Abstract
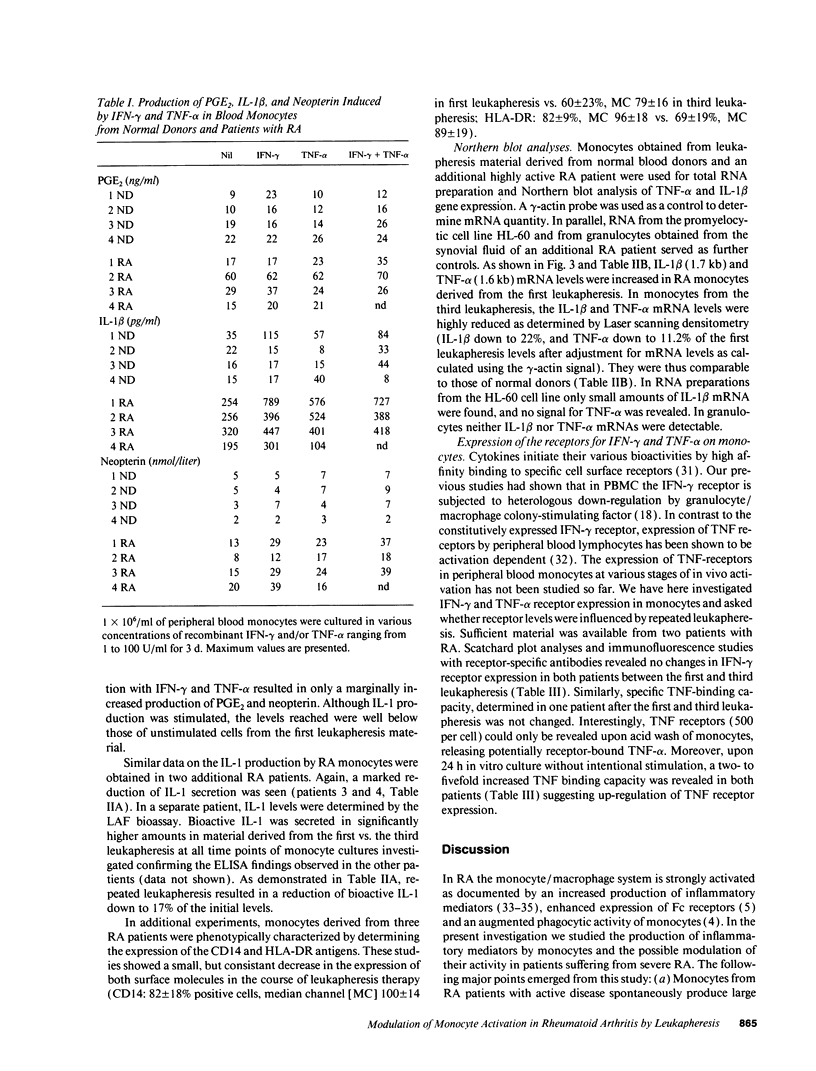
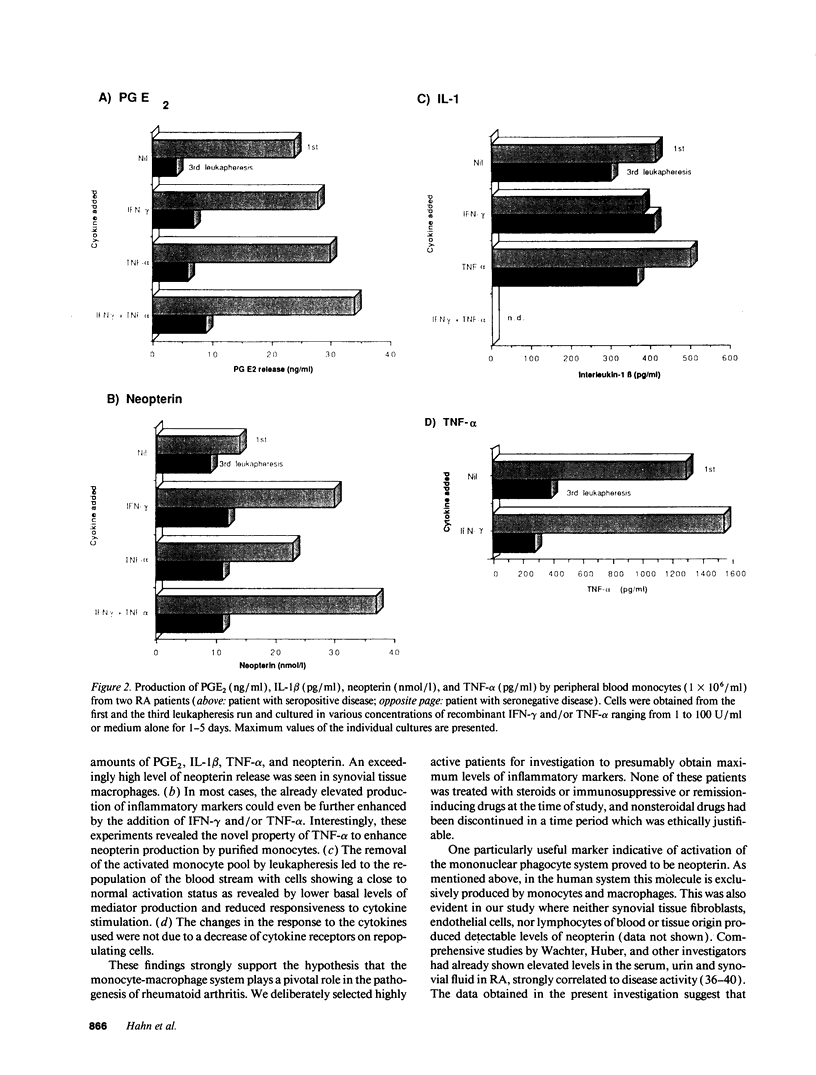
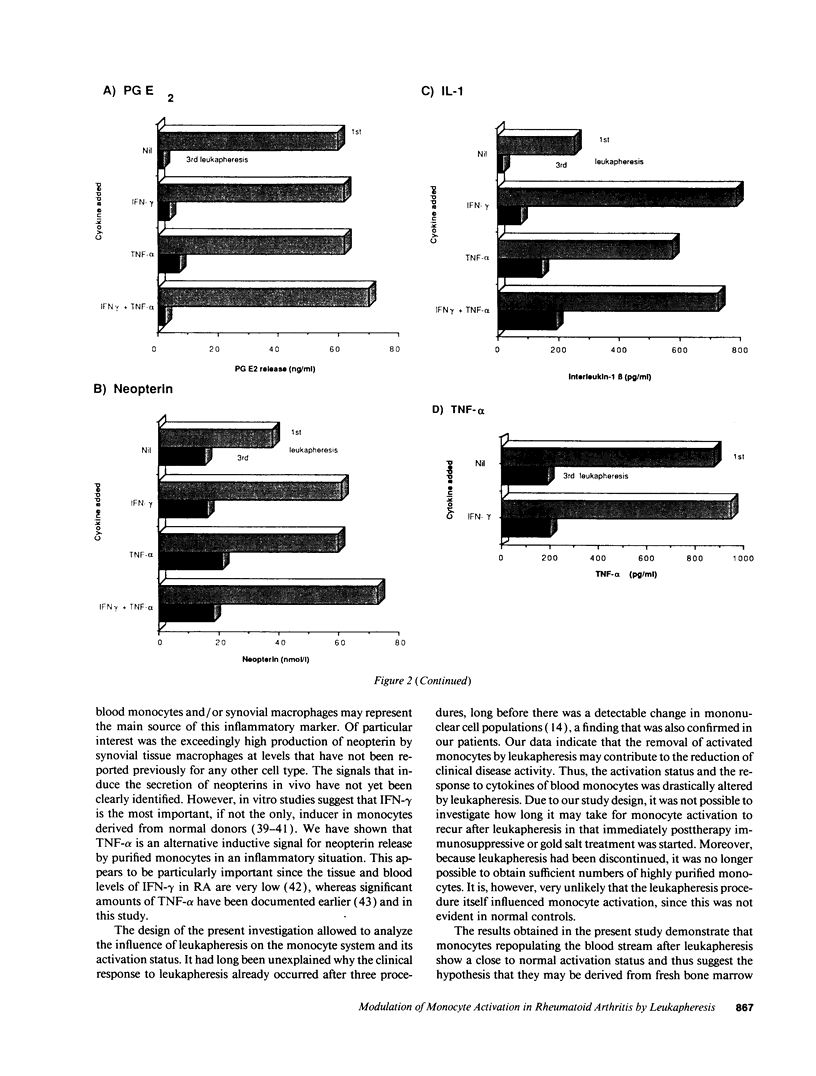
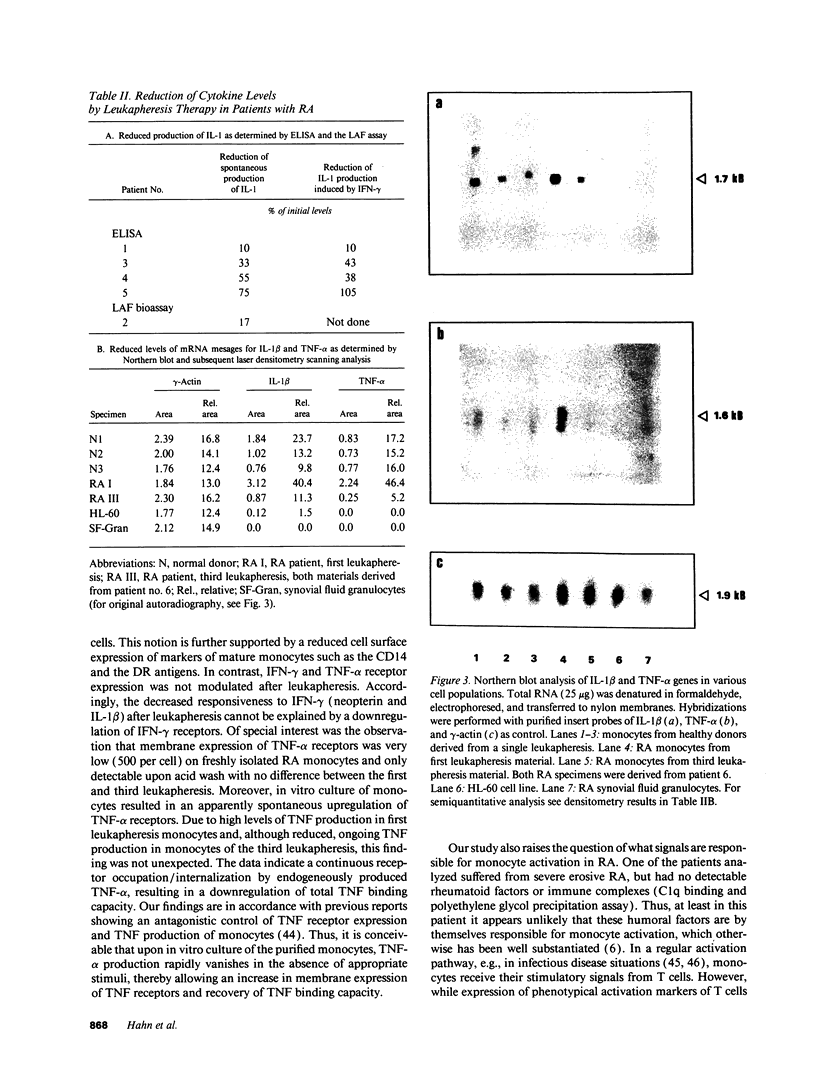
One of the hallmarks in rheumatoid arthritis (RA) is the intense activation of the monocyte-macrophage system. In the present investigation, the modulation of blood monocyte activation was studied with regard to the secretion of cytokines and inflammatory mediators, and to the expression of cytokine receptors. Patients with severe active RA underwent repeated leukapheresis procedures that removed all circulating monocytes. Highly enriched monocyte preparations from the first and third leukapheresis were studied. There were striking differences between the two monocyte populations. Cells obtained from the first leukapheresis constitutively released large amounts of prostaglandin E2 (PGE2), neopterin, interleukin 1 beta (IL-1 beta) and tumor necrosis factor-alpha (TNF-alpha). In particular, IL-1 beta and neopterin production were further enhanced by stimulation with either interferon-gamma (IFN-gamma) or TNF-alpha without a synergistic effect. In contrast, cells derived from the third leukapheresis procedure showed a close to normal activation status with only low levels of cytokine and mediator production as well as a reduced response to cytokine stimulation. The number of the receptors for IFN-gamma and TNF-alpha was not changed between first and third leukapheresis. However, TNF-binding capacity was only detectable upon acid treatment of freshly isolated monocytes. A further upregulation was noted upon 24 h in vitro culture, suggesting occupation of membrane receptors and receptor down-regulation by endogenously produced TNF-alpha. Northern blot analysis of cytokine gene expression was in good correlation with the amount of mediators determined on the protein level. These data indicate that cells of the monocyte-macrophage system are already highly activated in the peripheral blood in RA patients with active disease. These cells can be efficiently removed by repeated leukapheresis and are replenished by monocytes that have, with respect to cytokine and mediator production, a considerably lower activation status.
Full text
PDF

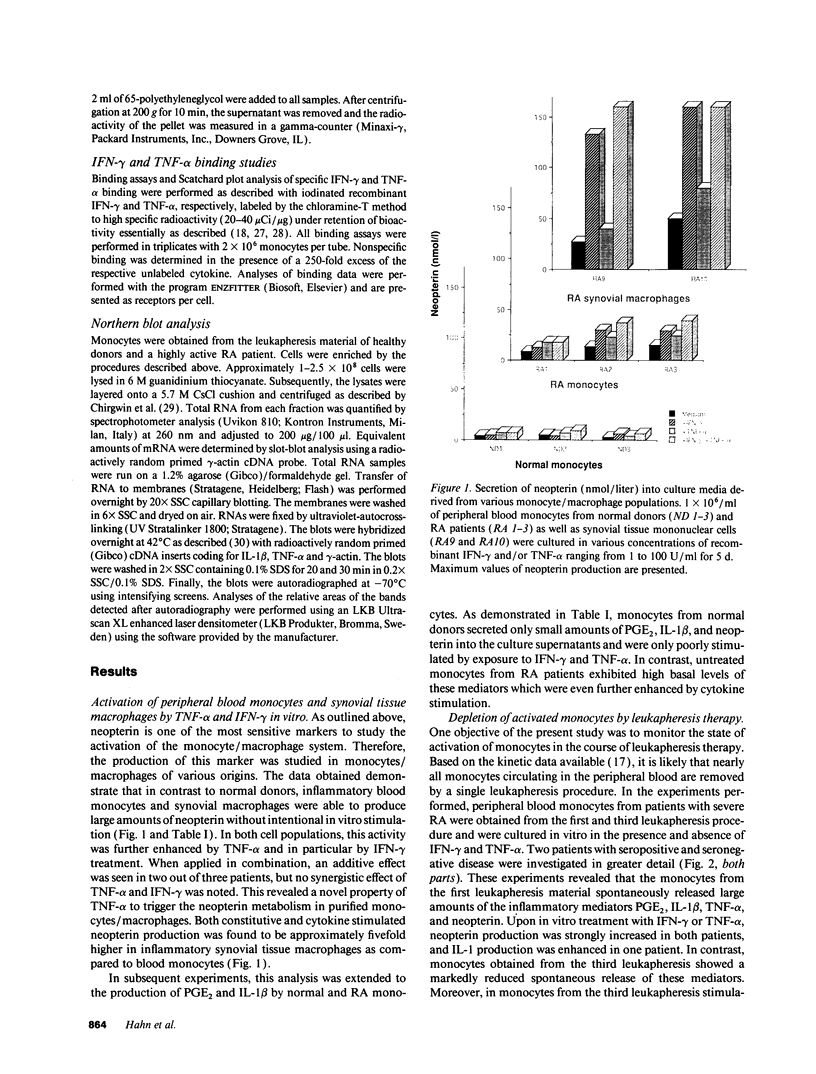


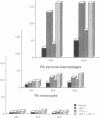
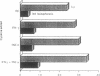
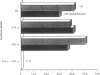
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Merlin G. Purification of human gamma interferon receptors by sequential affinity chromatography on immobilized monoclonal antireceptor antibodies and human gamma interferon. J Exp Med. 1987 Apr 1;165(4):988–999. doi: 10.1084/jem.165.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bitterlich G., Szabó G., Werner E. R., Larcher C., Fuchs D., Hausen A., Reibnegger G., Schulz T. F., Troppmair J., Wachter H. Selective induction of mononuclear phagocytes to produce neopterin by interferons. Immunobiology. 1988 Feb;176(3):228–235. doi: 10.1016/S0171-2985(88)80055-X. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A. M., Maini R. N., Feldmann M. Cytokine production in culture by cells isolated from the synovial membrane. J Autoimmun. 1989 Jun;2 (Suppl):177–186. doi: 10.1016/0896-8411(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Rohwer P., Zacher J., Winchester R. J., Kalden J. R. Differential expression of Ia antigens by rheumatoid synovial lining cells. J Clin Invest. 1987 Sep;80(3):595–604. doi: 10.1172/JCI113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Locher P., Koch B., Winchester R. J., Dimitriu-Bona A., Kalden J. R., Mohr W. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. I. The localization of the major synovial cell populations as detected by monoclonal reagents directed towards Ia and monocyte-macrophage antigens. Rheumatol Int. 1983;3(4):173–181. doi: 10.1007/BF00541597. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Carter S. D., Bourne J. T., Elson C. J., Hutton C. W., Czudek R., Dieppe P. A. Mononuclear phagocytes in rheumatoid arthritis: Fc-receptor expression by peripheral blood monocytes. Ann Rheum Dis. 1984 Jun;43(3):424–429. doi: 10.1136/ard.43.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dimitriu-Bona A., Burmester G. R., Waters S. J., Winchester R. J. Human mononuclear phagocyte differentiation antigens. I. Patterns of antigenic expression on the surface of human monocytes and macrophages defined by monoclonal antibodies. J Immunol. 1983 Jan;130(1):145–152. [PubMed] [Google Scholar]
- Feige U., Karbowski A., Rordorf-Adam C., Pataki A. Arthritis induced by continuous infusion of hr-interleukin-1 alpha into the rabbit knee-joint. Int J Tissue React. 1989;11(5):225–238. [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987 Aug;30(8):864–871. doi: 10.1002/art.1780300804. [DOI] [PubMed] [Google Scholar]
- Fischer T., Thoma B., Scheurich P., Pfizenmaier K. Glycosylation of the human interferon-gamma receptor. N-linked carbohydrates contribute to structural heterogeneity and are required for ligand binding. J Biol Chem. 1990 Jan 25;265(3):1710–1717. [PubMed] [Google Scholar]
- Fischer T., Wiegmann K., Böttinger H., Morens K., Burmester G., Pfizenmaier K. Regulation of IFN-gamma-receptor expression in human monocytes by granulocyte-macrophage colony-stimulating factor. J Immunol. 1990 Nov 1;145(9):2914–2919. [PubMed] [Google Scholar]
- Flescher E., Bowlin T. L., Ballester A., Houk R., Talal N. Increased polyamines may downregulate interleukin 2 production in rheumatoid arthritis. J Clin Invest. 1989 Apr;83(4):1356–1362. doi: 10.1172/JCI114023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs D., Hausen A., Reibnegger G., Reissigl H., Schönitzer D., Spira T., Wachter H. Urinary neopterin in the diagnosis of acquired immune deficiency syndrome. Eur J Clin Microbiol. 1984 Feb;3(1):70–71. doi: 10.1007/BF02032832. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D. C., Sheldon J., Riches P., Hobbs J. R. Cytokine induction of neopterin production. Clin Exp Immunol. 1991 Mar;83(3):479–482. doi: 10.1111/j.1365-2249.1991.tb05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Huber C., Batchelor J. R., Fuchs D., Hausen A., Lang A., Niederwieser D., Reibnegger G., Swetly P., Troppmair J., Wachter H. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984 Jul 1;160(1):310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häupl T., Burmester G. R., Hahn G., Feige U., Rordorf-Adam C., Kalden J. R. Differential immunological response of patients with rheumatoid arthritis towards two different Epstein-Barr virus strains: inhibition of interleukin-1 release by the B95-8, but not the P3HR-1 virus strain. Rheumatol Int. 1989;9(3-5):153–160. doi: 10.1007/BF00271873. [DOI] [PubMed] [Google Scholar]
- Karsh J., Wright D. G., Klippel J. H., Decker J. L., Deisseroth A. B., Flye M. W. Lymphocyte depletion by continuous flow cell centrifugation in rheumatoid arthritis: clinical effects. Arthritis Rheum. 1979 Oct;22(10):1055–1059. doi: 10.1002/art.1780221002. [DOI] [PubMed] [Google Scholar]
- Krause A., Protz H., Goebel K. M. Correlation between synovial neopterin and inflammatory activity in rheumatoid arthritis. Ann Rheum Dis. 1989 Aug;48(8):636–640. doi: 10.1136/ard.48.8.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon S. K., Starkebaum G. Monocyte Fc receptor function in rheumatoid arthritis. Enhanced cell-binding of IgG induced by rheumatoid factors. Arthritis Rheum. 1987 May;30(5):498–506. doi: 10.1002/art.1780300503. [DOI] [PubMed] [Google Scholar]
- Maerker-Alzer G., Diemer O., Strümper R., Rohe M. Neopterin production in inflamed knee joints: high levels in synovial fluids. Rheumatol Int. 1986;6(4):151–154. doi: 10.1007/BF00541281. [DOI] [PubMed] [Google Scholar]
- Merigan T. C. Is recombinant interleukin-2 the best way to deliver interferon-gamma in human disease? J Interferon Res. 1987 Oct;7(5):635–639. doi: 10.1089/jir.1987.7.635. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Stern J. J., Welte K., Rubin B. Y., Carriero S. M., Nathan C. F. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J Immunol. 1987 Apr 1;138(7):2290–2297. [PubMed] [Google Scholar]
- Peters K. M., Dommaschk J., Grundmann R., Schaadt M., Schicha H. Monitoring of tumor necrosis factor therapy with neopterin. Arzneimittelforschung. 1990 Apr;40(4):508–510. [PubMed] [Google Scholar]
- Pfizenmaier K., Wiegmann K., Scheurich P., Krönke M., Merlin G., Aguet M., Knowles B. B., Ucer U. High affinity human IFN-gamma-binding capacity is encoded by a single receptor gene located in proximity to c-ros on human chromosome region 6q16 to 6q22. J Immunol. 1988 Aug 1;141(3):856–860. [PubMed] [Google Scholar]
- Potocnik A. J., Menninger H., Yang S. Y., Pirner K., Krause A., Burmester G. R., Bröker B. M., Hept P., Weseloh G., Michels H. Expression of the CD2 activation epitope T11-3 (CD2R) on T cells in rheumatoid arthritis, juvenile rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, and Lyme disease: phenotypic and functional analysis. Scand J Immunol. 1991 Sep;34(3):351–358. doi: 10.1111/j.1365-3083.1991.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Reibnegger G., Egg D., Fuchs D., Günther R., Hausen A., Werner E. R., Wachter H. Urinary neopterin reflects clinical activity in patients with rheumatoid arthritis. Arthritis Rheum. 1986 Sep;29(9):1063–1070. doi: 10.1002/art.1780290902. [DOI] [PubMed] [Google Scholar]
- Reibnegger G., Egg D., Fuchs D., Günther R., Hausen A., Werner E. R., Wachter H. Urinary neopterin reflects clinical activity in patients with rheumatoid arthritis. Arthritis Rheum. 1986 Sep;29(9):1063–1070. doi: 10.1002/art.1780290902. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Köbrich G., Pfizenmaier K. Antagonistic control of tumor necrosis factor receptors by protein kinases A and C. Enhancement of TNF receptor synthesis by protein kinase A and transmodulation of receptors by protein kinase C. J Exp Med. 1989 Sep 1;170(3):947–958. doi: 10.1084/jem.170.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Köbrich G., Pfizenmaier K. Antagonistic control of tumor necrosis factor receptors by protein kinases A and C. Enhancement of TNF receptor synthesis by protein kinase A and transmodulation of receptors by protein kinase C. J Exp Med. 1989 Sep 1;170(3):947–958. doi: 10.1084/jem.170.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Seitz M., Hunstein W. Enhanced prostanoid release from monocytes of patients with rheumatoid arthritis and active systemic lupus erythematosus. Ann Rheum Dis. 1985 Jul;44(7):438–445. doi: 10.1136/ard.44.7.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz M., Hunstein W. Enhanced prostanoid release from monocytes of patients with rheumatoid arthritis and active systemic lupus erythematosus. Ann Rheum Dis. 1985 Jul;44(7):438–445. doi: 10.1136/ard.44.7.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum J., Urowitz M. B., Keystone E. C., Dwosh I. L., Curtis J. E. Leucapheresis in severe rheumatoid arthritis. Ann Rheum Dis. 1979 Feb;38(1):40–44. doi: 10.1136/ard.38.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wilder R. L., Katona I. M., Wahl L. M., Allen J. B., Scher I., Decker J. L. Leukapheresis in rheumatoid arthritis. Association of clinical improvement with reversal of anergy. Arthritis Rheum. 1983 Sep;26(9):1076–1084. doi: 10.1002/art.1780260904. [DOI] [PubMed] [Google Scholar]
- Yeadon C., Karsh J. Lymphapheresis in rheumatoid arthritis. The clinical and laboratory effects of a limited course of cell depletion. Clin Exp Rheumatol. 1983 Apr-Jun;1(2):119–124. [PubMed] [Google Scholar]