Abstract
This study was to investigate the responses of phospholipase D (PLD) and lipoxygenase (LOX) to mechanical wounding in postharvest cucumber (Cucumis sativus L. cv. Biyu-2) fruits. Membrane-associated Ca2+ content, activities and gene expression of PLD and LOX, and contents of phosphatidylcholine (PC), phosphatidylinositol (PI), and phosphatidic acid (PA) were determined in cucumber fruits following mechanical wounding. Results show that PLD and LOX activities increased with the PLD and LOX mRNAs which are upregulated upon wounding, while membrane-associated Ca2+ content decreased. Accompanying with the increase of PLD and LOX activities, accumulation of PA and losses of PC and PI were observed in all fruits, but there were differences of degrees between wounded and control fruits. Results suggest that PLD and LOX might be the main hydrolytic enzymes of phospholipids in postharvest cucumber fruits participating in the mechanical wounding injury. The activation of PLD and LOX might be the result of gene expression, which could be stimulated by the Ca2+ flowing from the membrane to the cytoplasm upon receiving the wounding signals.
Keywords: Cucumber, Mechanical wounding, Phospholipase D, Lipoxygenase, Phospholipid
1. Introduction
Mechanical damages are the main cause of losses in the postharvest horticultural products (Durigan and Mattiuz, 2007), not only because external damage renders the fruit less attractive for consumption, but also because defects are preferred entry sites for pathogens and fungi. Hence, external damage creates a threat for food safety (van Linden et al., 2008). Mechanical wounding is also considered as a type of stress, which produces signals that migrate through cells into uninjured tissue and induces a number of physiological responses (Saltveit, 2000).
Previous studies have indicated that phospholipase D (PLD) and lipoxygenase (LOX) in plants play an important role in phospholipid catabolism, initiating a lipolytic cascade in membrane deterioration during senescence and stress (Paliyath and Thompson, 1987; Wang, 2005; Bargmann and Munnik, 2006; Bargmann et al., 2009). Increased PLD-mediated hydrolysis occurs under various stress conditions, such as frost, senescence, and wounding (Wang, 2000). It has been proposed that the liberated polyunsaturated fatty acids serve as substrates for LOX that produces activated oxygen and lipid peroxides leading to membrane damage (de Bruxelles and Roberts, 2001). After wounding, plants accumulate phosphatidic acid (PA) and unesterified fatty acids that are released from lipids, presumably by the action of wound-inducible phospholipases of types D and A2 (Conconi et al., 1996; Ryu and Wang, 1996; Lee et al., 1997; Narvaez-Vasquez et al., 1999; Bargmann et al., 2009). LOX mRNA levels altered in soybean leaflets within 8 h after wounding (Creelman et al., 1992; Saravitz and Siedow, 1996). Calcium has been shown to protect fruit against stress (Martínez-Romero et al., 1999) through regulating enzyme activities (Lein and Saalbach, 2001). Some evidence reported that wounding-induced activation of PLD could result from translocation of PLD to the membrane, which is mediated by an increase in cytoplasmic calcium and stimulated by low micromolar calcium levels (Paliyath and Droillard, 1992; Ryu and Wang, 1996; Yuan et al., 2005). So far, there is no direct evidence of activation by wounding for the lipid hydrolytic enzymes in postharvest fruits and vegetables. This experiment investigated the induction of PLD and LOX, and the mechanism of enzyme induction in response to wounding in harvested cucumber fruits.
2. Materials and methods
2.1. Plant materials and postharvest treatment
About 10 d after full bloom, cucumber (Cucumis sativus L. cv. Biyu-2) fruits were collected from Zhejiang Institute of Vegetable Science, Hangzhou, Zhejiang, China, at commercial maturity, and were transported to the laboratory immediately. Cucumber fruits of similar size, same mature period, without infection and physical injuries were chosen and randomly divided into wounded and control groups, 90 fruits each. In wounded group, cucumbers were punctured at 3-cm intervals from the calyx to the stalk end by a 10-mm diameter stainless steel puncher. The fruits in control group were not punctured. Both groups were stored at 25 °C and 90% relative humidity (RH). Each 15 cucumber fruits were taken every 2 h (0, 2, 4, 6, 8, and 10 h) after wounding for the determination.
2.2. Determination of Ca2+ content
Determination of membrane-associated Ca2+ content was based on Yapa et al. (1986). Cucumber fruit tissue (5 g) was homogenized in 15 ml of extraction buffer containing 0.25 mol/L sucrose, 20 mmol/L Tris-HCl buffer (pH 7.5), 3 mmol/L ethylenediamine tetra-acetic acid (EDTA), 3 mmol/L 2-mercapto-thanol, and 1 g insoluble polyvinylpyrrolidone (PVP). The homogenate was passed through four layers of cheesecloth and then centrifuged at 1500 r/min for 15 min. The supernatant was decanted and then centrifuged at 15 000 r/min for 30 min to yield a pellet of the membranes. The membrane fraction in precipitate was homogenized in the suspension buffer containing 0.125 mol/L sucrose, 1 mmol/L Tris-MES buffer (pH 7.2), and 3 mmol/L 2-mercapto-ethanol, the ratio being 10 g fresh weight (FW) per 1.0 ml of buffer. HCl was added to each suspension to a final concentration of 0.1 mol/L and the mixture was shaken for 60 min. Ca2+ was extracted by centrifuging at 15 000 r/min for 10 min and then the supernatant was determined at 422.7 nm by an atomic absorption spectrometer (Analyst 800, Perkin Elmer, USA) (Mao et al., 2007a).
2.3. PLD and LOX activity assay
Cucumber fruit tissue (50 g) was homogenized in 300 ml of water for 5 min at 4 °C. The homogenate was filtered through muslin cloth and centrifuged at 13 000 r/min for 30 min at 4 °C. After centrifugation, the supernatant was separated and was used as PLD extract (Khatoon et al., 2008). PLD activity assay was based on the enzymatic determination of the release of choline by the hydrolysis of phosphatidylcholine (PC) by PLD. The substrate solution was prepared by adding 40 mg of PC to 1 ml of chloroform and 5 ml of water containing 1.44 mg/ml of sodium dodecyl sulfate (SDS). In a typical experiment, 10 μl of PC and 30 μl of 0.5 mol/L sodium acetate buffer (pH 5.5) containing 5 mmol/L CaCl2 were taken. The reaction was started by the addition of 30 μl of the enzyme extract, followed by incubation at 37 °C for 10 min, and terminated by adding 20 μl of 1 mol/L Tris-HCl buffer (pH 8.0) containing 0.1 mol/L EDTA. This reaction mixture was then immediately incubated with 30 μl of the following mixture: peroxidase reagent (containing 0.2 U peroxidase, 1.5 mmol/L 4-aminoantipyrine, and 2.1 mmol/L phenol) and choline oxidase (3 U) in 10 mmol/L Tris-HCl buffer (pH 8.0), at 37 °C for 45 min (Khatoon et al., 2008). The quinoneimine dye formed was measured at 492 nm (UltroSpec 2100 Pro UV/visible spectrophotometer, USA). One unit of PLD is defined as the increase in absorption at 492 nm of 0.01 in 1 min (3-min period). Protein in the enzyme extract was determined by Coomassie brilliant blue G-250 reagent (Li and Jiao, 1980).
For LOX extraction, the method of Mao et al. (2007b) was followed. Each 5 g of cucumber tissue was homogenized with 5 ml of 50 mmol/L Tris-HCl (pH 8.0) containing 10 mmol/L KCl, 500 mmol/L sucrose, and 0.5 mmol/L phenylmethylsulfonyl fluoride (PMSF), and centrifuged at 15 000 r/min for 30 min. The standard assay mixture contained 200 μl of Tween 20 and 40 μl of linoleic acid in 40 ml of 0.1 mol/L phosphate buffer (pH 7.0). To 500 μl of 0.1 mol/L phosphate buffer (pH 7.0) in a cuvette were added 500 μl of assay mixture and 200 μl of LOX extract. One unit of LOX is defined as the increase in absorption at 234 nm of 0.01 in 1 min (3-min period) at 25 °C (Todd et al., 1990). Protein in the enzyme extract was determined by Coomassie brilliant blue G-250 reagent (Li and Jiao, 1980).
2.4. Real-time reverse transcription-polymerase chain reaction (RT-PCR)
2.4.1. Extraction of total RNAs
Total RNAs were extracted from 100 mg of fresh cucumber tissues. TRIzol reagent (Invitrogen, USA) was used according to the manufacturer’s directions, followed by RNase-free DNase treatment (TaKaRa, Dalian, China). Total RNA concentrations were measured at 260 and 280 nm by NanoDrop™ 1000 spectrophotometer (USA) and the purity of the total RNA extracted was determined as the ratio of optical density at 260 nm to that at 280 nm (OD260/OD280) with values between 1.8 and 2.1. The integrity of the total RNAs was determined by electrophoresis on 1% (w/v) agarose gel. Isolated RNA was dissolved in 50 µl of RNase free H2O and stored at −80 °C (Feng et al., 2009).
2.4.2. Primer design
Gene-special primers were designed using Vector NTI Suite 5.5 software to detect the otherness of PLD and LOX gene relative expression to respond the mechanical wounding. Primer sequences are shown in Table 1.
Table 1.
Primers of genes used for real time RT-PCR assay
| GenBank accession No.a | Gene name | Primer sequences 5′-3′ |
| X92890 | lipoxygenase (LOX) | Sense primer: TCAAGTGCCCCCCATGGACTTAG |
| Antisense primer: TCATGTTTCTTGTCAGCGTGGCC | ||
| EF363796 | Phospholipase D (PLD) | Sense primer: AAGTCAAGAGGTCGGGAGAATA |
| Antisense primer: GTTCATACCATAGAGCCATTCG | ||
| AF206894 | 18S ribosomal RNAb | Sense primer: GGCGGATGTTGCTTTAAGGA |
| Antisense primer: GTGGTGCCCTTCCGTCAAT |
GenBank accession number of gene to the National Center for Biotechnology Information (NCBI) database
According to reference Gil-Salas et al. (2009)
2.4.3. Analysis of gene expression
PLD and LOX cDNA syntheses and real-time PCR were performed according to the TaKaRa manufacturer specifications (TaKaRa SYBR® PrimeScript™ RT-PCR Kit, Dalian, China). The SYBR Green PCR cycling was denaturation at 95 °C for 10 s, and 40 cycles of 95 °C for 5 s and 60 °C for 30 s were executed by iQ™ 5 Multicolor real-time PCR detection system (Bio-RAD, USA). In this experiment, 18S ribosomal RNA (18S rRNA) gene of Cucumis sativus L. was used as a housekeeping gene (Gil-Salas et al., 2009). The threshold cycle (C T) values were determined by iQ™ 5 Multicolor real-time PCR detection system, and the changes of each gene were calculated by the comparative 2−ΔΔCT, where ΔΔC T=(C T,target−C T,18S rRNA)treatment−(C T,target−C T,18S rRNA)control (Livak et al., 2001).
2.5. Analysis of phospholipids by high performance liquid chromatography
In this experiment, the standards of phosphatidylinositol (PI), PC, and PA were purchased from Sigma (USA). Methanol and acetonitrile were of high performance liquid chromatography (HPLC) grade and other chemicals were of analytical grade.
Extraction of phospholipids was based on the method described by Yang et al. (2008). Namely, 10 g fresh tissue was ground to power in liquid N2 with a mortar and pestle, and solved with 15 ml Folch reagent in 50 ml screw cap test tube. The mixed solution was extracted absolutely in ultrasonic instrument (KQ 5200E, Jiangsu, China) at 4 °C for 1 h, and centrifuged at 10 000 r/min under 4 °C for 20 min. Supernatant was placed in a 50-ml test tube, recentrifuged at 10 000 r/min under 4 °C for 20 min. The aqueous phase was discarded and the chloroform phase was put into a 10-ml tube. Then 1 ml of acetone was added and vortexed for 2 min. The mixture was then dried under nitrogen and dissolved in 2 ml Folch reagent for phospholipid detection.
The separation of phospholipid compounds was achieved on an Agilent 1200 series Rapid Resolution LC System (Agilent Technologies, Palo Alto, CA) using Si 60 column (250 mm×4.6 mm, 5 μm, Dalian Yilite, China). The mobile phase consisted of acetonitrile-methanol-85% phosphoric acid (130:370:1, v/v/v) at a flow rate of 1.5 ml/min, and the 2 µl injection was detected at 205 nm. Sample was filtrated by 0.22-μm micropore filter before detection (Yang et al., 2008). Data were collected on Agilent ChemStation software.
2.6. Statistical analysis
The data were reported as mean±standard deviation (SD) for triplicate determinations. Graphs were described using Microsoft Excel 2003 (Microsoft Corp.). Analysis of variance and least significant difference tests (SPSS for Windows, SPSS Inc., Chicago, IL) were conducted to identify differences among means. Statistical significance was declared at P<0.05.
3. Results
3.1. Decrease in membrane-associated Ca2+ content
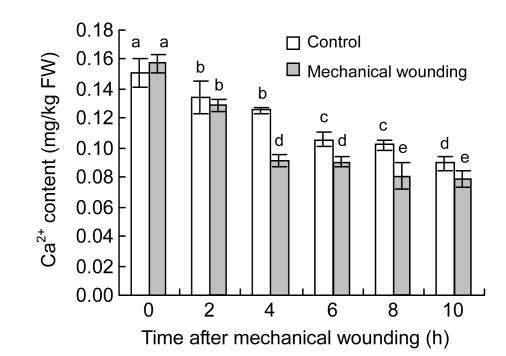
Membrane-associated Ca2+ content decreased in both wounded and control fruits (Fig. 1). Wounded fruits had a much lower content of membrane Ca2+ than control fruits after 4 h of wounding. After 4, 6, 8, or 10 h of wounding treatment, content of membrane Ca2+ in wounded fruits decreased significantly by 31.49%, 18.99%, 42.32%, or 30.07%, respectively, compared with control fruits (P<0.05).
Fig. 1.
Changes of membrane-associated Ca2+ content in cucumber fruits
Values represent mean±SD of three replicates derived from composite samples of five fruits each
3.2. Activation of PLD and LOX by mechanical wounding
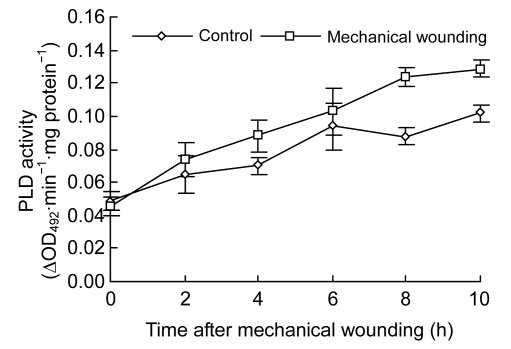
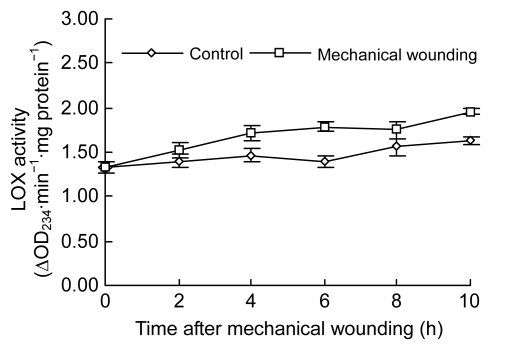
Changes of PLD activities demonstrated a rapid increase tendency in both control and wounded fruits (Fig. 2). While maximized at 10 h in both groups, much higher PLD activities were observed in the wounded than in the control. At 4, 8, and 10 h after wounding, PLD activities were increased by 25.84% (0.08 ∆OD492∙min−1∙mg protein−1), 40.97% (0.12 ∆OD492∙min−1∙mg protein−1), and 26.73% (0.13 ∆OD492∙min−1∙mg protein−1), respectively (P<0.05). Similarly, LOX activities gradually increased in both control and wounded fruits (Fig. 3). In contrast, higher activities of LOX were observed in wounded fruits at 4, 6, 8, and 10 h, respectively (P<0.05), and activities of LOX reached the maximum at 10 h in both control and wounded fruits.
Fig. 2.
Changes of PLD activity in cucumber fruits
Values represent mean±SD of three replicates derived from composite samples of five fruits each
Fig. 3.
Changes of LOX activity in cucumber fruits
Values represent mean±SD of three replicates derived from composite samples of five fruits each
3.3. PLD and LOX mRNA levels
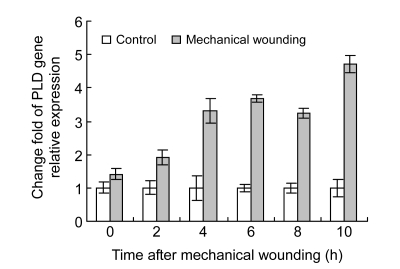
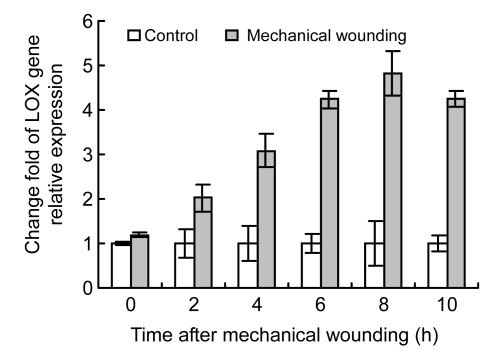
In order to identify whether PLD and LOX gene expression was affected by wounding, difference of PLD and LOX gene expression between wounded and control cucumber fruits was analyzed after mechanical wounding. Results show higher values of PLD and LOX mRNAs in wounded fruits than in control fruits, and the difference reached maximum at 10 h after wounding (Figs. 4 and 5). PLD and LOX mRNAs were induced instantly by wounding, and rapidly increased after 2 h. PLD mRNA level increased 1.40–4.69 times higher in the wounded than in the control from 4 to 10 h and LOX mRNA level also increased 1.18–4.82 times higher 2 h post wounding. Both PLD and LOX gene expression levels increased rapidly in the wounded fruits 2 h post wounding.
Fig. 4.
Relative expression levels of PLD mRNA in cucumber fruits using real-time RT-PCR
Gene expression values of control cucumber fruits were presented as 1. Values represent mean±SD of three replicates derived from composite samples of five fruits each
Fig. 5.
Relative expression levels of LOX mRNA in cucumber fruits using real-time RT-PCR
Gene expression values of control cucumber fruits were presented as 1. Values represent mean±SD of three replicates derived from composite samples of five fruits each
3.4. Changes of phospholipid compounds
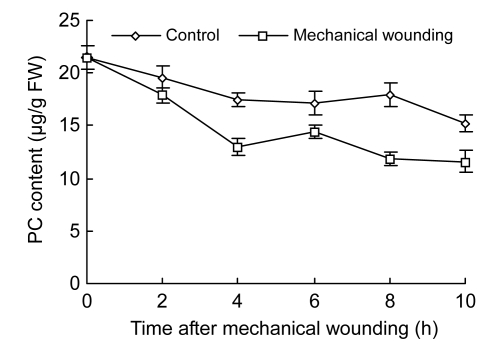
PC, PI, and PA were measured in the experiment in order to confirm whether mechanical wounding actually induces phospholipid hydrolysis. PC was rapidly decreased by mechanical wounding in wounded fruits and PC content declined sharply within the first 4 h after wounding in both wounded and control fruits (Fig. 6). Decreased ratio of PC was maximal at 10 h, 46.15% (11.60 µg/g FW) in wounded fruits and 29.30 % (15.19 µg/g FW) in control (P<0.05).
Fig. 6.
Changes of PC content in cucumber fruits
Values represent mean±SD of three replicates derived from composite samples of five fruits each
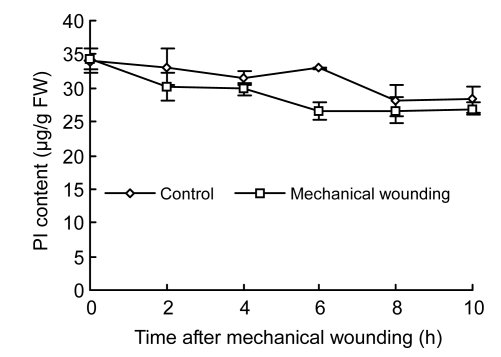
PI content in both control and wounded fruits displayed a slight decline trend with increase of testing time (Fig. 7). There was no significant difference in PI content between wounded and control fruits; however, the PI content in wounded fruits (26.65 µg/g FW) significantly decreased by 22.49% compared with control fruits (32.99 µg/g FW) at 6 h (P<0.05).
Fig. 7.
Changes of PI content in cucumber fruits
Values represent mean±SD of three replicates derived from composite samples of five fruits each
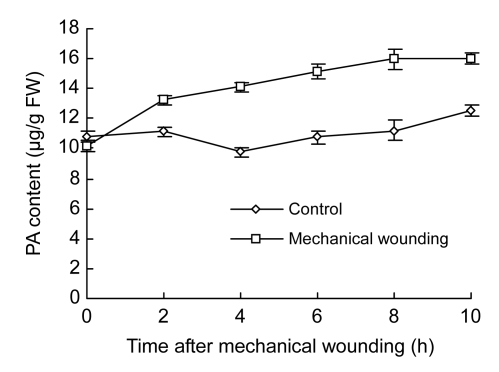
PA content was sharply increased in wounded fruits within the early 2 h, and then maintained at the high levels during the whole testing period (Fig. 8). Increased ratio of PA maximized at 56.49% (15.98 µg/g FW) in wounded fruits and 16.35% (12.55 µg/g FW) in control fruits at 10 h. There were significantly higher contents of PA in wounded fruits than in the control after 2 h (P<0.05).
Fig. 8.
Changes of PA content in cucumber fruits
Values represent mean±SD of three replicates derived from composite samples of five fruits each
4. Discussion
The current study demonstrated that membrane-associated Ca2+ content decreased more rapidly in wounded cucumber fruits than in control during experiment time after mechanical wounding (Fig. 1). Ca2+ plays a crucial role in many physiological processes in plants, such as warding off membrane damage and leakiness and strengthening wall structure (Yapa et al., 1986). Ca2+ is also an important secondary messenger in plant cells (Bush, 1995), and has long been suspected to be a regulator of plant PLD (Pinhero et al., 1998). PLD activity is stimulated by Ca2+-channel blockers and calmodulin antagonists, which are known to increase cellular Ca2+ levels (de Vrije and Munnik, 1997). Elevated PLD activity was observed with increased cytoplasmic calcium during senescence of broccoli florets and strawberry (Deschene et al., 1991; Yuan et al., 2005). The results in this study indicate that mechanical wounding stimulates the flux of Ca2+ from the membrane into the cytoplasm, which is likely enough to activate the activities of PLD and LOX (Figs. 2 and 3) and that Ca2+ signal transduction plays a role in wounding for postharvest cucumber fruits.
The higher gene expression of PLD and LOX was observed in wounded fruits accompanying higher PLD and LOX activities (Figs. 4 and 5). These results indicate that PLD and LOX activities in wounded fruits were associated with its mRNA expression. PLD and LOX induced by wounding might stimulate corresponding physiological reactions related with deterioration and senescence through increased gene expression.
While PA content increased rapidly during a 10-h period after wounding, PC and PI contents decreased (Figs. 6–8). Higher PA and lower PC and PI in wounded fruits were compared with the control. Several pieces of evidence confirmed that PLD hydrolyzes phospholipids to PA and free head groups, which serve as substrates for LOX and entail the loss of cell-membrane integrity (Ryu and Wang, 1996; Wang, 2000; Bargmann et al., 2009). LOX plays an important role in generating peroxidative damage in membrane lipids through peroxidation reactions on plasma membrane-lipids (Lee et al., 2005). Furthermore, our results show that synergistic effect of PLD and LOX might accelerate phospholipid hydrolysis and peroxidation, eventually causing membrane integrity damage. PC might be main substrate for PLD in harvested cucumbers and PA might play a key role in response to mechanical wounding signals.
Footnotes
Project (No. 30771513) supported by the National Natural Science Foundation of China
References
- 1.Bargmann BOR, Munnik T. The role of phospholipase D in plant stress responses. Current Opinion in Plant Biology. 2006;9(5):515–522. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann BOR, Laxalt AM, Ter Riet B, Testerink C, Merquiol E, Mosblech A, Leon-Reyes A, Pieterse CMJ, Haring MA, Heilmann I, et al. Reassessing the role of phospholipase D in the Arabidopsis wounding response. Plant Cell & Environment. 2009;32(7):837–850. doi: 10.1111/j.1365-3040.2009.01962.x. [DOI] [PubMed] [Google Scholar]
- 3.Bush DS. Calcium regulation in plant-cells and its role in signaling. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46(1):95–122. doi: 10.1146/annurev.pp.46.060195.000523. [DOI] [Google Scholar]
- 4.Conconi A, Miguel M, Browse JA, Ryan CA. Intracellular levels of free linolenic and linoleic acids increase in tomato leaves in response to wounding. Plant Physiology. 1996;111(3):797–803. doi: 10.1104/pp.111.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proceedings of the National Academy of Sciences. 1992;89(11):4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bruxelles GL, Roberts MR. Signals regulating multiple responses to wounding and herbivores. Critical Reviews in Plant Sciences. 2001;20(5):487–521. doi: 10.1016/S0010-8545(02)00029-2. [DOI] [Google Scholar]
- 7.Deschene A, Paliyath G, Lougheed EC, Dumbroff EB, Thompson JE. Membrane deterioration during postharvest storage of broccoli florets: modulation by temperature and controlled atmosphere storage. Postharvest Biology and Technology. 1991;1(1):19–31. doi: 10.1016/0925-5214(91)90016-5. [DOI] [Google Scholar]
- 8.de Vrije T, Munnik T. Activation of phospholipase D by calmodulin antagonists and mastoparan in carnation petals. Journal of Experimental Botany. 1997;48(9):1631–1637. doi: 10.1093/jxb/48.9.1631. [DOI] [Google Scholar]
- 9.Durigan MFB, Mattiuz BH. Effect of mechanical injuries on the quality of watermelon stored at room temperature. Horticultura Brasileira. 2007;25(2):296–300. doi: 10.1590/S0102-05362007000200033. [DOI] [Google Scholar]
- 10.Feng JL, Wang K, Liu X, Chen SN, Chen JS. The quantification of tomato microRNAs response to viral infection by stem-loop real-time RT-PCR. Gene. 2009;437(1-2):14–21. doi: 10.1016/j.gene.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Gil-Salas FM, Colyer A, Boonham N, Cuadrado IM, Janssen D. Resistance screening against cucumber vein yellowing virus using a real-time (TaqMan) RT-PCR assay in cucumber (Cucumis sativus) Crop Protection. 2009;28(1):109–112. doi: 10.1016/j.cropro.2008.09.005. [DOI] [Google Scholar]
- 12.Khatoon H, Talat S, Younus H. Phospholipase D from Allium sativum bulbs: a highly active and thermal stable enzyme. International Journal of Biological Macromolecules. 2008;42(4):380–385. doi: 10.1016/j.ijbiomac.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Suh S, Kim S, Crain RC, Kwak JM, Nam HG, Lee Y. Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. The Plant Journal. 1997;12(3):547–556. doi: 10.1046/j.1365-313X.1997.00547.x. [DOI] [Google Scholar]
- 14.Lee SH, Ahn SJ, Im YJ, Cho K, Chung GC, Cho BH, Han O. Differential impact of low temperature on fatty acid unsaturation and lipoxygenase activity in fig leaf gourd and cucumber roots. Biochemical and Biophysical Research Communications. 2005;330(4):1194–1198. doi: 10.1016/j.bbrc.2005.03.098. [DOI] [PubMed] [Google Scholar]
- 15.Lein W, Saalbach G. Cloning and direct G-protein regulation of phospholipase D from tobacco. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2001;1530(2-3):172–183. doi: 10.1016/S1388-1981(00)00182-7. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Jiao XZ. Measurement of protein content by the agency of protein stain Coomassie Brilliant Blue G250. Plant Physiology Communications. 1980;6:52–55. [Google Scholar]
- 17.Livak KJ, Thomas D, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Mao LC, Wang GZ, Zhu CG, Pang HQ. Involvement of phospholipase D and lipoxygenase in response to chilling stress in postharvest cucumber fruits. Plant Science. 2007;172(2):400–405. doi: 10.1016/j.plantsci.2006.10.002. [DOI] [Google Scholar]
- 19.Mao LC, Pang HQ, Wang GZ, Zhu CG. Phospholipase D and lipoxygenase activity in cucumber fruit in response to chilling stress. Postharvest Biology and Technology. 2007;44(1):42–47. doi: 10.1016/j.postharvbio.2006.11.009. [DOI] [Google Scholar]
- 20.Martínez-Romero D, Serrano M, Riquelme F. Effects of postharvest putrescine and calcium treatments on reducing mechanical damage and polyamines and ABA levels during lemon storage. Journal of the Science of Food and Agriculture. 1999;79(12):1589–1595. doi: 10.1002/(SICI)1097-0010(199909)79:12<1589::AID-JSFA403>3.0.CO;2-J. [DOI] [Google Scholar]
- 21.Narvaez-Vasquez J, Florin-Christensen J, Ryan CA. Positional specificity of a phospholipase A activity induced by wounding, systemin and oligosaccharide elicitors in tomato leaves. The Plant Cell Online. 1999;11(11):2249–2260. doi: 10.1105/tpc.11.11.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paliyath G, Thompson JE. Calcium and calmodulin regulated breakdown of phospholipid by microsomal membranes from bean cotyledons. Plant Physiology. 1987;83(1):63–68. doi: 10.1104/pp.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paliyath G, Droillard MJ. The mechanisms of membrane deterioration and disassembly during senescence. Plant Physiology and Biochemistry. 1992;30:789–812. [Google Scholar]
- 24.Pinhero RG, Paliyath G, Yada RY, Murr DP. Modulation of phospholipase D and lipoxygenase activities during chilling: relation to chilling tolerance of maize seedlings. Plant Physiology and Biochemistry. 1998;36(3):213–224. doi: 10.1016/S0981-9428(97)86878-7. [DOI] [Google Scholar]
- 25.Ryu SB, Wang X. Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1996;1303(3):243–250. doi: 10.1016/0005-2760(96)00096-3. [DOI] [PubMed] [Google Scholar]
- 26.Saltveit ME. Wound-induced changes in phenolic metabolism and tissue browning are altered by heatshock. Postharvest Biology and Technology. 2000;21(1):61–69. doi: 10.1016/S0925-5214(00)00165-4. [DOI] [Google Scholar]
- 27.Saravitz DM, Siedow JN. The differential expression of wound-inducible lipoxygenase gene in soybean leaves. Plant Physiology. 1996;110(1):287–299. doi: 10.1104/pp.110.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todd JF, Paliyath G, Thompson JE. Characteristics of a membrane associated lipoxygenase in tomato fruit. Plant Physiology. 1990;94(3):1225–1232. doi: 10.1104/pp.94.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Linden V, Sila DN, Duvetter T, Baerdemaeker JD, Hendrickx M. Effect of mechanical impact-bruising on polygalacturonase and pectinmethylesterase activity and pectic cell wall components in tomato fruit. Postharvest Biology and Technology. 2008;47(1):98–106. doi: 10.1016/j.postharvbio.2007.06.006. [DOI] [Google Scholar]
- 30.Wang XM. Multiple forms of PLD in plant: the gene family, catalytic and regulatory properties, and cellular functions. Progress in Lipid Research. 2000;39(2):109–149. doi: 10.1016/S0163-7827(00)00002-3. [DOI] [PubMed] [Google Scholar]
- 31.Wang XM. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development and stress responses. Plant Physiology. 2005;139(2):566–573. doi: 10.1104/pp.105.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang WL, Yang YH, Yan XF, Wang X. Determination of phospholipid content in tree leaves by high performance liquid chromatography. Journal of Northeast Forestry University. 2008;36(9):61–62. (in Chinese) [Google Scholar]
- 33.Yapa PAJ, Kawasaki T, Matsumoto H. Changes of some membrane-associated enzyme activities and degradation of membrane phospholipids in cucumber roots due to Ca2+ starvation. Plant and Cell Physiology. 1986;27(2):223–232. [Google Scholar]
- 34.Yuan HY, Chen LG, Paliyath G, Sullivan A, Murr DP. Characterization of microsomal and mitochondrial phospholipase D activities and cloning of a phospholipase D alpha cDNA from strawberry fruits. Plant Physiology and Biochemistry. 2005;43(6):535–547. doi: 10.1016/j.plaphy.2005.04.003. [DOI] [PubMed] [Google Scholar]