(2):257–263. doi: 10.1016/j.carbpol.2004.09.017. [DOI] [Google Scholar]
- 22.Zhang W, Qu W, Zhang X. The anti-hyperglycemic activity of polysaccharides from Tremella aurantialba mycelium. Acta Nutrimenta Sinica. 2004;26(4):300–303. (in Chinese) [Google Scholar]