Abstract
Selenocosmia huwena and Selenocosmia hainana are two tarantula species found in southern China. Their venoms contain abundant peptide toxins. Two new neurotoxic peptides, huwentoxin-III (HWTX-III) and hainantoxin-VI (HNTX-VI), were obtained from the venom using ion-exchange chromatography and reverse-phase high performance liquid chromatography (RP-HPLC). The mechanism of action of HWTX-III and HNTX-VI on insect neuronal voltage-gated sodium channels (VGSCs) was studied via whole-cell patch clamp techniques. In a fashion similar to δ-atracotoxins, HNTX-VI can induce a slowdown of current inactivation of the VGSC and reduction in the peak of Na+ current in cockroach dorsal unpaired median (DUM) neurons. Meanwhile, 10 µmol/L HNTX-IV caused a positive shift of steady-state inactivation of sodium channel. HWTX-III inhibited VGSCs on DUM neurons (concentration of toxin at half-maximal inhibition (IC50)≈1.106 µmol/L) in a way much similar to tetrodotoxin (TTX). HWTX-III had no effect on the kinetics of activation and inactivation. The shift in the steady-state inactivation curve was distinct from other depressant spider toxins. The diverse effect and the mechanism of action of the two insect toxins illustrate the diverse biological activities of spider toxins and provide a fresh theoretical foundation to design and develop novel insecticides.
Keywords: Insect neurotoxin, Dorsal unpaired median neurons, Sodium channel, Whole-cell patch clamp technique
1. Introduction
Strong neurotoxins, and other bioactive chemicals that are toxic to insects and mammals, are present in spider venoms. The sodium channels in cell membranes are the site of action for many toxins from spider venom, and could be potential targets for drug development. Previous investigations have indicated that partial damage to the central nervous system by spider venoms is because of the induced malfunction of ion channels (Haigeny et al., 1994; Taylor and Meldrum, 1995; Meng and Nie, 2005). Voltage-gated sodium channels (VGSCs) are widely present in the most excitable tissues, playing multiple roles in important physiological processes. They are complex proteins containing a functional pore-forming α-subunit (260 kDa) and up to four auxiliary β-subunits (21–23 kDa). The α-subunit and β-subunits interact with each other through covalent or non-covalent bonds (Catterall, 2000; Yu et al., 2003).
More than nine mammalian subtypes (Nav1.1–1.9 and Nav x) and three insect subtypes of VGSCs have been successfully cloned and expressed (Warmke et al., 1997; Goldin et al., 2000; Nicholson, 2007). Despite the similarities in electrophysiology, primary structure, and allocation of functional domains of VGSCs, the pharmacological properties of insect VGSCs are distinct from those of vertebrate subtypes (Zlotkin, 1999).
Two new neurotoxic peptides, huwentoxin-III (HWTX-III) and hainantoxin-VI (HNTX-VI), were recently isolated from the venoms of two Chinese tarantulas, Selenocosmia huwena and Selenocosmia hainana, respectively. They are identified to be ion channel toxins composed of 33 and 34 residues, respectively, and each includes 6 cystein amino acid residues, which are connected by multiple disulfide bonds (I-IV, II-V, and III-VI). Preliminary studies have indicated that the two toxins reversibly paralyze cockroaches for several hours, and HNTX-VI paralyzed a rat and blocked the neuromuscular transmission in the rat (Pan et al., 2002; Huang et al., 2003). The mechanism behind these effects remains unknown. The objective of this study is to determine the mechanism of action of HWTX-III and HNTX-VI on insect VGSCs.
2. Materials and methods
2.1. Venoms and animals
The venoms from female adult S. huwena and S. hainana were collected based on the method described by Shu and Liang (1999). The cockroach Periplaneta americana was from our laboratory stock.
2.2. Toxins and reagents
HWTX-III and HNTX-VI were purified from venoms of Chinese tarantulas S. huwena and S. hainana via a combination of ion-exchange chromatography and reverse-phase high performance liquid chromatography (RP-HPLC) using the methodology adapted from Pan et al. (2002) and Huang et al. (2003). The purity of the two toxins was over 98%, as assessed by RP-HPLC and mass spectrometry analyses. All reagents used were of analytical grade.
2.3. Cell isolation procedure
Dorsal unpaired median (DUM) neurons were acutely dissociated from the terminal abdominal ganglion (TAG) of the cockroach P. americana. The dissociated neuron cells were cultivated in a short-term primary culture for 2–3 h. Briefly, mature cockroaches were sacrificed using 75% (v/v) alcohol and washed in a saline solution (200 mmol/L NaCl, 4 mmol/L MgCl2, 3.1 mmol/L KCl, 10 mmol/L hydroxyethyl piperazine ethanesulfonic acid (HEPES), and 50 mmol/L sucrose at pH 7.4). After cleaning the enteron, the TAG was excised, digested, and incubated in a saline solution (200 mmol/L NaCl, 4 mmol/L MgCl2, 3 mmol/L KCl, 5 mmol/L CaCl2, 50 mmol/L sucrose, and 10 mmol/L HEPES at pH 7.4), with 0.5 mg/ml collagenase (type IA) and 0.5 mg/ml trypsin (type III) for 3 min at room temperature. The enzymatic digestion was halted using culture medium in culture dishes. The culture medium was composed of 200 mmol/L NaCl, 4 mmol/L MgCl2, 3 mmol/L KCl, 5 mmol/L CaCl2, 50 mmol/L sucrose, 10 mmol/L HEPES, 5% (w/v) fetal bovine serum, 50 U/ml streptomycin, and 50 U/ml penicillin (pH 6.8). The isolated DUM cells were fostered in a 5% CO2 incubator at 28 °C for 2–3 h before the experiment.
2.4. Solution preparation
The sodium currents from cockroach DUM neurons were recorded using an external solution containing 100 mmol/L NaCl, 4 mmol/L KCl, 2 mmol/L CaCl2, 10 mmol/L D-glucose, 10 mmol/L HEPES, 50 mmol/L choline-Cl, 20 mmol/L tetraethylammonium chloride (TEA-Cl), 1 mmol/L 4-aminopyridine (4-AP), and 0.02 mmol/L CdCl2·2.5H2O at pH 6.8, and an internal solution of 100 mmol/L CsF, 40 mmol/L CsCl, 10 mmol/L HEPES, 3 mmol/L MgCl2·6H2O, 10 mmol/L ethyleneglycol bis(2-aminoethyl ether) tetraacetic acid (EGTA) and 10 mmol/L TEA-Cl, at pH 7.0.
2.5. Electrophysiological recordings
The electrophysiological recordings of HNTX-VI and HWTX-III were obtained according to the methodology described by Xiao and Liang (2003b). Sodium currents were recorded from experimental cells using an EPC-9 patch-clamp amplifier (HEKA Electronics, Germany) under a whole-cell patch clamp configuration at room temperature (22–25 °C).
2.6. Data analysis
Experimental data were obtained and statistical analysis was conducted using Pulse+Pulsefit 8.0 (HEKA Electronics, Germany) and SigmaPlot 9.0 (Sigma, USA). The data in the study are described as mean±standard error (SE). The fast inactivation was assayed by calculating the I 5 ms/I peak ratio, where I 5 ms is the current measured at the depolarization of 5 ms (Alami et al., 2003), and I peak is the peak amplitude. The statistical significance of the toxin effect was determined using paired Student’s t-test with P<0.05 considered significant.
The curves were fit with concentration-dependent inhibition (%) and steady-state sodium channel inactivation (I test/I max) obtained from the Boltzmann equations given below:
| Inhibition (%)=100/[1+exp(C−IC50)/K], | (1) |
| Itest/Imax=1/[1+exp(V−V1/2)/K]. | (2) |
In Eq. (1), IC50 is the concentration of toxin at half-maximal inhibition, K is the slope factor, and C is the toxin concentration. In Eq. (2), I test is the peak amplitude of I Na at −10 mV test pulse from a holding potential of −130 to −20 mV, and I max is the maximal peak amplitude. V 1/2 is the voltage at half-inactivation, and V is the test voltage.
3. Results
3.1. Effects of HNTX-VI and HWTX-III on insect sodium currents
The HNTX-VI and HWTX-III action on insect VGSCs was characterized in P. americana DUM neurons. Multiple ion channels are expressed in the insect DUM neurons, where underlie spontaneous electrical activities. All VGSCs in cockroach DUM neurons, however, are tetrodotoxin-sensitive (TTX-S) isoforms (Szeto et al., 2000).
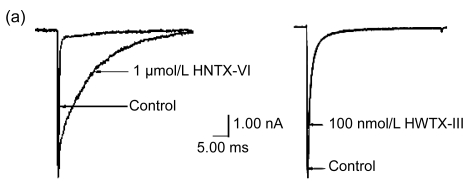
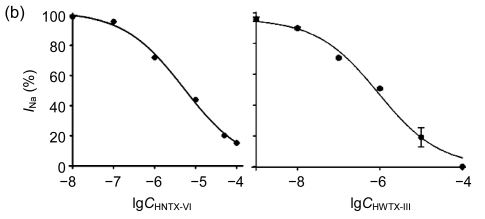
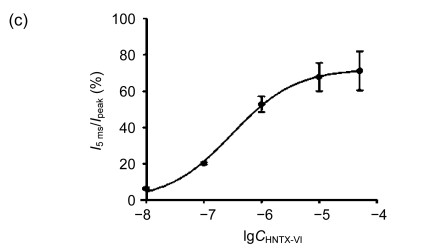
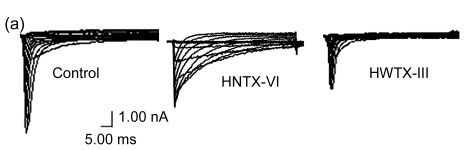
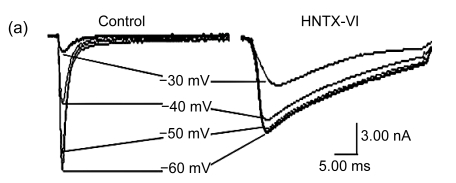
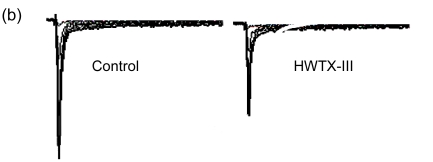
The effects of HNTX-VI and HWTX-III on insect DUM neuron Na+ currents are shown in Fig. 1 (n=6). The sodium currents were induced by a 50-ms step depolarization ranging from −80 to −10 mV every second. We found that HNTX-VI inhibited sodium channel inactivation and reduced control peak current amplitudes, while HWTX-III only inhibited inward sodium currents and did not alter activation or inactivation (Fig. 1a). The efficiency of HNTX-VI on inactivation was determined by calculating the I 5 ms/I peak ratio. The I 5 ms/I peak ratio is an estimate of the probability for the channel that is not inactivated after 5 ms. The HNTX-VI at 10 μmol/L delayed the channel inactivation of currents by (67.8±7.7)% (Fig. 1c). In parallel, HNTX-VI reduced the peak current amplitude by (56.1±9.8)%, while the inhibition and reduction were facilitated in a dose-dependent manner; their IC50 values were determined to be 0.3 and 5.2 μmol/L, respectively (Fig. 1b left and Fig. 1c). Furthermore, the reduction of HWTX-III on the current amplitude also followed a dose-dependent pattern. The IC50 estimated from the concentration-effect curve was 1.106 μmol/L (Fig. 1b, right). The rat dorsal root ganglion contains all types of mammalian subtype sodium channels except Nav1.5 and Nav1.4. Therefore, HWTX-III was tested on the sodium channels from adult rat dorsal root ganglion neurons; however, no effect was detected (data not shown). Furthermore, HWTX-III displayed strong effect on insect sodium channel with IC50 about 1 µmol/L. Therefore, HWTX-III could be an insect-specific neurotoxin.
Fig. 1.
Effects of HNTX-VI and HWTX-III on insect DUM neuron sodium currents
All current traces were induced by depolarizing the cell from a holding potential ranging from −80 to −10 mV. The duration of the test pulse was 20 ms. (a) 1 μmol/L of HNTX-VI and 100 nmol/L of HWTX-III changed the sodium currents in insect neurons; (b) The concentration-dependent effect of HNTX-VI and HWTX-III on sodium currents in insect DUM neurons; (c) Dose-dependent inhibition of sodium current inactivation by HNTX-VI (mean±SE) measured from 5–8 separated experimental cells. The points were fit based on Boltzmann Eq. (1). C HNTX-VI and C HWTX-III are the concentrations (mol/L) of HNTX-VI and HNTW-III, respectively
3.2. Effects of HNTX-VI and HWTX-III on the current-voltage relationship of insect sodium channels
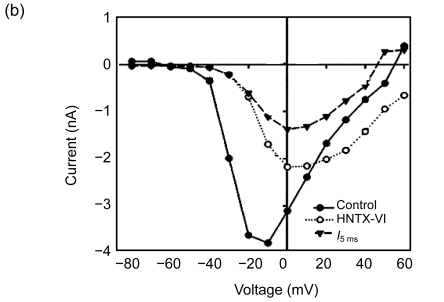
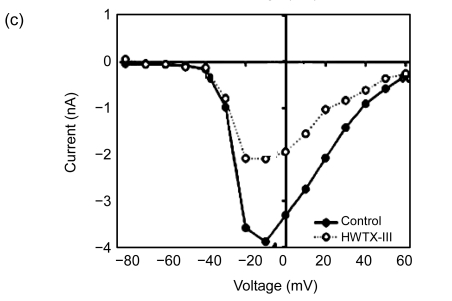
Current-voltage plots were measured using test voltage pulses ranging from −80 to +60 mV in a 10-mV step from a holding potential of −80 mV. As shown in Fig. 2 (n=6), 10 µmol/L of the HNTX-VI treatment failed to shift the threshold potential of sodium channel activation. This treatment, however, also caused an increase in the threshold potential around 10 mV in the active voltage of the peak inward current and the reverse potential. The data points clearly show that peak I Na was decreased and I 5 ms was induced by toxin treatment between −50 and +45 mV (Fig. 2b). No significant changes were observed, however, in the HWTX-III activation threshold and the active voltage of inward peak currents. Similarly, there was no membrane reversal potential observed (Fig. 2c). HWTX-III showed no effect on the activation and inactivation kinetics of the insect neuron VGSCs, and also no change in the ion selectivity of the channels.
Fig. 2.
Effects of HNTX-VI and HWTX-III on the current-voltage relationship of sodium channels
(a) Na+ currents were elicited by a series of 20 ms depolarizations ranging from −80 to +60 mV in 10-mV steps applied from a holding potential of −80 mV; (b) The I/V curves showed the relationships between current traces of control and after addition of 10 µmol/L HNTX-VI. I 5 ms was the current inactivated at 5 ms; (c) The I/V curves show the relationship between current traces of control and after addition of HWTX-III
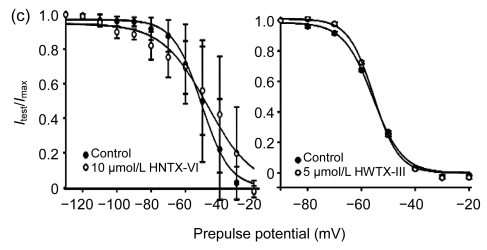
3.3. Effects of HNTX-VI and HWTX-III on steady-state inactivation of insect sodium channels
The effects of these toxins on steady-state inactivation were investigated using standard two-pulse protocol as shown in Fig. 3 (n=6). After HNTX-VI treatment, the midpoint voltage (V 1/2) was shifted only by +3.6 mV (−50.7 mV→−47.1 mV), implying that a modulation of HNTX-VI on the steady-state inactivation potential of the sodium channels was not significant. In addition, HNTX-VI at 10 μmol/L showed a non-inactivating component at prepulse test potential to a depolarization deeper than −55 mV (Fig. 3c, left), revealing an incomplete inactivation of the insect sodium currents. HWTX-III did not affect the steady-state inactivation kinetics of the VGSC currents. With HWTX-III at 5 µmol/L, the midpoint voltage (V 1/2) and slope value (K) were (−55.72±0.38) mV and (−5.43±0.46), respectively. Similarly, the control treatment also indicated the values for V 1/2 and K to be (−55.48±0.45) mV and (−4.67±0.35), respectively (Fig. 3c, right). These results suggest that HWTX-III did not interact with the VGSCs in an inactivated state.
Fig. 3.
Effects of HNTX-VI and HWTX-III on the steady-state inactivation of sodium channels
A conventional two-pulse voltage-clamp protocol was used. Sodium currents were elicited by a 50-ms depolarizing potential of −10 mV from various prepulse potentials for 500 ms ranging from −130 to −20 mV with a 10-mV increment. (a) Typical current traces were obtained following a 50-ms test pulse to −10 mV from prepulse potentials of −60, −50, −40, and −30 mV; (b) Typical current traces were obtained following a 50-ms depolarization to −10 mV from prepulse potentials of −90, −60, −50, and −40 mV; (c) Ratios of I test to I max were fit using the Boltzmann equation
4. Discussion
Sodium channels are widely distributed within insect neuron membranes. They represent an important structural factor that controls cellular excitability in biological systems. Therefore, sodium channels are targeted by a variety of animal toxins to immobilize and/or kill their prey. Many animals have evolved a venom gland to secrete a wide variety of these toxins.
Our preliminary studies show that cockroaches are reversibly paralyzed by HNTX-VI and HWTX-III for several hours. Therefore, the activity of these toxins is likely to target the sodium channel in the insect neuron cells. VGSCs toxins are classified into two groups based on their immunological properties: blockers and modulators. The blockers include tetrodotoxin (TTX), saxitoxin, and μ-conotoxins that occlude the channel pore by binding to site 1 to block inward sodium currents (Shon et al., 1998). The modulators include δ-atracotoxin, sea anemone toxins, and scorpion α-toxins that modulate the processes of VGSCs kinetics by binding to neuronal sites 2–6 (Rogers et al., 1996; Nicholson et al., 1998; Richard Benzinger et al., 1999).
The action of HNTX-VI and HWTX-III on VGSCs in cockroach Periplaneta americana DUM neurons was characterized using the whole-cell voltage clamp technique. We found the mechanism of action of the two insect toxins on VGSCs to be different. HNTX-VI caused a significant slowdown in the Na+ current inactivation and reduction in peak current amplitudes on insect DUM neurons (Fig. 1). These observations suggested that HNTX-VI might modulate the activities of insect sodium channels in a manner similar to δ-atracotoxins, which increased the recovery rate from channel inactivation by binding to neuronal site 3 (Nicholson et al., 1998). In the presence of HNTX-VI, no significant shift was detected on steady-state inactivation kinetics; however, the production of a non-inactivating component at prepulse potential was found to be more positive than −55 mV, similar to δ-ACTX-Hv1a (Grolleau et al., 2001), but different from the spider toxin Tx4 (6-1) (de Lima et al., 2002). These actions suggest that HNTX-VI could be an excitatory spider toxin (Li et al., 2004). HWTX-III was found, however, to depress the amplitude of the Na+ currents on cockroach DUM neurons, which is different in effect from that of HNTX-VI. No changes in the activation voltage threshold and the membrane reversal potential of HWTX-III were observed, indicating that HWTX-III did not change the ion selectivity in the channels. HWTX-III has no effect on the activation and inactivation kinetics. Therefore, we infer that HWTX-III belongs to the class of depressant insect toxins. Furthermore, HWTX-III did not cause significant change in the steady-state inactivation curve, indicating the activity of HWTX-III to be distinct from other depressant spider toxins, such as HNTX-I (Li et al., 2003), HWTX-I (Wang et al., 2007), HNTX-V (Xiao and Liang, 2003a), and HNTX-III (Xiao and Liang, 2003b).
In conclusion, HNTX-VI and HWTX-III are two insect toxins that alternate VGSCs. Their action is complex, with HNTX-VI slowing sodium channel inactivation and HWTX-III selectively blocking insect sodium channels. These two toxins cause diverse effects and their mechanism of action demonstrate the diversity of spider toxins, providing a theoretical foundation for designing and developing more effective and safer insecticides.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 30500146) and the National Basic Research Program (973) of China (No. 2006CB708508)
References
- 1.Alami M, Vacher H, Bosmans F, Devaux C, Rosso JP, Bougis PE, Tytgatt J, Darbon H, Martin-Eauclaire MF. Characterization of Amm VIII from Androctonus mauretanicus mauretanicus: a new scorpion toxin that discriminates between neuronal and skeletal sodium channels. Biochemical Journal. 2003;375(3):551–560. doi: 10.1042/BJ20030688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. doi: 10.1016/S0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 3.de Lima ME, Stankiewicz M, Hamon A, Figueiredo SG, Cordeiro MN, Diniz CR, Martin-Eauclaire MF, Pelhate M. The toxin Tx4 (6-1) from the spider Phoneutria nigriventer slows down Na+ current inactivation in insect CNS via binding to receptor site 3. Journal of Insect Physiology. 2002;48(1):53–61. doi: 10.1016/S0022-1910(01)00143-3. [DOI] [PubMed] [Google Scholar]
- 4.Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28(2):365–368. doi: 10.1016/S0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 5.Grolleau F, Stankiewicz M, Birinyi-Strachan LC, Wang XH, Nicholson GM, Pelhate M, Lapied B. Electrophysiological analysis of the neurotoxic action of a funnel-web spider toxin, δ-atracotoxin-HV1a on insect voltage-gated Na+ channels. The Journal of Experimental Biology. 2001;204(4):711–721. doi: 10.1242/jeb.204.4.711. [DOI] [PubMed] [Google Scholar]
- 6.Haigeny MC, Lakatta EG, Stern MD, Silverman HS. Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation. 1994;90(1):391–399. doi: 10.1161/01.cir.90.1.391. [DOI] [PubMed] [Google Scholar]
- 7.Huang RH, Liu ZH, Liang SP. Purification and characterization of a neurotoxic peptide huwentoxin-III and a natural inactive mutant from the venom of the spider Selenocosmia huwena Wang (Ornithoctonus huwena Wang) Acta Biochimica et Biophysica Sinica. 2003;35(11):976–980. [PubMed] [Google Scholar]
- 8.Li D, Xiao Y, Hu W, Xie J, Bosmans F, Tytgat J, Liang S. Function and solution structure of hainantoxin-I, a novel insect sodium channel inhibitor from the Chinese bird spider Selenocosmia hainana. FEBS Letters. 2003;555(3):616–622. doi: 10.1016/S0014-5793(03)01303-6. [DOI] [PubMed] [Google Scholar]
- 9.Li DL, Xiao YC, Xu X, Xiong X, Lu SY, Liu ZH, Zhu Q, Wang MC, Gu XC, Liang SP. Structure-activity relationships of hainantoxin-IV and structure determination of active and inactive sodium channel blockers. Journal of Biological Chemistry. 2004;279(36):37734–37740. doi: 10.1074/jbc.M405765200. [DOI] [PubMed] [Google Scholar]
- 10.Meng ZQ, Nie AF. Enhancement of sodium metabisulfite on sodium currents in acutely isolated rat hippocampal CA1 neurons. Environmental Toxicology and Pharmacology. 2005;20(1):35–41. doi: 10.1016/j.etap.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson GM. Insect-selective spider toxins targeting voltage-gated sodium channels. Toxicon. 2007;49(4):490–512. doi: 10.1016/j.toxicon.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson GM, Walsh R, Little MJ, Tyler MI. Characterisation of the effects of robustoxin, the lethal neurotoxin from the Sydney funnel-web spider Atrax robustus, on sodium channel activation and inactivation. Pflügers Archiv-European Journal of Physiology. 1998;436(1):117–126. doi: 10.1007/s004240050612. [DOI] [PubMed] [Google Scholar]
- 13.Pan JY, Hu WJ, Liang SP. Purification, sequencing and characterization of hainantoxin-VI, a neurotoxin from the Chinese bird spider Selenocosmia hainana . Zoological Research. 2002;23(4):280–283. (in Chinese) [Google Scholar]
- 14.Richard Benzinger G, Tonkovich GS, Hanck DA. Augmentation of recovery from inactivation by site-3 Na channel toxins. A single-channel and whole-cell study of sustained currents. The Journal of General Physiology. 1999;113(2):333–346. doi: 10.1085/jgp.113.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. Journal of Biological Chemistry. 1996;271(27):15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- 16.Shon KJ, Olivera BM, Watkins M, Jacobsen RB, Gray WR, Floresca CZ, Cruz LJ, Hillyard DR, Brink A, Terlau H, et al. μ-Conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. The Journal of Neuroscience. 1998;18(12):4473–4481. doi: 10.1523/JNEUROSCI.18-12-04473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu Q, Liang SP. Purification and characterization of huwentoxin-II, a neurotoxic peptide from the venom of the Chinese bird spider Selenocosmia huwena. Journal of Peptide Research. 1999;53(5):486–491. doi: 10.1034/j.1399-3011.1999.00039.x. [DOI] [PubMed] [Google Scholar]
- 18.Szeto TH, Birinyi-Strachan LC, Smith RW, Connor M, Christie MJ, King G, Nicholson GM. Isolation and pharmacological characterisation of δ-atracotoxin-Hv1b, a vertebrate-selective sodium channel toxin. FEBS Letters. 2000;470(3):293–299. doi: 10.1016/S0014-5793(00)01339-9. [DOI] [PubMed] [Google Scholar]
- 19.Taylor CP, Meldrum B. Na+ channels as targets for neuroprotective drugs. Trends in Pharmacological Sciences. 1995;16(9):309–316. doi: 10.1016/S0165-6147(00)89060-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang MC, Guan X, Liang SP. The cross channel activities of spider neurotoxin huwentoxin-I on rat dorsal root ganglion neurons. Biochemical and Biophysical Research Communications. 2007;357(3):579–583. doi: 10.1016/j.bbrc.2007.02.168. [DOI] [PubMed] [Google Scholar]
- 21.Warmke JW, Reenan RA, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, van der Ploeg LH, et al. Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology. The Journal of General Physiology. 1997;110(2):119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao YC, Liang SP. Purification and characterization of hainantoxin-V, a tetrodotoxin-sensitive sodium channel inhibitor from the venom of the spider Selenocosmia hainana . Toxicon. 2003;41(6):643–650. doi: 10.1016/S0041-0101(02)00280-5. [DOI] [PubMed] [Google Scholar]
- 23.Xiao YC, Liang SP. Inhibition of neuronal tetrodotoxin-sensitive Na+ channels by two spider toxins: hainantoxin-III and hainantoxin-IV. European Journal of Pharmacology. 2003;477(1):1–7. doi: 10.1016/S0014-2999(03)02190-3. [DOI] [PubMed] [Google Scholar]
- 24.Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, et al. Sodium channel β4, a new disulfide-linked auxiliary subunit with similarity to β2. The Journal of Neuroscience. 2003;23(20):7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zlotkin E. The insect voltage-gated sodium channel as target of insecticides. Annual Review of Entomology. 1999;44(1):429–455. doi: 10.1146/annurev.ento.44.1.429. [DOI] [PubMed] [Google Scholar]