Abstract
Synapses between nociceptive dorsal root ganglion (DRG) neurons and spinal cord dorsal horn neurons represent the first loci for transmission of painful stimuli. Our knowledge of the molecular organization and development of these synapses is sparse due, partly, to a lack of a reliable model system that reconstitutes synaptogenesis between these two neuronal populations. To address this issue, we have established an in vitro assay system consisting of separately purified DRG neurons and dorsal horn neurons on astrocyte micro-islands. Using immunocytochemistry, we have found that 97%, 93%, 98%, 96%, and 94% of DRG neurons on these microislands express markers often associated with nociceptive neurons including Substance P, TRPV1, calcitonin-gene related peptide (CGRP), TrKA, and peripherin, respectively. Triple labeling with these nociceptive-like markers, synaptic vesicle marker Vglut2 and using MAP2 as a dendritic marker revealed the presence of nociceptive-like markers at synaptic terminals. Using this immunocytochemical approach, we counted contact points as overlapping MAP2/Vglut2 puncta and showed that they increased with time in culture. Single and dual patch clamp recordings showed that overlapping Vglut2/MAP2 puncta observed after a few days in culture are likely to be functional synapses between DRG and dorsal horn neurons in our in vitro assay system. Taken together, these data suggest our co-culture microisland model system consists of mostly nociceptive-like DRG neurons that express presynaptic markers and form functional synapses with their dorsal horn partners. Thus, this model system may have direct application for studies on factors regulating development of nociceptive DRG/dorsal horn synapses.
Keywords: Electrophysiology, synaptogenesis, immunocytochemistry, nociceptive, microisland, dorsal horn, dorsal root ganglia
1. Introduction
Investigating the complexity of developing CNS synapses has relied heavily on studies of cultured CNS neurons. Studies on synapses between nociceptive dorsal root ganglion (DRG) neurons and their targets in the dorsal horn of the spinal cord, however, are rare. This is due, in part, to lack of a well defined model system in which the timing of synapse formation between individual neurons can be carefully quantified. Nociceptive DRG/dorsal horn synapses in vivo represent primary sites for pain processing, making its recapitulation in vitro an important model system for studying sensory processing and synapse formation. Mature nociceptors express many unique receptors and ion channels that are essential for detection of noxious stimuli (McCleskey and Gold, 1999). Many of these are developmentally regulated in terms of expression patterns and physiological properties (Woolf and Ma, 2007). Some of these membrane proteins may also have additional functions during the development of DRG/dorsal horn synapses.
Synapse formation is a multistep process that includes generation of neurons from neural stem cells, migration of newly generated neurons toward genetically determined locations, extension of axons and dendrites, and establishment of precise neuronal connectivity (Spitzer, 2006). Much of our current knowledge about the development of synapses is based on studies at the neuromuscular junction (NMJ). Recent studies on synapse formation using dissociated CNS neurons, however, have shown that this process entails additional complexity beyond that at the NMJ. This increase in complexity is partly due to the multitude of neurotransmitters, diversity of receptors, and numerous choices of potential partners for CNS neuronal synapses (Lipton and Kater, 1989; Cohen-Cory, 2002; Scheiffele, 2003; Craig & Boudin, 2001; Juttner & Rathjen, 2005; McAllister, 2007). Recent work has begun to identify early cellular events in synapse formation as well as molecular signals that initiate this complex process (McAllister, 2007). However, despite these advances in our understanding of synapse formation, several important issues concerning synapse formation remain unresolved, including the mechanisms that endow neurons with the ability to form selective connections with their synaptic partners.
In the present study, we devised a culture system that offers optimal conditions for cell maturation and synapse formation while providing researchers with simple nociceptive circuitry suitable for studies into factors regulating nociceptive synapse development. We used collagen dots to grow small microislands of astrocytes (Segal and Furshpan, 1990) as substrates to co-culture separately dissociated rat embryonic DRG and dorsal horn neurons. Under these culture conditions, the few DRG and dorsal horn neurons that settle on the collagen dots are forced to communicate with each other as opposed to neurons on neighboring dots. DRG-DH neurons cultured on microislands for 2-4 weeks have been previously used by our laboratory to study the role of ATP P2X (Gu and MacDermott 1997) and presynaptic AMPA and KA receptors (Lee et al., 2004) in modulating evoked glutamate release by DRG onto DH neurons. However, the phenotype of these DRG neurons and their time course for synapse formation with their DH partners have not been previously characterized. Using immunocytochemistry with antibodies to nociceptive markers, here we show that most DRG neurons on microislands expressed all of the nociceptive markers tested. Further, synaptic labeling of these cultures showed that the nociceptive markers are expressed at synapses and these synapses increased in numbers during development. Using conventional and dual patch clamp recordings, we show synaptic puncta at 5 DIV are likely to be functional synapses. Together, these results establish our microisland assay system as useful for studies into the factors influencing synapse formation in the dorsal horn of the spinal cord.
2. Materials and methods
2.1. Preparation of microisland co-cultures
2.1.1 Device
Detailed instructions for making a microisland stamp and for culture preparation have recently been published (Albuquerque et al., 2009a-d). Briefly, collagen micro-island dots were patterned by a homemade stamp that was used in combination with a picospritzer to generate a matrix of collagen microdots on coverslips. The patterns were made using a 0.2mm template as a guide to make a matrix of holes in a piece of plastic with number 10 insect pins. We used 0.1- 0.2 mm polypropylene membranes. Upon generating this matrix, the plastic was glued onto a 30ml plastic syringe that had been cut ∼10 mm from the point where the syringe shaft begins to taper to form the tip (Fig 1A).
Fig. 1.
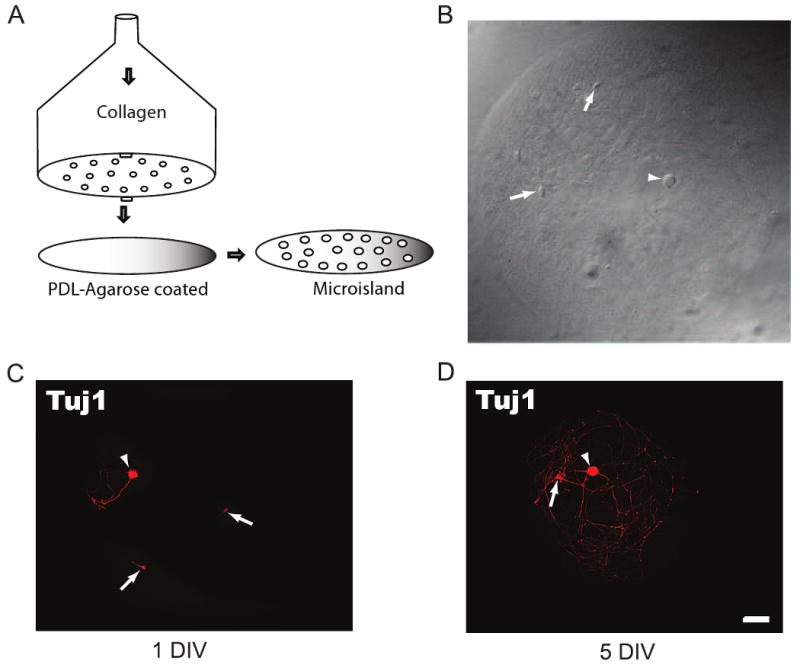
DRG/dorsal horn neuron microisland assay system. (A) Schematic of the homemade device for making microisland coverslips showing the perforations through which collagen is pushed out by air to generate a matrix of dots on a PDL-agarose pre-coated coverslip. (B) Example of a microisland with a DRG (Arrowhead) and two dorsal horn neurons (Arrows). (C, D) DRG (Arrowheads) and dorsal horn neurons (Arrows) at day 1 (C) and 5 (D) in vitro are positive for the neuronal marker Tuj1. Neurons avoid the nonpermissive agarose and are confined to the permissive substrate (collagen/cortical astrocytes). Note that a DRG neuron is easily identified by its size difference from the dorsal horn neuron. Scale bar is 50μm.
2.1.2. Preparation of collagen microisland coverslips
Coverslips were washed in 1N nitric acid and stored in 95 EtOH before they were used for culturing. Microislands were prepared as follows: cleaned coverslips were pre-coated with Poly-D-Lysine (PDL) at 10μg/ml and then 0.2% agarose (type II, medium electroendosmosis; Sigma) and allowed to dry for 1hr. Once dry, the dishes were stamped with rat-tail collagen (3 mg/ml) using the homemade stamp described above. This structure or stamp was loaded with collagen (3mg/ml), which was then pushed out of the stamp in the pattern of the holes by an air pressure control device (Fig 1A). The dishes were then sterilized by ultraviolet irradiation for 1hr just prior to plating of astrocytes.
2.1.3. Cell Culture
Microisland cultures of rat DRG/ dorsal horn neurons were prepared and maintained as described previously (Gu and MacDermott, 1997; Lee et al., 2004). In brief, astrocyte feeder wells enriched in rat cortical astrocytes were first prepared from postnatal day 1 rat cortical tissue (Albuquerque et al., 1999; 2009b). After dissection and removal of the choroid plexus and meninges, the material was dissociated and trypsinized (0.25%). Cells were grown in culture medium consisting of DMEM with 4.5 g/liter glucose (Invitrogen), 10% fetal bovine serum (Invitrogen), 100 IU/ml penicillin/streptomycin (Invitrogen). Cells were kept in an incubator at 37°C in a 5% CO2-enriched atmosphere. Once a monolayer was achieved, cells were shaken for 12 hours so that only astrocytes remained attached to the flask bottom. Astrocytes were trypsinized and re-plated onto collagen-coated (microislands) glass coverslips for 3–7 days before neuronal plating.
DRG and dorsal horn neurons were isolated from rat Sprague-Dawley rat embryos aged 17 days in utero as previously described (Gu and MacDermott, 1997; Lee et al., 2004). Briefly, pregnant rats were killed by CO2 asphyxiation followed by cervical dislocation, and the embryos were removed and transferred to ice-cold Leibowitz-15 medium (Invitrogen). Animals were sacrificed according to the guidelines approved by the Columbia University Institutional Animal Care and Use Committee. Isolated DRGs and dorsal horns were incubated in separate dishes at 37°C in calcium-magnesium free MEM (Gibco) containing 0.25% trypsin for 20 min. After enzymatic digestion, cells were gently triturated with a silicon-coated Pasteur pipette and centrifuged at 800×g for 8 min to remove the enzymes. Isolated cells were suspended in MEM plating medium (Invitrogen) containing 4.5g/L of glucose, MEM vitamins (Gibco), and 4% B-27 supplement before plating on astrocyte microislands. Separately dissociated DRG and dorsal horn neurons were plated on top of the astrocytes. The plating density was usually 6,000–8,000 dorsal horn neurons per dish, and 10,000–15,000 DRG neurons per dish. 2.5S nerve growth factor (NGF; 10ng/ml) and glial-derived neurotrophic factors (GDNF; 50ng/ml) were added at the time of plating. 5-fluoro-2′-deoxyuridine (10 μM) was added after two days in culture. Cells were kept in an incubator at 37°C in a 5% CO2-enriched atmosphere and cultured until use in each experiment. DRG neurons were easily distinguished from dorsal horn neurons by their distinct larger diameter, phase bright soma, and finer neurites.
2.2. Immunocytochemistry
DRG/dorsal horn cultures prepared as described above were washed with PBS two to three times before and after each of the following steps. Cultures were fixed with 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 for 1hr, incubated overnight with appropriate nociceptive markers in combination with guinea pig anti-Vglut2 (1:1500 dilution; Synaptic System) and mouse anti-MAP2 (1:2000 dilution; Chemicon), and secondary antibody (1hr at room temperature) diluted in the same permeabilization solution. The typical nociceptive markers used in combination with Vglut2 and MAP2 included Substance P, TRPV1, TrkA, CGRP, and peripherin (1:1000 dilution each; Chemicon) followed by secondary antibodies conjugated to Alexa 488 (1:400 dilution), Alexa 568 (1:800 dilution), and Alexa 647 (1:300 dilution; all from Invitrogen), respectively. The cultures were mounted on microscope slides with Fluomount-G (southernbiotech) and imaged. Phase-contrast and fluorescence images were captured with an Axiocam CCD camera mounted on a Zeiss Axioplan 2 microscope with a 20× 1.4 numerical aperture objective (Zeiss) using Openlab imaging software (Improvision). Images were prepared using Adobe Photoshop 7.2 software (Adobe Systems).
2.3. Image analysis
For quantification of nociceptive synapses, digital images from 30–35 microislands were acquired randomly from 10 dishes chosen from 5 different batches of cultures. DRG to dorsal horn synapses were labeled with Vglut2, TrKA, and MAP2 as described above and images were processed for analysis. Each single-channel image was first subjected to a user-defined intensity threshold to select labeling from the background (with an intensity between 80 and 120, depending on the experiment, equal to 5 times the background level) using Metamorph software. Corresponding channel images were then merged to create a color [RGB (red–green–blue)] image containing the three single-channel images as individual color channels. Then co-localized (overlapping) puncta of TrkA, Vglut2, and MAP2 were manually counted for each microisland. For quantification of the percentage of nociceptive neurons, appropriate nociceptive positive and negative neurons were counted from 10 dishes over 5 different cultures. For each dish, the count was on microislands across 10 random microscopic fields. Additional analyses were performed using Microsoft, Excel and Graphpad Prism. Statistical comparisons of immunofluorescence were made using Student's unpaired t test and one-way ANOVA test. All data are reported as mean ±SEM.
2.4. Electrophysiology
All electrophysiological recordings were carried out as previously described (Lee et al., 2004). Briefly, coverslips were transferred to the stage of a Nikon microscope and continuously perfused (approximately 1 ml/min) with bath recording solution containing in mM: 145 NaCl, 5 KCl, 2 CaCl2, 10 HEPES, 2 MgCl2, and 5.5 glucose at pH 7.3 and 325mOsm. Synaptic responses from DRG and dorsal horn pairs were recorded after sequentially patching the DRG neuron in the perforated-patch current clamp mode and the dorsal horn neuron in the whole-cell voltage clamp mode. For perforated-patch current clamp recordings, the pipette solution consisted of 25 μg/ml gramicidin D (50μg/mL; Sigma-Aldrich), 100mM K-methanesulfonate, 10mM NaCl, 0.1mM CaCl2, and 10mM HEPES; pH was adjusted to pH 7.1 with KOH, and osmolarity was adjusted to 310mOsm with sucrose. For whole-cell voltage clamp recordings, the pipette solution contained in mM: of 120 Cs-methanesulfonate, 10 HEPES, 0.2 EGTA, 10 NaCl, 0.1 CaCl2, 2 MgATP, 0.5 LiGTP; pH was adjusted to 7.1 with CsOH and osmolarity adjusted to 310mOsm with sucrose. The recording pipettes were made from glass capillaries (World Precision Instruments), with a resistance of 4–6 MΩ for all recordings. Liquid junction potentials between external and internal solutions were corrected in the bath before GΩ seal formation for each cell. Membrane currents were recorded with an Axopatch 200A amplifier (Molecular Devices, Foster City, CA), filtered at 2k Hz, and digitized at 2-5 kHz with pClamp 9-acquisition software (Molecular Devices). Membrane voltages were recorded with an Axoclamp 2A patch-clamp amplifier (Molecular Devices) in bridge mode. Action potentials were stimulated in DRG neurons by injecting currents ranging from 1 pA to approximately 100 pA for 5 ms. Synaptic connections were assessed by the presence or absence of evoked excitatory postsynaptic currents (eEPSCs) approximately 10ms following stimulation of the DRG neuron. In some recordings, miniature EPSC (mEPSC) events were recorded from dorsal horn neurons in the presence of 0.5 mM lidocaine, 10 μM SR95531, and 1 μM strychnine. Unless specified, the membrane potential was held at −70 mV throughout all whole cell voltage-clamp experiments. All experiments were carried out at room temperature (22–25°C).
Evoked EPSCs were analyzed using Clampex and Clampfit software (Version 9.0.1, Axon Instruments). Peak eEPSC amplitudes were measured as the difference between the baseline and the first peak of the eEPSC. Recordings of mEPSCs were analyzed using Mini Analysis (Synaptosoft, Inc.). Based on our recording baseline noise levels, the amplitude threshold for mEPSC detection was set at 5 pA. The amplitude and frequency of synaptic events were determined over a 2 min sampling period. Unless specified, average values are expressed as mean ± SEM.
2.5. Materials
NGF was from Boehringer Mannheim and GDNF from Promega. Lidocaine, gramicidin D, and other chemicals were from Sigma. All drugs were maintained as frozen stocks and were freshly diluted in bath solution to their final concentrations before experiments.
3. Results
3.1. Morphological differentiation of DRG and dorsal horn neurons in the microisland assay system
Our microisland assay system was established by spraying collagen on a glass coverslips through a homemade patterned device. This created a matrix of collagen dots or microislands (442 ± 68 μm diameter) on the coverslip (Fig 1A, B). Because the collagen dots, a substrate permissive for astrocyte attachment, were applied to a non-permissive agarose background (Fig. 1A), the subsequently plated cortical astrocytes, DRG and dorsal horn neurons were confined to the permissive area or microisland (Fig 1B). DRG neurons were easily distinguished from dorsal horn neurons by their distinct larger diameter (Fig 1B), phase bright soma, and finer neurites. By day 1 in vitro, the DRG and dorsal horn neurons grown on these microislands were firmly attached to the previously plated cortical astrocytes and extended neurites that were restricted to the microisland (Fig 1C). By day 5 in vitro, the neuritic processes remained restricted to the collagen dots but had grown more extensively (Fig. 1D).
3.2. Nociceptive-specific markers are expressed by DRG neurons
Mature DRGs are composed of heterogeneous populations of sensory neurons whose phenotypes can be distinguished on the basis of several factors. These include sensory modality, neurotrophic factor dependency, central and peripheral innervation targets, and protein expression (Scott, 1992; Snider & Silos-Santiago, 1996; Clifford & Ma, 2007; Marmigere & Ernfors, 2007; Zhang & Bao, 2007; Priestly et al., 2002). For our in vitro system, we used expression of markers, including growth factor receptors, to characterize the DRG neurons growing on the microislands. In vivo, small diameter peripheral sensory neurons primarily associated with detection of noxious or temperature stimuli express growth factor receptors TrkA and Ret, making them sensitive to NGF and GDNF, respectively (Davies, 1986; Bennett et al., 1996; Molliver et al., 1995, 1997; Chen and Frank, 1999; Bibel and Barde, 2000; Ernfors, 2001). Many TrkA expressing neurons express peptides such as substance P and CGRP while the Ret expressing cells express the surface marker IB4 (Davies, 1986; Bennett et al., 1996; Molliver et al., 1995, 1997; Chen and Frank, 1999; Bibel and Barde, 2000; Ernfors, 2001).
We quantified expression of the markers TrkA, CGRP and SP in our embryonic neuronal assay system by immunocytochemistry. Immunoreactive profiles for the peptidergic markers substance P, CGRP, and TrkA were seen in 96%, 97%, 96.5% of DRG neurons, respectively (example for TrkA in Fig. 2A; summary in Fig. 2B). These values and expression patterns are in contrast with values reported for immunostaining with TrkA, substance P and CGRP in intact postnatal rat ganglia, where 20% (Lawson et al., 1997; Salt & Hill, 1983; Traub, 1996; Lee et al., 1985a), 40% (Lee et al., 1985b), and 47% (Molliver et al., 1995; Averill et al., 1995) of DRG neurons stained for SP, CGRP, and TrkA, respectively.
Fig. 2.
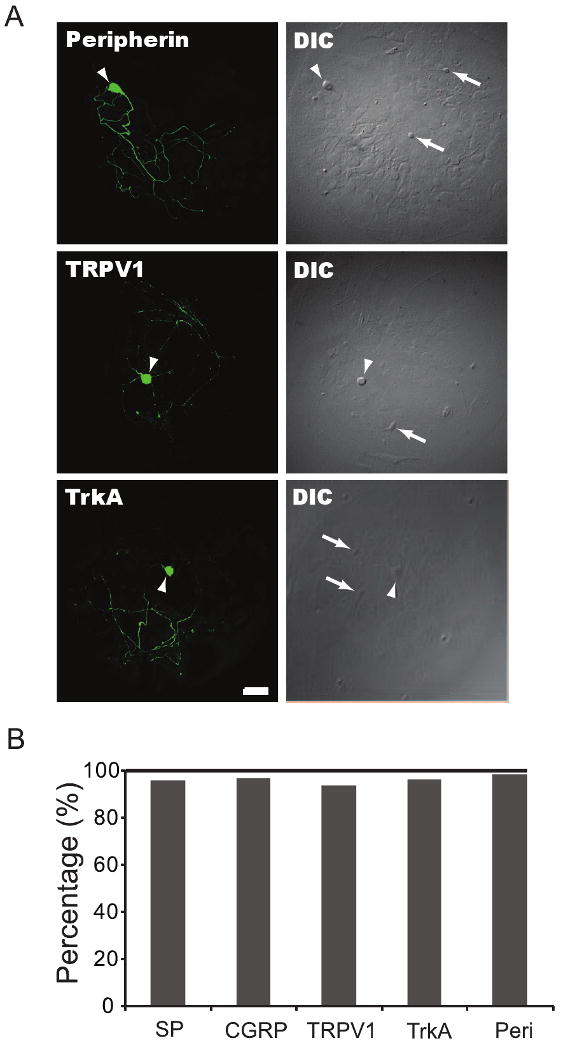
DRG neurons on microislands are predominantly nociceptive. DRG/dorsal horn microisland cultures were fixed and immunostained with antibodies against markers associated with nociceptors. (A) Arrowheads point to DRG neurons positively stained for the nociceptive markers peripherin, TRPV1, and TrkA, whereas arrows in DIC images point to unstained dorsal horn neurons. (B) Shows that most DRG neurons in our microisland culture system are nociceptive with 96% (913/947) expressing substance P (SP), 97% (658/680) CGRP, 96.5% (860/890) TrkA, 94% (851/903) TRPV1, and 98.5% (957/972) peripherin. Cells were counted over 10 different cultures. Scale bar is 50 μm.
We also investigated expression of the noxious heat receptor TRPV1 (Julius & Basbaum, 2001) and nociceptor associated intermediate filament, peripherin (Lawson, 1992; Belecky-Adams et al., 2003). Expression of the putative nociceptor marker, TRPV1, was seen in 94% of DRG neurons on the microislands (Fig 2A and B). This broad expression pattern was also in contrast to expression distribution that was previously reported for intact postnatal rat DRGs (ranging from 32 to 58%) (Michael & Priestley, 1999; Guo et al., 2001; Carlton & Hargett, 2002). Moreover, 98.5% of the DRG neurons in microisland assay system expressed the distinct neuronal intermediate filament protein, peripherin (Fig 2A and B). This expression level is much higher than the 66% of DRG neurons that have been reported to be peripherin positive in intact DRGs (Lawson, 1992; Belecky-Adams et al., 2003).
3.3. Synapse formation on the collagen microisland
The clustering of synaptic vesicles around active zones is the initial structural sign of synapse formation and, accordingly, the number of vesicles in these clusters increases in parallel with changes in the architectural complexity of synaptic boutons during synapse maturation (Dyson and Jones, 1980; Amaral and Dent, 1981; Blue and Parnavelas, 1983; Vaughn, 1989). The vesicular glutamate transporter 1 (VGluT1) and VGluT2 accumulate neurotransmitter glutamate into synaptic vesicles at presynaptic terminals, and they have become presynaptic axon terminal markers of choice for many studies into synapse formation (Prange et al., 2004; Graf et al., 2004).
After characterizing the population of DRG neurons in our culture system by expression of a panel of markers, we examined the temporal pattern of synapse formation between these DRG neurons and dorsal horn neurons. To do this, we assessed co-localization of a presynaptic marker, a marker for nociceptors and a postsynaptic marker. We used the presynaptic marker VGluT2 to label synaptic vesicles at axonal terminals. To selectively identify presynaptic terminals of DRG neurons, double staining with TrkA was performed (Fig 3A, B). Using this staining approach, we evaluated the co-localization of DRG presynaptic VGluT2 clusters with the dendritic marker MAP2 (Caceres et al., 1984) (Fig 3A, B). This provided a way to quantify the number of synapses between nociceptive DRG and dorsal horn neurons on the collagen microislands. Cultures were assayed at 1 day in vitro (DIV) through day 5 after plating (5 DIV). We used microislands with at most 3 neurons, consisting of at least 1 DRG neuron and 1 dorsal horn neuron. In the 1 DIV cultures, Vglut2 staining was primarily localized to the soma, with some staining throughout the neuritic processes (Fig 3A, B). By contrast, in the 5 DIV cultures, Vglut2 staining profile showed intense puncta along processes (Fig 3B). The number of synaptic boutons and contact points increased with time in culture starting at 2 DIV, with the 1 DIV cultures showing few or no synaptic boutons or contact points (Fig 3A, C). By days 2 and 3, averages of 3±0.30 and 7.7±0.60 synaptic boutons were counted, respectively. On day 4, the number of synaptic boutons counted increased to 13.4±1.1, while at 5 DIV, synaptic bouton number increases again to 16.4±1 (Fig 3C).
Fig. 3.
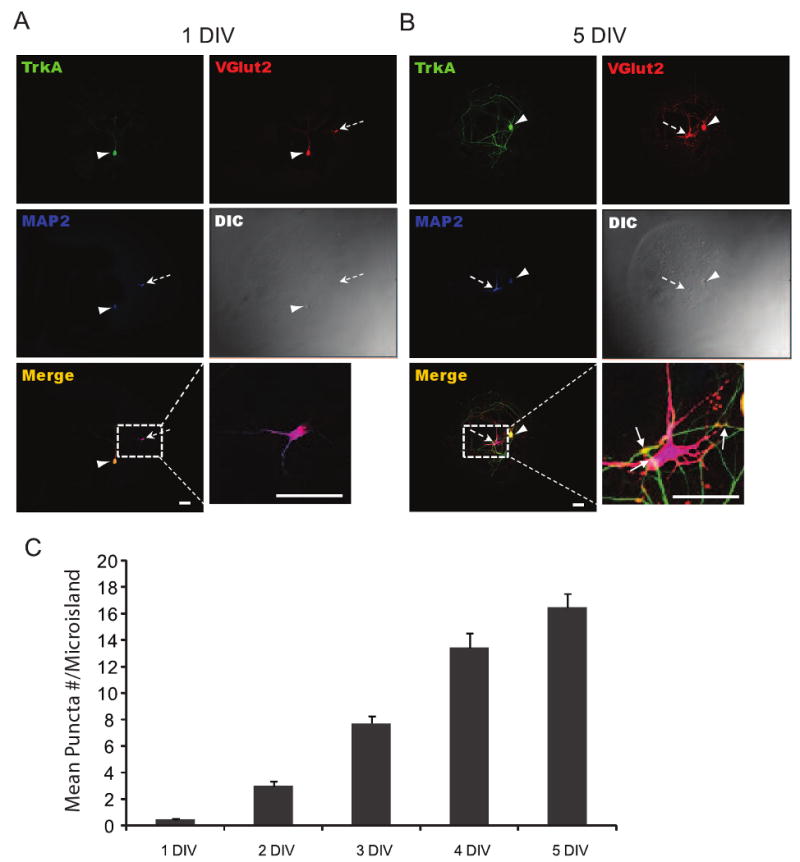
Time-course for accumulation of nociceptive DRG and dorsal horn synaptic boutons. (A, B) representative immunostaining images at day 1 and 5 against TrkA, VGlut2, and MAP2. Arrowhead and broken arrow point to a DRG and dorsal horn neuron, respectively. Insets in lower left and right panels of A and B illustrate the triple-labeled boutons (Arrows) quantified in C. The number of synaptic boutons increased over time in culture (0.48 ± 0.1, 3 ± 0.30, 7.7 ± 0.64, 13.4 ± 1.1, 16.4 ± 1.02 for 1-5 DIV cultures, respectively; P<0.0001 by ANOVA test, n≥27 for each time point). All values are given in ± SEM and scale bars are 50μm.
3.4. Synaptic boutons are functional synapses
Having shown that the number of synaptic boutons increased with time in culture, we examined whether these synaptic boutons were functional at 5 DIV by recordings of action potential-independent mEPSCs and eEPSCs. As with the synaptic boutons experiments, we restricted our recordings to microislands with two or three cells, with at least one dorsal horn neuron and one DRG neuron. Under these conditions, synaptic contributions from other dorsal horn neurons are minimized, though contributions from autaptic connections cannot be ruled out.
We recorded mEPSCs from 6 dorsal horn neurons at 5 DIV by voltage-clamping the cells at -70 mV. Under these conditions, we observed mEPSCs with fast kinetics (1.4±0.06 ms rise time) (Fig 4A, lower trace), suggesting that they may be AMPA receptor-mediated mEPSCs. The mean frequency of these events was 0.2±0.005Hz, n=6 (Fig 4A, top trace) and the mean amplitude of the distribution shown in Fig. 4B was 13.8±1.8 pA (Fig 4B). These recordings demonstrate the presence of functional synapses on dorsal horn neurons in our culture system by 5 DIV.
Fig. 4.
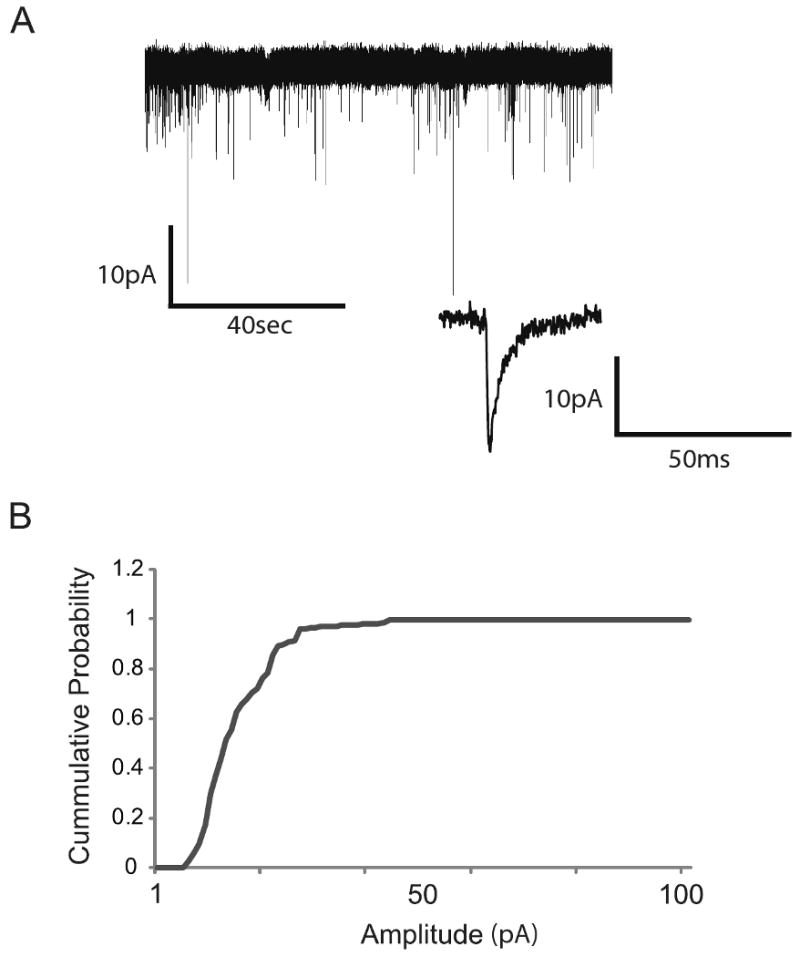
DRG and dorsal horn neurons on microisland assay system form functional synapses. (A) Representative compressed trace showing a view of all the events during the sampling period. The trace below is an expanded view of an mEPSC event. (B) Cumulative amplitude distribution of mEPSCs recorded from dorsal horn neurons after 5 days in culture (13.8 ± 1.8pA, n=6).
We next examined whether functional synaptic connections exist between the DRG and dorsal horn neurons on microislands at 5 DIV using simultaneous whole-cell recordings from individual pairs of DRG and dorsal horn. Synaptic connectivity between a pair of DRG and dorsal horn neurons was obtained by patching a DRG neuron under perforated current clamp mode and a dorsal horn neuron under whole cell voltage-clamped mode at −70mV (Fig 5A). We then established the threshold for evoking action potentials in the DRG neurons by brief current injections (1 ± 0.02 nA) (Fig 5B). Subthreshold stimulation of DRG neurons almost always resulted in no eEPSCs in the postsynaptic dorsal horn neurons (Fig 6C, top two traces). In contrast, suprathreshold stimulation of DRG neurons always resulted in eEPSCs in the postsynaptic dorsal horn neurons at 5 DIV (Fig 6D bottom traces, 5/5 pairs). The mean amplitude of eEPSCs was 120±10.02 pA. These results suggest that DRG and dorsal horn neurons readily form functional synapses on the collagen microisland assay system.
Fig. 5.
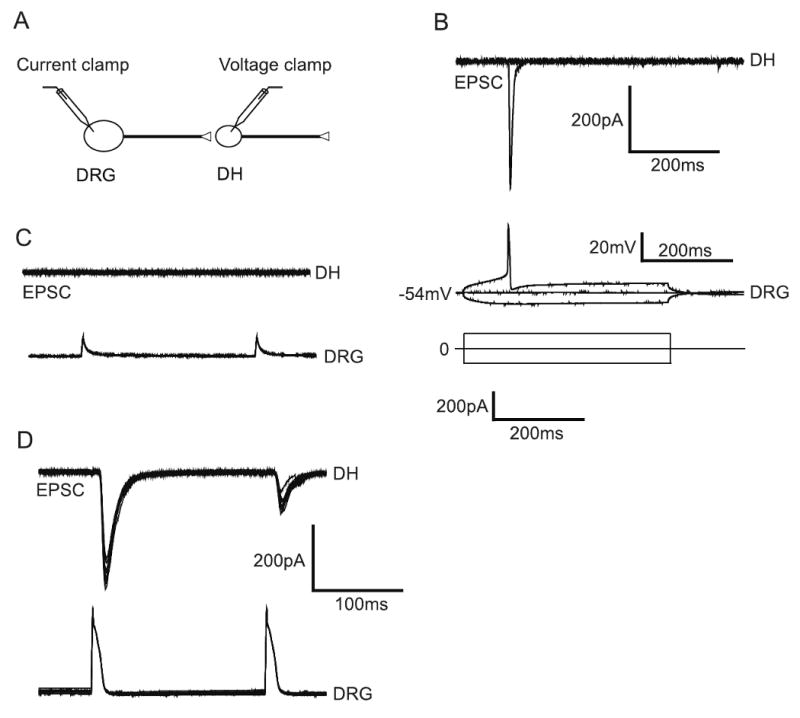
Paired-recording assay. (A) Typically, a microisland with a DRG neuron and a dorsal horn neuron (Maximum numbers of cell is 3) is selected for patching. DRG neuron is patched in the current-clamp mode and the dorsal horn neuron in the voltage clamp mode. (B) Current steps are given to DRG neurons to assess threshold for action potential and synaptic connection. (C) Sub-threshold stimulation of DRG neurons generally fails to generate action potentials and eEPSCs in the synaptically coupled dorsal horn neuron. (D) Suprathreshold stimulation of DRG neurons generates action potentials, resulting in eEPSCs in the dorsal horn neuron (120 ± 10.02 pA, n=5).
4. Discussion
Although the microisland assay has been used for hippocampus (Segal and Furshpan, 1990; Bekkers and Stevens, 1991; Harms et al., 2005) and DRG/ dorsal horn co-cultures as model systems for studies in synaptic transmission, it has not been characterized as a model system for synaptogenesis. For the DRG and dorsal horn synapses, this is particularly important as the understanding of the mechanisms of synapse formation is far less advanced than in the hippocampus. We have now developed culture conditions in which nociceptive DRG and dorsal horn neurons undergo extensive differentiation over the first 5 days after plating and form synapses in a time dependent manner.
To produce a consistent matrix of collagen microislands, we constructed a stamp (Albuquerque et al, 2009a) rather than spraying random sized collagen dots as described in some studies with hippocampal cultures (Segal and Furshpan, 1990; Bekkers and Stevens, 1991; Harms et al., 2005). Using the stamp approach, our resulting collagen dots on glass coverslips had a mean diameter of 442±68μm (Fig 1A, n=100), making them ideal for synaptogenesis studies where the distance between possible synaptic pairs may otherwise be an impediment to the study. On this substrate, as we expected from previous studies (Gu and MacDermott, 1997; Lee et al., 2004), the DRG and dorsal horn neurons extended neurites that remained on the permissive area regardless of the extensive outgrowth of the neurites (Fig 1B, C). This pattern of differentiation in a restricted space is similar to that observed in hippocampal microisland cultures (Segal and Furshpan, 1990; Bekkers and Stevens, 1991; Harms et al., 2005).
4.1. DRG neurons in the microisland cultures are nociceptor-like
We characterized the expression of the nociceptive markers TRPV1, TrkA, peripherin, CGRP, and SP in our embryonic neuronal assay system by immunocytochemistry and found the percentage distribution for each marker to be above 90% (Fig 2C, D). The immunoreactive profiles of these markers in our culture system are not consistent with the 20% (Lawson et al., 1997; Salt & Hill, 1983; Traub, 1996; Lee et al., 1985a), 40% (Lee et al., 1985b), and 47% (Molliver et al., 1995; Averill et al., 1995) values reported for SP, CGRP, and TrkA, respectively from intact postnatal rat ganglia. Similarly, the immunoreactive profiles in our culture system are not consistent with the 32-58% (Michael & Priestley, 1999; Guo et al., 2001; Carlton & Hargett, 2002) and the 66% (Belecky-Adams et al., 2003) values reported for TRPV1 and peripherin, respectively. These results suggest that there is some abnormal overlapping of nociceptive markers within individual DRG neurons in our cultures that may be due to embryonic DRG neurons not having reached their mature phenotypes prior to culturing (Goldstein et al, 1996). Alternatively, our culture conditions, with generous concentrations of NGF and GDNF, may simply enhance the survival of nociceptive-like DRG neurons as opposed to large, neurofilament-positive cells. In support of this notion, the nerve growth factor (NGF) has been shown to be essential for survival of small TrkA-positive neurons (Davies, 1986; Chen and Frank, 1999; Bibel and Barde, 2000; Ernfors, 2001), whereas GDNF has been shown to be essential for the survival of small IB4+ neurons (Bennett et al., 1996; Molliver et al, 1997). The lectin IB4 could not be used on our culture system due to strong staining of astrocytes, which made it difficult to follow positively-stained neuronal processes.
4.2. Synapse formation
We used triple labeling to allow us to identify synapses that include presynaptic DRG terminals and postsynaptic dorsal horn dendrites. TrkA was used to uniquely identify the DRG neurites, MAP2 uniquely identified dorsal horn dendrites and VGluT2 identified sites of synaptic glutamate release. VGluT2 is expressed by dorsal horn neurons and some DRG neurons (Todd et al, 2003; Alvarez et al, 2004). While the pattern of VGluT2 expression by DRG subpopulations remains unclear in vivo, our data show strong colocalization with TrkA expressing DRG neurons as well as dorsal horn neurons.
Immunoreactivity for VGluT2 assumed a punctate profile in our 5 DIV cultures and many of these puncta were preferentially localized at the site where the presumed axon of a DRG neuron contacted the dendrite of a dorsal horn neuron (Fig 3B). This suggests that the neurons formed synapses at those points of contact. For quantification, we counted nociceptive presynaptic TrkA/VGluT2 and MAP2 clusters and showed that the number of synaptic boutons increased with time in culture starting at 2 DIV (Fig 3C). This pattern of increased synapse formation is in agreement with data from studies on various mass cultured neurons (Scheiffele, 2003; McAllister, 2007).
Given that we observed the most synaptic boutons and contact points at 5 DIV, we examined dorsal horn neurons at that age to determine whether the synaptic boutons are functional. Conventional whole cell patch recording of dorsal horn neurons showed low frequency and modest amplitude mEPSCs (Fig 5A, B). Further, simultaneous whole-cell recordings from individual pairs of DRG and dorsal horn neurons showed that stimulation of action potentials in DRG neurons consistently generated a postsynaptic current in all pairs tested (Fig 5D). Together, these results show that our culture system represents a model system suitable for electrophysiological and immunocytochemical studies into the mechanisms of synapse formation between nociceptive DRG and dorsal horn neurons. These neuronal populations are easily identified in the cultures by their differences in morphology and are easily accessible for electrophysiological manipulations. Growing and branching axons of DRG and dorsal horn neurons are more confined than mass culture systems, allowing for reliable identification of synaptic pairs.
While the model culture system described here offers several advantages over the more traditional mass culture systems used in many synaptogenesis studies, there are several limitations worth mentioning. Just as with the mass cultures, there are inherent limitations of an artificial growth environment. The three-dimensional organization and normal intercellular signaling milieu are lost. Moreover, the temporal regulation of the developmental program may be altered as compared to the in vivo situation.
We used cortical astrocytes as a feeder layer in our DRG-DH culture system due to the inherent difficulty in obtaining substantial amount of spinal cord astrocytes during late embryonic and early postnatal ages. This approach has been used for retinal ganglion cells where cortical astrocytes were shown to be critical in the development of numerous and efficient synapses by early postnatal retinal ganglion cells (Steinmetz et al., 2006). However, recent studies have shown that astrocytes are comprised of a heterogenous population in terms of morphology as well as functional differences that appears to depend on brain regions and developmental stage (Matyash & Kettenmann, 2009). Thus, we cannot rule out the possibility that spinal cord and cortical glial cells may differentially affect synapse formation between DRG and DH neurons. This underscores the importance for comparative studies into astrocytic factors necessary for the establishment of DRG-DH synapses.
DRG and DH neurons are comprised of heterogenous subpopulations that can be characterized both morphologically and functionally in slice or in culture. We did not attempt to characterize the DH neurons in this paper, but Albuquerque at al. (1999) identified various subpopulations of DH neurons by immunocytochemistry using similar culture conditions as used in these studies. Identified subpopulations include both inhibitory (GABA and glycinergic) and excitatory (calbindin, calretinin, and NK1 receptor positive) neurons. The DRG neurons in our culture system appear to be homogenous in terms of nociceptive function. It is not clear how the presence of other classes of DRG neurons as occurs in vivo would affect the time course of synapse formation between classes of DRG and DH neurons described here. Nonetheless, our culture system offers functional synaptic connections in culture that develop over 5 DIV where experimental conditions can be well controlled. Given the clarity of analysis and the readiness of manipulation, this DRG/ dorsal horn culture system provides a powerful tool for studies into the development of nociceptive synapses.
Acknowledgments
This work was supported by NIH grants NS49964 to DJJ and NS40428 to ABM. The authors wish to thank C. Justin Lee and Cristovao Albuquerque for help at the beginning of these studies and Asya Haney for invaluable technical support.
Footnotes
Abbreviations: CNS; DRG; EPSC; GDNF; NGF; NMJ; PDL
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque C, Lee CJ, Jackson AC, MacDermott AB. Subpopulations of GABAergic and non-GABAergic rat dorsal horn neurons express Ca2+-permeable AMPA receptors. Eur J Neurosci. 1999;11:2758–2766. doi: 10.1046/j.1460-9568.1999.00691.x. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Preparation of coverslips for neuronal cultures. CSH Protoc. 2009 Aug 1;2009(8) doi: 10.1101/pdb.prot5272. pdb.prot5272. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Dissection, plating, and maintenance of cortical astrocyte cultures. CSH Protoc. 2009 Aug 1;2009(8) doi: 10.1101/pdb.prot5273. pdb.prot5273. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Dissection, plating, and maintenance of dorsal horn neuron cultures. CSH Protoc. 2009 Aug 1;2009(8) doi: 10.1101/pdb.prot5274. pdb.prot5274. [DOI] [PubMed] [Google Scholar]
- Albuquerque C, Joseph DJ, Choudhury P, MacDermott AB. Dissection, Plating and maintenance of dissociated dorsal root ganglion (DRG) neurons for mono-culture and for co-culture with dorsal horn neurons. CSH Protoc. 2009 Aug 1;2009(8) pdb.prot5275. [Google Scholar]
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991;88:7834–7838. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belecky-Adams T, Wight DC, Kopchick JJ, Parysek LM. Intragenic sequences are required for cell type-specific and injury-induced expression of the rat peripherin gene. J Neurosci. 1993;13:5056–5065. doi: 10.1523/JNEUROSCI.13-12-05056.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur J Neurosci. 1996;8:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 14:2919–2937. doi: 10.1101/gad.841400. 2000. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The formation and maturation of synapses in the visual cortex of the rat. II. Quantitative analysis. J Neurocytol. 1983;12:697–712. doi: 10.1007/BF01181531. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Stereological analysis of Ca(2+)/calmodulin-dependent protein kinase II alpha -containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- Chen HH, Frank E. Development and specification of muscle sensory neurons. Curr Opin Neurobiol. 1999;9:405–409. doi: 10.1016/S0959-4388(99)80061-0. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S. The developing synapse: construction and modulation of synaptic structures and circuits. Science. 2002;298:770–776. doi: 10.1126/science.1075510. [DOI] [PubMed] [Google Scholar]
- Craig AM, Boudin H. Molecular heterogeneity of central synapses: afferent and target regulation. Nat Neurosci. 2001;4:569–578. doi: 10.1038/88388. [DOI] [PubMed] [Google Scholar]
- Davies AM. The survival and growth of embryonic proprioceptive neurons is promoted by a factor present in skeletal muscle. Dev Biol. 1986;115:56–67. doi: 10.1016/0012-1606(86)90227-7. [DOI] [PubMed] [Google Scholar]
- Dyson SE, Jones DG. Quantitation of terminal parameters and their inter-relationships in maturing central synapses: a perspective for experimental studies. Brain Res. 1980;183:43–59. doi: 10.1016/0006-8993(80)90118-3. [DOI] [PubMed] [Google Scholar]
- Goldstein ME, Grant P, House SB, Henken DB, Gainer H. Developmental regulation of two distinct neuronal phenotypes in rat dorsal root ganglia. Neurosci. 1996;71:243–258. doi: 10.1016/0306-4522(95)00404-1. [DOI] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–26. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Guo A, Simone DA, Stone LS, Fairbanks CA, Wang J, Elde R. Developmental shift of vanilloid receptor 1 (VR1) terminals into deeper regions of the superficial dorsal horn: correlation with a shift from TrkA to Ret expression by dorsal root ganglion neurons. Eur J Neurosci. 2001;14:293–304. doi: 10.1046/j.0953-816x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Tovar KR, Craig AM. Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci. 2005;25:6379–6388. doi: 10.1523/JNEUROSCI.0302-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Juttner R, Rathjen FG. Molecular analysis of axonal target specificity and synapse formation. Cell Mol Life Sci. 2005;62:2811–2827. doi: 10.1007/s00018-005-5299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol. 1997;505(Pt 1):177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Takami K, Kawai Y, Girgis S, Hillyard CJ, MacIntyre I, Emson PC, Tohyama M. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985;15:1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kawai Y, Shiosaka S, Takami K, Kiyama H, Hillyard CJ, Girgis S, MacIntyre I, Emson PC, Tohyama M. Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat: immunohistochemical analysis. Brain Res. 1985;330:194–196. doi: 10.1016/0006-8993(85)90027-7. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Labrakakis C, Joseph DJ, Macdermott AB. Functional similarities and differences of AMPA and kainate receptors expressed by cultured rat sensory neurons. Neuroscience. 2004;129:35–48. doi: 10.1016/j.neuroscience.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12:265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- Matyash V, Kettenmann H. Heterogeneity in astrocyte morphology and physiology. Brain Res Rev. 2010 doi: 10.1016/j.brainresrev.2009.12.001. in press. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–13920. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. doi: 10.1139/y02-034. [DOI] [PubMed] [Google Scholar]
- Salt TE, Morris R, Hill RG. Distribution of substance P-responsive and nociceptive neurones in relation to substance P-immunoreactivity within the caudal trigeminal nucleus of the rat. Brain Res. 1983;273:217–228. doi: 10.1016/0006-8993(83)90846-6. [DOI] [PubMed] [Google Scholar]
- Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- Segal MM, Furshpan EJ. Epileptiform activity in microcultures containing small numbers of hippocampal neurons. J Neurophysiol. 1990;64:1390–1399. doi: 10.1152/jn.1990.64.5.1390. [DOI] [PubMed] [Google Scholar]
- Snider WD, Silos-Santiago I. Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Philos Trans R Soc Lond B Biol Sci. 1996;351:395–403. doi: 10.1098/rstb.1996.0034. [DOI] [PubMed] [Google Scholar]
- Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- Steinmetz CC, Buard I, Claudepierre T, Nagler K, Pfrieger FW. Regional variations in the glial influence on synapse development in the mouse CNS. J Physiol. 2006;577:249–261. doi: 10.1113/jphysiol.2006.117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain. 1996;67:151–161. doi: 10.1016/0304-3959(96)03076-X. [DOI] [PubMed] [Google Scholar]
- Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L. The development and modulation of nociceptive circuitry. Curr Opin Neurobiol. 2006;16:460–466. doi: 10.1016/j.conb.2006.06.002. [DOI] [PubMed] [Google Scholar]


