Abstract
Tea polyphenols have been shown to have ansticancer activity in many studies. In the present study, we investigated effects of theaflavin-3-3′-digallate (TF3), one of the major theaflavin monomers in black tea, in combination with ascorbic acid (AA), a reducing agent, and (−)-epigallocatechin-3-gallate (EGCG), the main polyphenol presented in green tea, in combination with AA on cellular viability and cell cycles of the human lung adenocarcinoma SPC-A-1 cells. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay showed that the 50% inhibition concentrations (IC50) of TF3, EGCG, and AA on SPC-A-1 cells were 4.78, 4.90, and 30.62 μmol/L, respectively. The inhibitory rates of TF3 combined with AA (TF3+AA) and EGCG combined with AA (EGCG+AA) at a molar ratio of 1:6 on SPC-A-1 cells were 54.4% and 45.5%, respectively. Flow cytometry analysis showed that TF3+AA and EGCG+AA obviously increased the cell population in the G0/G1 phase of the SPC-A-1 cell cycle from 53.9% to 62.8% and 60.0%, respectively. TF3-treated cells exhibited 65.3% of the G0/G1 phase at the concentration of its IC50. Therefore, TF3+AA and EGCG+AA had synergistic inhibition effects on the proliferation of SPC-A-1 cells, and significantly held SPC-A-1 cells in G0/G1 phase. The results suggest that the combination of TF3 with AA or EGCG with AA enhances their anticancer activity.
Keywords: Theaflavin-3-3′-digallate (TF3), (−)-epigallocatechin-3-gallate (EGCG), Ascorbic acid (AA), Synergism, SPC-A-1 cells, Cell cycle
1. Introduction
Epidemiologic studies have suggested that the consumption of tea is linked to a decreased incidence of various cancers (Yang C.S. et al., 2007). The inhibitory action of tea and tea products against carcinogenesis has been demonstrated in animal models, including skin, lung, esophagus, stomach, liver, small intestine, pancreas, prostate, and mammary gland cancers (Conney et al., 1999; Tu et al., 2004).
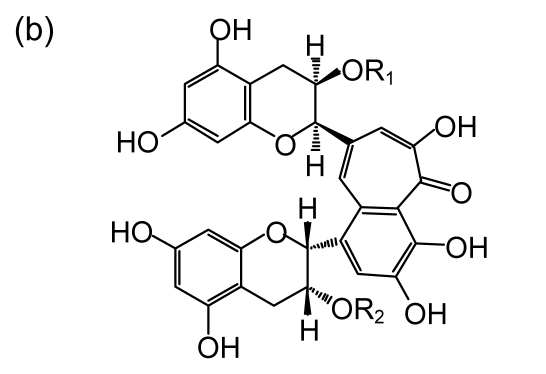
Both black tea and green tea have monomeric gallated catechins of the flavanol class. The main polyphenol presented in green tea is (−)-epigallo-catechin-3-gallate (EGCG) (Fig. 1a), which accounts for about 60%–70% of total catechins (Agarwal and Mukhtar, 1996). EGCG is a precursor of theaflavin-3-3′-digallate (TF3) (Fig. 1b). Previous studies showed that TF3 appears to be the most potent compound in tea on inhibition of proliferation and induction of apoptosis of cancer cells (Yang et al., 1998). Black tea, accounting for 78% of the world tea consumption, is the product of tea catechin oxidation and polymerization via fermentation of fresh tea leaves (Yang et al., 2009). The fermentation results in the formation of theaflavins. The major theaflavin monomers in black tea are theaflavin (TF1), theaflavin-3-gallate (TF2A), theaflavin-3′-gallate (TF2B), and theaflavin-3-3′-digallate (TF3). TF3 has two galloyl groups, TF2A and TF2B each have one, and TF1 has none. More specific investigations concerning with theaflavin structure reported that the antioxidative activities of theaflavin monomers depend on the number and position of hydroxyl groups within their molecules (Yang Z.Y. et al., 2007), e.g., the antioxidation activities following the sequence of TF3>TF2>TF1 (Leung et al., 2001).
Fig. 1.
Chemical structures of EGCG and theaflavins
(a) EGCG; (b) Theaflavins. TF1 (R1=R2=OH), TF2A (R1=gallate, R2=OH), TF2B (R1=OH, R2=gallate), TF3 (R1=R2=gallate)
The antioxidative activities of black tea theaflavins are also studied for their influence on activation of transcription factors such as nuclear factor kappa B (NFκB). Theaflavins, in particular TF3, could effectively inhibit the activation of NFκB, preventing the expression of inducible nitric oxide synthase (iNOS) gene or down-regulating the expression of iNOS mRNA, which led to a decrease of nitric oxide synthesis in RAW 264.7 cells (Hong et al., 2001). Ascorbic acid (AA) is an essential nutrient involved in many biochemical reactions and is known to protect some flavonoids against oxidative degradation during processing and storage of juices (Kaack and Austed, 1998). The biochemical roles of AA are related to its ability to act as an electron donor or reducing agent. As a free radical scavenger, AA can protect cellular biopolymers against the initiation and progression of carcinogenesis (Kim et al., 2006).
Lung cancer is one of the most common cancers worldwide. It has been suggested that there is an inverse association between tea intake and subsequent lung cancer incident (Yang et al., 2009). However, the synergic effects of TF3 or EGCG with AA on human epithelial lung adenocarcinoma SPC-A-1 cells have not been examined. In this study, we investigated in vitro the combined effects of TF3 with AA (TF3+AA) and EGCG with AA (EGCG+AA) on SPC-A-1 cells.
2. Materials and methods
2.1. Cell culture
Human lung adenocarcinoma SPC-A-1 cells were purchased from the Cell Bank of Chinese Academy of Science (Shanghai, China) and cultured in RPMI-1640 medium (GIBCO BRL, Grand Island, NY, USA) supplemented with 10% (w/v) fetal calf serum (GIBCO BRL) in a humidified atmosphere of 37 °C with 5% CO2.
2.2. Chemicals
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and AA were purchased from Sigma-Aldrich (St. Louis, MO, USA). EGCG was a generous gift from the Tea Science Department of Zhejiang University, with a purity of above 98% (analyzed by high performance liquid chromatography (HPLC)). Tea polyphenols were provided by Siming Natural Plant Co. (Zhejiang, China). All other chemicals were of analytical grade and were commercially obtained from local chemical suppliers.
2.3. Preparation and analysis of theaflavin monomers
The theaflavins (80% purity) were prepared by the enzymatic oxidation of tea polyphenols using immobilized polyphenol oxidase (PPO) according to the protocol of Tu and Xia (2004). TF2A, TF2B, TF1, and TF3 were separated using a Sephadex LH-20 gel (Pharmacia Fine Chemicals Inc., NJ, USA) column (Tu et al., 2004).
Theaflavin monomer was determined using our previously established method (Yang et al., 2008). Briefly, theaflavin monomers (0.4 mg) and EGCG (2.0 mg) were dissolved in 1.0 ml methanol, respectively. The concentrations of theaflavin monomer and EGCG were determined using a Shimadzu LC-2010A (Shimadzu, Kyoto, Japan).
2.4. Inhibition of tea polyphenols on SPC-A-1 cells
Cell viability was measured by quantitative colorimetric assay with MTT, which shows the mitochondrial activity of living cells as described by Mosmann (1983) and Denizot and Lang (1986). SPC-A-1 cells were seeded into 96-well plates 0.1 ml/well at a density of 1×105 cells/ml. After the cells attached, EGCG, TF2B, TF3, TF2A, or AA at different doses was added. After an exposure period, 10 μl MTT (5 mg/ml) was added to each well. The final concentrations for each of AA and TF2A added to SPC-A-1 were 5, 10, 50, 100, and 200 μmol/L, and the final concentrations for each of TF3, EGCG, and the compound of TF2B and TF3 were 0.5, 1, 5, 10, and 20 μmol/L. Six replicates were set for each treatment.
2.5. Analysis of synergistic effects
Using the median-effect method developed by Chou and Talalay (1984), the dose-response curve was plotted for each drug, and the multiple dose of a fixed-ratio combination was plotted by
| fa/fu=(D/Dm)m, | (1) |
where D is the dose administered, D m is the dose required for 50% inhibition of growth (IC50), f a is the fraction affected by dose D, f u is the unaffected fraction, and m is a coefficient signifying the sigmoidicity of the dose-response curve.
The dose-response curve was plotted using a logarithmic conversion of the equation to determine the m and D m values. Dx required for x percent effect (f a)x was then calculated by
| Dx=Dm[(fa)x/(fu)x]1/m. | (2) |
Thus, the combination index (CI) can be defined by the isobologram
| CI=(D)1/(Dx)1+(D)2/(Dx)2+α(D)1(D)2/[(Dx)1(Dx)2], | (3) |
where (Dx)1 is the dose of drug-1 for producing x percent effect and (D)1 for producing the same x percent effect in combination with drug-2; (Dx)2 and (D)2 correspond to drug-2. Consequently, CI<1 indicates synergism, CI>1 shows antagonism, and CI=1 points to additive effects. The CI values obtained from both the mutually non-excusive (α=1) and mutually exclusive (α=0) isobologram equations are presented in this work.
2.6. Detection of cell cycle by flow cytometry
Cell cycle analysis for the cell distribution in the sub-G1 region was determined by flow cytometric analysis after propidium iodide (PI) staining. Cells were treated with: AA (30.62 μmol/L), TF3 (4.78 μmol/L), EGCG (4.90 μmol/L), TF3 (4.78 μmol/L)+AA (30.62 μmol/L), and EGCG (4.90 μmol/L)+AA (30.62 μmol/L) according to their IC50 for 48 h. The cells in suspension (5×104 cells/ml) with or without drugs were fixed with 80% ethanol at 4 °C for 24 h and then stained with 25 μg/ml PI. After staining, the population of cells in each cell cycle phase was determined using the FAC Star flow cytometry (Becton-Dickinson, San Jose, California, USA).
2.7. Statistical analysis
All experiments were performed in triplicate. One-way analysis of variance (ANOVA) was used to estimate overall significance, followed by the post-hoc Tukey’s tests corrected for multiple comparisons. Data are presented as mean±standard deviation (SD). A probability level less than 5% (P<0.05) was considered significant.
3. Results
3.1. Effects of theaflavin monomers, AA, and EGCG on the proliferation of SPC-A-1 cells
Firstly, the effects of tea polyphenols (TF2A, TF2B+TF3, TF3, and EGCG) and AA on cell viability of SPC-A-1 were measured. The highest inhibition rate of TF2A on the proliferation of SPC-A-1 cells was 40.39% at 100 μmol/L, so we were not able to calculate the IC50 of TF2A. Thus, the TF2A was not considered in the following study.
Cells were continuously exposed to various drug treatments for 48 h and then tested for the cell viability using MTT assay. The lower the IC50 value, the higher the inhibition on the SPC-A-1 cells. TF2B+TF3, TF3, and EGCG showed the most potent inhibition on the SPC-A-1 cells, with the IC50 values of 6.70, 4.78, and 4.90 μmol/L, respectively. The IC50 value of AA was 30.6 μmol/L, which was significantly higher than that of tea polyphenols alone.
3.2. Cytotoxicities of EGCG+AA and TF3+AA on SPC-A-1 cells
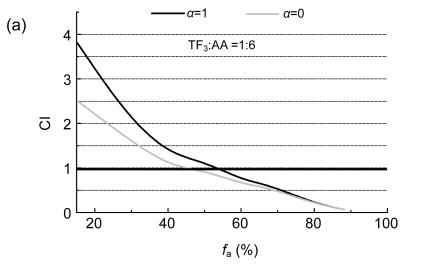
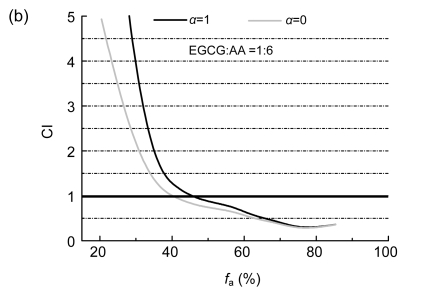
The interactions of EGCG with AA and TF3 with AA on SPC-A-1 were analyzed by the method of Chou and Talalay (1984). The molar ratios of TF3 to AA and EGCG to AA were both set at 1:6 based on their IC50 values. CI plots were constructed by computer analysis using Eq. (3); and both the mutually non-exclusive (α=1) and mutually exclusive (α=0) isobologram equations were used.
Biphasic effects of a drug combination were shown for TF3:AA and EGCG:AA at the molar ratio of 1:6 against SPC-A-1 cells, with a turning point appearing at 54.4% and 45.5% of f a values (Fig. 2). The combinations of AA with EGCG and AA with TF3 both enhanced their inhibition on SPC-A-1 cells at a higher concentration, which is indicated by CI<1.
Fig. 2.
Combination index (CI) plots of interactions between TF3, EGCG, and AA
SPC-A-1 cells were treated with tea polyphenol monomers and AA at a fixed molar ratio: (a) TF3:AA =1:6 and (b) EGCG:AA=1:6. Using the mutually exclusive or mutually non-exclusive isobologram equation, the affected fraction (f a)-CI plots for SPC-A-1 cells were constructed by computer analysis of the data generated from the median effect analysis. CI<1 occurred over a wide range of inhibition levels, indicating synergism
3.3. Effects of polyphenols and AA on the growth inhibition of SPC-A-1 cells and G1-phase arrest
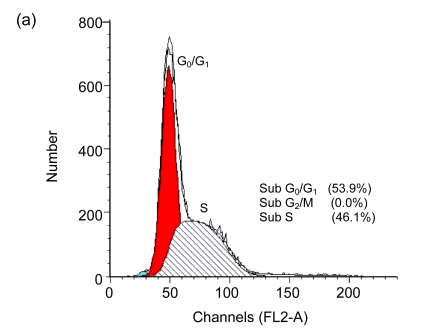
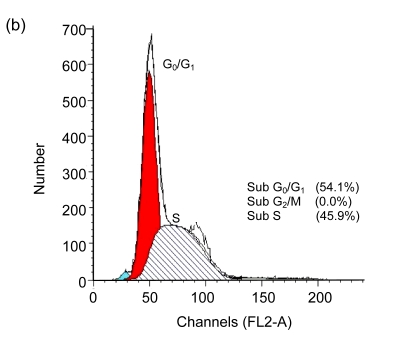
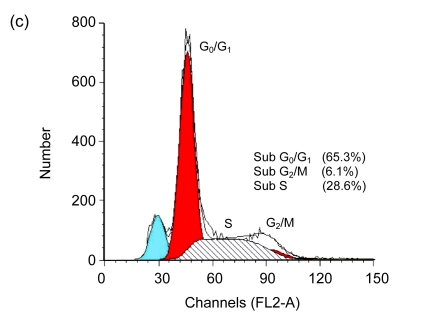
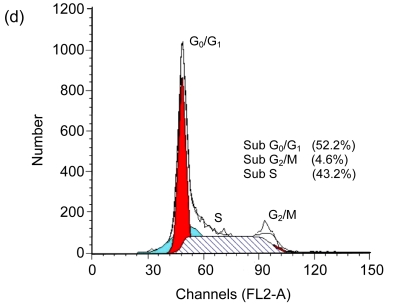
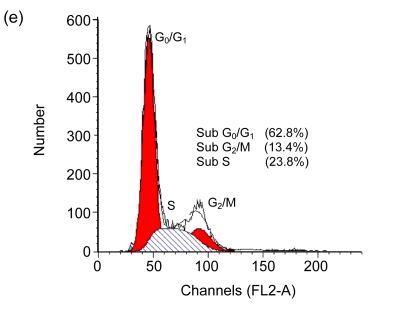
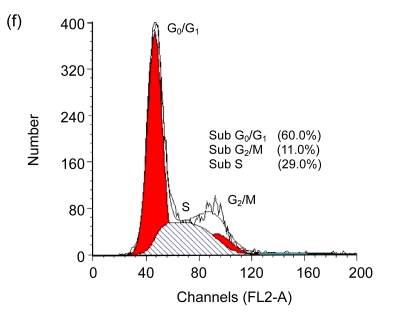
Cell cycle arrest is a characteristic of many anticancer drugs. Cells in G0/G1 phase arrest might go into apoptosis, or recover from the G0/G1 phase and enter into S phase. The effects of EGCG and TF3 on distribution of cells in the cell cycle were analyzed by the flow cytometry. The results show that while all treatments led to an increase of cell population in G0/G1 phase, TF3 promoted the biggest increase (Fig. 3c). TF3+AA and EGCG+AA obviously increased the cell population in G0/G1 phase of the SPC-A-1 cell cycle from 53.9% to 62.8% and 60.0%, respectively (Figs. 3a, 3e and 3f). TF3+AA reduced the cell population in S phase from 46.1% to 23.8% (Figs. 3a and 3e), and increased apoptosis from 1.0% to 22.3%. Interestingly, TF3-treated cells exhibited 65.3% of G0/G1 phase at the IC50.
Fig. 3.
Cell cycle analysis of SPC-A-1 cells treated with the indicated concentrations of drugs for 48 h by flow cytometry analysis
After cells were fixed and stained with propidium iodide, and the DNA content was measured by flow cytometry. Cell cycle distribution was analyzed using the FAC Star flow cytometry. (a) Control; (b) Ascorbic acid (30.62 μmol/L); (c) TF3 (4.78 μmol/L); (d) EGCG (4.90 μmol/L); (e) TF3 (4.78 μmol/L)+AA (30.62 μmol/L); (f) EGCG (4.90 μmol/L)+AA (30.62 μmol/L)
4. Discussion
EGCG displayed strong growth inhibitory effects against lung cancer lines H661 and H1299, with an estimated IC50 value of 22 μmol/L (Yang et al., 1998), which is much higher than that of TF3 or EGCG in lung cancer lines SPC-A-1 in the present study. Moon et al. (2007) demonstrated that EGCG was a strong inducer of early growth response gene-1 expression and mediated early growth response gene-1 nuclear translocation via extracellular signal-regulated protein kinase signaling pathway in A549 cells. TF3 had stronger inhibition on I kappa B (IκB) kinase (IKK) activity in activated macrophages than other polyphenols. TF3 strongly inhibited both IKK1 and IKK2 activities and prevented the degradation of IκBα and IκBβ in activated macrophage cells (Pan et al., 2000). This suggests that the inhibition of IKK activity by TF3 could occur by a direct effect on IKKs or on upstream events in the signal transduction pathway.
Growth inhibitory activities of EGCG have been demonstrated by Yang et al. (2009), which is also confirmed in this study. Whereas green tea contains EGCG, black tea contains theaflavins. In the present study, we showed that TF3 was as effective as EGCG. We extended our studies to the synergistic inhibition of TF3 in combination with AA and EGCG with AA on human lung cancer SPC-A-1 cells, and found that AA enhanced the inhibitory abilities of TF3 and EGCG on lung cancer cells. When EGCG or TF3 was combined with AA at a lower concentration, the combination significantly enhanced the antagonism (CI>1). Polyphenols are generally unstable under oxidative conditions, so EGCG levels decrease rapidly, even in solutions of neutral pH (Hatano et al., 2003). The synergism in this study suggests that AA may protect EGCG from oxidation. The chemical structure of TF3 is bulkier and has two A-rings linked by a fused seven-member ring (Yang Z.Y. et al., 2007). Theaflavin monomers are transformed from tea catechins through oxidation catalyzed by enzyme and chemical condensation, which makes theaflavin monomers more stable than EGCG. Some experiments demonstrated that the theaflavins presented in black tea have at least the same antioxidant potential as catechins presented in green tea, and that the conversion of catechins to theaflavins during black tea production does not alter significantly their free radical-scavenging activity (Leung et al., 2001). More studies indicated that the synergistic anticancer effects of AA and green tea extract on several cancer cell lines in tissue culture were higher than those of the individual nutrients (Roomi et al., 2006).
AA as a well-known antioxidant can protect tea polyphenols from oxidation. We also found that the synergistic effects of TF3+AA and EGCG+AA on SPC-A-1 cells were through cell cycle arrest, but the EGCG or TF3 alone can lead to more apoptosis. In synergistic cultures, the relative number of apoptotic cells did not increase; a maximum of 2.1% of apoptotic cells were observed. TF3 can increase the cell cycle at the G0/G1 phase. When AA is added, it can produce a large amount of H2O2 by reducing the concentration of peroxidase (Zheng et al., 2002). TF3 shows the most potent hydroxyl radicals-scavenging ability (Yang et al., 2008).
Using human lymphoma cells, Saeki et al. (1999) revealed that TF3 and TF1 had equivalent apoptosis-inducing activities in vitro. Especially, Tu et al. (2004) suggested that TF2B was a more powerful inhibitor to the growth of human liver cancer cells, gastric cancer cells, and leukemia cells than TF3. Apparently, these findings suggest that multiple mechanisms may be responsible for the anticancer effects of theaflavins on different types of cancers.
In this study, we found that AA with EGCG or TF3 acts synergistically to suppress cell proliferation of SPC-A-1 in vitro. Presently, it is not clearly defined how black tea theaflavins exert their cytotoxic effects (Lung et al., 2004), cell cycle arrest (Chung et al., 1999), and inducing of apoptosis (Lu et al., 2000). Studies show that cancer cells are more sensitive than normal cells to the in vitro anti-proliferation effects of EGCG (Mittal et al., 2004) and black tea polyphenol extracts (Weisburg et al., 2004). After entering the cells, EGCG may produce reactive oxygen species (ROS) by an unknown mechanism (Yang et al., 2009).
In conclusion, this paper showed that TF3, one of the major theaflavin monomers in black tea, in combination with AA, a reducing agent, and EGCG, the main polyphenol presented in green tea, in combination with AA could synergistically inhibited the proliferation of SPC-A-1 cells, and increased its cell population in G0/G1 phase of cell cycle. It suggested that the combination of EGCG with AA and TF3 with AA may be potent anticancer agents for cancer therapy.
Footnotes
Project supported by the Key Program of Science and Technology of Zhejiang Province (No. 2007C12068) of China and the National Natural Science Foundation of China (No. 30901002)
References
- 1.Agarwal R, Mukhtar H. Cancer chemoprevention by polyphenols in green tea and artichoke. Advances in Experimental Medicine and Biology. 1996;401:35–50. doi: 10.1007/978-1-4613-0399-2_4. [DOI] [PubMed] [Google Scholar]
- 2.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 3.Chung JY, Huang CS, Meng XF, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Research. 1999;59(18):4610–4617. [PubMed] [Google Scholar]
- 4.Conney AH, Lu YP, Lou YR, Xie JP, Huang MT. Inhibitory effect of green and black tea on tumor growth. Proceedings of the Society for Experimental Biology and Medicine. 1999;220(4):229–233. doi: 10.1046/j.1525-1373.1999.d01-39.x. [DOI] [PubMed] [Google Scholar]
- 5.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of Immunological Methods. 1986;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 6.Hatano T, Kusuda M, Hori M, Shiota S, Tsuchiya T, Yoshida T. Theasinensin A, a tea polyphenol formed from (−)-epigallocatechin gallate, suppresses antibiotic resistance of methicillin-resistant Staphylococcus aureus . Planta Medica. 2003;69(11):984–989. doi: 10.1055/s-2003-45142. [DOI] [PubMed] [Google Scholar]
- 7.Hong JG, Smith TJ, Ho CT, August DA, Yang CS. Effects of purified green and black tea polyphenols on cyclooxygenase and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochemical Pharmacology. 2001;62(9):1175–1183. doi: 10.1016/S0006-2952(01)00767-5. [DOI] [PubMed] [Google Scholar]
- 8.Kaack K, Austed T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods for Human Nutrition. 1998;52(3):187–198. doi: 10.1023/A:1008069422202. [DOI] [PubMed] [Google Scholar]
- 9.Kim KN, Pie JE, Park JH, Park YH, Kim HW, Kim MK. Retinoic acid and ascorbic acid act synergistically in inhibition human breast cancer cell proliferation. The Journal of Nutritional Biochemistry. 2006;17(7):454–462. doi: 10.1016/j.jnutbio.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Leung LK, Su Y, Chen R, Zhang Z, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. Journal of Nutrition. 2001;131(9):2248–2251. doi: 10.1093/jn/131.9.2248. [DOI] [PubMed] [Google Scholar]
- 11.Lu JB, Ho CT, Ghai G, Chen KY. Differential effects of theaflavin monogallates on cell growth, apoptosis, and Cox-2 gene expression in cancerous versus normal cells. Cancer Research. 2000;60(22):6465–6471. [PubMed] [Google Scholar]
- 12.Lung HL, Ip WK, Chen ZY, Mak NK, Leung KN. Comparative study of the growth-inhibitory and apoptosis-inducing activities of black tea theaflavins and green tea catechin on murine myeloid leukemia cells. International Journal of Molecular Medicine. 2004;13(3):465–471. [PubMed] [Google Scholar]
- 13.Mittal A, Pate MS, Wylie RC, Tollefsbol TO, Katiyar SK. EGCG down-regulates telomerase in human breast carcinoma MCF-7 cells, leading to suppression of cell viability and induction of apoptosis. International Journal of Oncology. 2004;24(3):703–710. [PubMed] [Google Scholar]
- 14.Moon Y, Lee M, Yang H. Involvement of early growth response gene 1 in the modulation of microsomal prostaglandin E synthase 1 by epigallocatechin gallate in A549 human pulmonary epithelial cells. Biochemical Pharmacology. 2007;73(1):125–135. doi: 10.1016/j.bcp.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Pan MH, Lin-Shiau SY, Ho CT, Lin JH, Lin JK. Suppression of lipopolysaccharide-induced nuclear factor-kappa B activity by theaflavin-3,3'-digallate from black tea and other polyphenols through down-regulation of I kappa B kinase activity in macrophages. Biochemical Pharmacology. 2000;59(4):357–367. doi: 10.1016/S0006-2952(99)00335-4. [DOI] [PubMed] [Google Scholar]
- 17.Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. Inhibition of matrix metalloproteinase-2 secretion and invasion by human ovarian cancer cell line SK-OV-3 with lysine, proline, arginine, ascorbic acid and green tea extract. Journal of Obstetrics and Gynaecology Research. 2006;32(2):148–154. doi: 10.1111/j.1447-0756.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 18.Saeki K, Sano M, Miyase T, Nakamura Y, Hara Y, Aoyagi Y, Isemura M. Apoptosis-inducing activity of polyphenol compounds derived from tea catechins in human histiolytic lymphoma U937 cells. Bioscience Biotechnology and Biochemistry. 1999;63(3):585–587. doi: 10.1271/bbb.63.585. [DOI] [PubMed] [Google Scholar]
- 19.Tu YY, Xia HL. Immobilized Polyphenol Oxidase Catalyze Purified Tea Polyphenols into High Quality Theaflavins. 02136982.8 ZL. China Patent. 2004
- 20.Tu YY, Tang AB, Watanabe N. The theaflavin monomers inhibit the cancer cells growth in vitro. Acta Biochimica et Biophysica Sinica. 2004;36(7):508–512. doi: 10.1093/abbs/36.7.508. [DOI] [PubMed] [Google Scholar]
- 21.Weisburg JH, Weissman DB, Sedaghat T, Babich H. In vitro cytotoxicity of epigallocatechin gallate and tea extracts to cancerous and normal cells from the human oral cavity. Basic & Clinical Pharmacology & Toxicology. 2004;95(4):191–200. doi: 10.1111/j.1742-7843.2004.pto_950407.x. [DOI] [PubMed] [Google Scholar]
- 22.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicology and Applied Pharmacology. 2007;224(3):265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nature Reviews Cancer. 2009;9(6):429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19(4):611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 25.Yang Z, Jie G, Dong F, Xu Y, Watanabe N, Tu Y. Radical-scavenging abilities and antioxidant properties of theaflavins and their gallate esters in H2O2-mediated oxidative damage system in the HPF-1 cells. Toxicology in Vitro. 2008;22(5):1250–1256. doi: 10.1016/j.tiv.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Yang ZY, Tu YY, Xia HL, Jie GL, Chen XM, He PM. Suppression of free-radicals and protection against H2O2-induced oxidative damage in HPF-1 cell by oxidized phenolic compounds present in black tea. Food Chemistry. 2007;105(4):1349–1356. doi: 10.1016/j.foodchem.2007.05.006. [DOI] [Google Scholar]
- 27.Zheng QS, Zhang YT, Zheng RL. Ascorbic acid induces redifferentiation and growth inhibition in human hepatoma cells by increasing endogenous hydrogen peroxide. Pharimazie. 2002;57(11):753–757. [PubMed] [Google Scholar]