Abstract
Background
The rostral anterior cingulated cortex (rACC) and the amygdala consistently emerge from neuroimaging studies as brain regions crucially involved in normal and abnormal fear processing. The aim of this study is to investigate a possible top-down modulation of a specific rACC sub-region on the acquisition of auditory fear conditioning.
Methods
We performed excitotoxic lesions, temporal inactivation and activation of a specific sub-region of the rACC that we identified by tracing studies as supporting most of the connectivity with the basolateral amygdala (rAmy-ACC). The effects of these manipulations over amygdala function were investigated using a classical tone-shock associative fear conditioning in the rat.
Results
Excitotoxic lesions and transient inactivation of the rAmy-ACC pre-training selectively produced deficits in the acquisition of the tone-shock associative learning (but not context). This effect was specific for the acquisition phase and did not required protein synthesis in the rAmy-ACC. However, it could be overcome if an overtraining procedure was applied. Conversely, pre-training transient activation of the rAmy-ACC facilitated tone-shock associative learning and interfered with further extinction processes.
Conclusion
Our results suggest that a subregion of the rACC is key to gating the efficiency of amygdala-dependent auditory fear conditioning learning. Since rAmy-ACC inputs were confirmed to be glutamatergic, we propose that recruitment of this brain area might modulate overall BLA excitatory tone during CS-US concomitant processing. In the light of clinical research, our results provide new insight on the effect of inappropriate rACC recruitment on emotional behaviours.
Keywords: anxiety, amygdala, fear acquisition, fear conditioning, anterior cingulate cortex, medial prefrontal modulation
Introduction
It is now well established that anxiety disorders arise as a consequence of alterations in amygdala-dependent fear learning processes [1–5]. Fear learning has been investigated using classical Pavlovian fear conditioning paradigms, which consists of pairing a neutral conditioned stimulus (CS) such as a tone, with an aversive unconditioned stimulus (US) such as a footshock. Upon subsequent exposure, the CS is then perceived as aversive and provokes a fear response [6]. Using this paradigm, brain areas engaged in the perception and response to threat were identified [1–4,7,8]. While the amygdala was found to be central for the CS to acquire aversiveness, the hippocampus primarily processes information associated with the fear context [7,9,10]. In addition, cortical areas such as the medial prefrontal cortex (mPFC) have been shown to play a critical role in the processing of emotional and cognitive information [11–15]. Of all cortical areas, the rostral anterior cingulate cortex (rACC), the affective division of the ACC [16–18], is ideally positioned between limbic and cortical structures to integrate emotion and cognition and is thereby primed to influence amygdala-dependent learning [17–25]. Neuroimaging studies have revealed a key role of the rACC in the normal processing of emotion and learning [16], but abnormal responses following provocation studies in patients suffering from various psychopathologies were also reported [26–30]. Interestingly, in fear conditioning studies, activation of the rACC occurs following the learning of repeated CS-US pairings [31–33], suggesting some functional interplay between the rACC and the amygdala specifically during the CS-US association. That the rACC may somehow control aspects of amygdala-dependent fear learning is further indicated by the coincident changes in neuronal activity detected within both these structures in affect-related processing [15,17,31,32,34–39].
Previous studies reported that the rACC is involved in the consolidation of inhibitory avoidance memories [40,41], in the acquisitions of fear avoidance learning [42,43], conditional visual discrimination [44] and appetitive conditioning [45]. Although there is a the large corpus of anatomical data on ACC-amygdala connectivity [18–22,46–49], very little is known about the rACC-amygdala interaction during the acquisition of auditory fear conditioning may be due to the fact that the ACC is involved in several processes such as anticipation, pain, attention, error monitoring and effortful recall [17,29,50–54]. Furthermore, lesions of this area led to different outcomes depending on the site and extent of the lesion, as well as the behavioural task used. However, in light of the above mentioned clinical reports studies focused on such interactions are especially prescient.
Here, we focused on the effects of permanent or temporary inactivation of a subregion of the rACC on the acquisition or expression of amygdala-dependent fear learning. We also sought to confirm that this subregion encompasses the full area of reciprocal connectivity between the two structures. Together our data demonstrate that a subregion of the rACC, which we term rAmy-ACC, is key for establishing the efficacy and strength of auditory-associated fear learning.
Methods and Materials
Animals
Male Sprague-Dawley rats (Charles River, France) weighing 275–300g were group-housed on a 12-hour light-dark cycle with food and water ab libitum. The animals were allowed to habituate for 4 days before surgery. All experiments were carried out in accordance with the Veterinary Authority of Basel-Stadt, Switzerland.
Reagents
Ibotenic Acid (1.5M, IBO, Sigma Aldrich) was dissolved in PBS (0.1M) and adjusted to a PH of 7.2–7.4 using 1.0M NaOH. Muscimol (10nmol/μl, Sigma Aldrich) and Bicuculline Methiodine (50pmol/μl, Sigma Aldrich) were dissolved in NaCl. Anisomycin (50ug/0.5 μl, Sigma Aldrich) was diluted in equimolar HCL, diluted 50/50 with ACSF and the PH was adjusted to 7 with NaOH. DiI crystals were from Molecular Probes (USA) and Fast Blue (3–5% solution in ddH2O) from Polysciences (Germany).
Surgery
Rats were anesthetized with a isoflurane-oxygen/nitrous oxide mixture. Surgeries were performed using a TSE Systems stereotaxic apparatus (Germany). For Tracing experiments, a 28 gauge injection cannula (Plastics One, Roanoke, VA) connected via a 0.28mm ID polythene tubing (SIMS Portex) to a microinfusion pump (CMA, Sweden) was either filled with DiI crystals diluted in PBS or a Fast Blue solution. The cannula was directed towards the rACC using the following coordinates from bregma (Paxinos and Watson – 4th edition): AP: +2.7, +1.7, +0.7, 0; ML: ± 0.5, DV: 2.2, 2.8 and a total volume of 0.3μl was injected over a 5 minute period bilaterally. Tracers were allowed to diffuse for 3–5 weeks before the animals were sacrificed. For lesion studies, IBO (0.2μl) or 0.1M PBS were microinjected at the following coordinates: AP: +2.7, +1.7; ML: ±0.5; DV: 2.2, 2.8, over 5 minutes. The cannula was left in place for another 5 minutes to allow for complete diffusion. For intra-rACC injections, two chronic guide cannulae per side (33-gauge, Plastic ones) were inserted at the above AP and ML coordinates until they reached the surface of the Dura. The guide cannulae were then secured to the skull using surgical screws and bone cement. Stainless steel dummy cannulae reaching out to the tip of the guide were inserted to keep it free of contamination during recovery time. The dummies were checked everyday to avoid blockage and to habituate the animals with insertion and removal procedures. All animals (tracing, lesion, sham and microinjection) were handled and monitored for their weight and behavioural testing started a minimum 10 days later.
Behavioural training and microinjections
Apparatus
The fear conditioning and testing experiments were performed using a computerized fear-conditioning system (TSE, Bad Homburg, Germany). The conditioning test chamber A (transparent Perspex) was placed inside a detection sensor frame. Each chamber is located into a sound-attenuating box equipped with loud speaker (for auditory stimulus delivery and background white noise), light (10 watts), a ventilation fan and a camera mounted on the ceiling. The floor of the conditioning test chambers consisted of a removable stainless foot-shock grid connected to a shock delivery unit.
Conditioned stimulus (CS) and unconditioned stimulus (US) were controlled via the main computer. To dissociate between context and cue-induced freezing different chambers made of black Perspex with different grids and floors, were used on testing days (Chamber B). Freezing behaviour was automatically recorded and was defined as the absence of any movement for 1 sec.
Locomotor activity
The possible effects of manipulating rACC functionality on locomotor activity was assessed as previously described [55]. Briefly the animals were put in transparent Plexiglas boxes (dimensions: 19 × 31 × 16 cm) and the horizontal activity was detected using infrared light beam along the x-, y-, and z-axes (TSE Moti system TSE, Bad Homburg, Germany) registering the animal’s total immobility time and total distance traveled over ninety minutes.
Pain Behaviour
Changes in pain sensitivity to the US following all treatments were determined by measuring the induction of rapid movements to increasing footshock intensities from 0 to 0.6 mA. Cumulative probability plots correspond to the probability that a specific footshock intensity induces movement reaction (1 being 100% of the time).
Conditioning
Rats were handled for 3 days before behavioural manipulations. On day 1, the rats were habituated for 15 min to chamber A prior to conditioning. Conditioning trial consisted of a 15 sec, 4.5 kHz, 85 dB tone (CS) that co-terminated with a 0.6mA, 1 sec footshock (US). Pairing was repeated 6 times at random inter-trial intervals between 30 and 90 sec. After the last pairing, rats were returned to their home cage. Conditioning test chambers were cleaned with 70% ethanol after each trial. Unless stated otherwise each result expressed in bar graphs in the same graph figure result from separate experiments.
Testing
On day 2, rats that underwent training on day 1 were split in two groups. One group was placed in chamber B and presented with 3 tones and the other group in the original training chamber A for contextual testing. Rats were videotaped for subsequent behavioural scoring. Chambers B were cleaned with Thedra (a window cleaner) between each trial and Chamber A with ethanol.
Extinction
Each extinction trial (one per day over 3 days) consisted of 6 presentations of the CS alone (see above). On day 4 % freezing to 3 tone presentations was assessed and averaged.
Microinjections
For all experiments, after removal of the dummy, injectors which were connected to a microinfusion pump (CMA, Sweden) were inserted in the 4 guide cannulae (2 per side). Drugs or vehicle were microinjected at a rate of 0.2μl/2 min (0.2μl total volume par injection site). The cannula was left in place for a remaining of 5 min. For intra-ACC inactivation, activation, vehicle and protein synthesis blockade experiments, microinjections were performed 5 minutes prior to the training session. The habituation phase of the training protocol was then used as a baseline activity and locomotor measurement between the drug and control groups. For experiment 4, muscimol and PBS were injected 5 min prior to testing.
Tracing and histology
Rats were anesthetized with a combination of 75 mg/kg ketamine (Narketan, Vétoniquol, Switzerland) and 5 mg/kg xylazin (Rompun Bayer, Switzerland) and then perfused transcardially with 0.1 M PBS followed by 4% parafolmadehyde. The brains were post-fixed 4 hours in cold 4% paraformaldehyde, cryoprotected in sucrose gradients and frozen for cryosectioning. Twenty μm thick coronal sections were collected onto pre-coated slides, rehydrated in PBS, incubated for 1 hour with blocking solution containing 10% normal donkey serum, 0.2% bovine serum albumen and 0.3% Triton-X100 in PBS, and then incubated overnight with anti-vGLUT-1 or anti-vGAT (Synaptic Systems, 1,500) in blocking solution. Sections were then repeatedly rinsed in PBS and incubated with species-specific Cy3-conjugated antibodies (1:1,000; Molecular Probes) combined with Hoechst 33342 (10 μg/m, Sigma) in PBS and mounted. Pictures were collected with a Zeiss CCD digital camera connected to a Zeiss Axioplan2 microscope and processed using Adobe Photoshop 7.0. Hoechst and ethidium bromide (EthBr) were used to label all nuclei. The extent of DiI and fast blue labelling within the anterior-posterior axis of the BLA was examined using a Zeiss microscope interface with camera lucida. Each tenth Nissl-staining section was used to define boundaries of BLA cytoarchitecture and was superimposed to the adjacent section containing the fluorescent labelling for counting. For microinjection experiments toluedine blue was injected intra-rACC prior to killing for verification of the injection sites. Only animals with clearly defined bilateral lesions in the region of interest as shown by gliosis and neuronal loss were included in the analysis.
Statistical analysis
All reported statistical analyses were performed using the program SYSTAT (SPSS Inc, version 11). Effect of the lesions/drug microinjections on the mean percent freezing scores across each test trial were analyzed using a two-factors ANOVA (treatment × time) with time as a repeated measure. If significant, each time point was analyzed using a Student’s t-test. Freezing scores were averaged across trials for each rat and expressed as a percentage of the total tone presentation time. Total amount of time spent freezing during the conditioning and the memory retention test (24hrs and 5 days later following extinction) were compared using a Student’s t-test. All values are expressed as means ± s.e.m. statistical significance for all experiments was P< 0.05.
RESULTS
Effects of rAmy-ACC lesions on fear learning
To investigate the physiological relevance for the rAmy-ACC amygdala connectivity, we performed excitotoxic lesions of the rAmy-ACC region and subjected the animals to an auditory fear conditioning protocol. Two bilateral infusions of ibotenic acid (IBO) resulted in specific fibre sparing lesions (Fig. 1a, b). Only animals that exhibited no damage to motor, limbic and prelimbic areas, and in which, the extent of the lesion was similar to that of the fluorescent dyes encompassing the area of rAmy-ACC connectivity, were selected for analysis. The fear conditioning training consisted of six CS-US pairings (CS; 4.5 kHz, 15 s, 85dB – US; 0.6mA, 1s) separated by random time intervals to avoid anticipatory behaviours. The time course of learning was measured by the amount of freezing induced during each tone presentation. While both groups developed freezing behaviour as the number of associative pairing increased (Fig. 1c), the lesion group exhibited a slower acquisition across pairings (Fig. 1c). ANOVA analysis revealed a significant effect of pairings (F5,100 = 5.5, *P < 0.05) in which pairing 5 and 6 were significantly different (*P < 0.05) as well as a treatment effect (F1,20 = 6.044, *P < 0.05) and a significant interaction between pairings × treatment (F5,100 =7.977, *P < 0.05). The learning impairment was also reflected by a deficit in the total time spent freezing (averaged over the 6 pairings) across the entire acquisition session (lesion: 14.2 ± 2.5%; n = 10 sham: 31.2 ± 4.1%; n = 12; *P < 0.05; Fig. 1d). 24 hours later, rats were tested for either contextual or cue fear expression. Consistent with reduced levels of initial learning, fear expression to the CS+ presentation was lower in the lesion group compared to sham (18.1 ± 4.5%; n = 10 vs. 48.3 ± 5.4%; n = 12; **P < 0.05; Fig. 1e). However, the expression of contextual fear was not affected by lesion of the rAmy-ACC (sham: 51.1 ± 3.4% vs. lesioned: 49.2 ± 4.7%; n = 10; P >0.05; Fig. 1f). The rACC has an important role in motor function and in pain processing [16–18], we then asked whether changes in locomotor activity or in US perception could account for the learning impairment following rAmy-ACC lesion. No differences were observed in immobility behaviour and total distance travelled between groups (Fig. 1g). Similarly, US-induced movements across pairings were not affected by rAmy-ACC the lesion (Fig. 1h). Finally, no difference was found in the amount of exploratory behaviour during the baseline periods, prior to training, and on day two, when introduced to a new context (baseline groups of the cue expression test, Fig. 1d, e). Taken together, these results suggest that pre-training lesions of the rAmy-ACC specifically modulates amygdala-dependent CS-US acquisition without interfering with the further expression of contextual fear.
Figure 1.
Lesion of the rAmy-ACC produce deficit in fear acquisition. (a) Examples of the larger (light grey) and smaller (dark grey) extend of the lesions. (b) Representative phase contrast image showing a site specific lesion of the Cg1 (c) Lesion of the rAmy-ACC (black squares: n = 10) exhibit a slower acquisition time course than sham (white dots: n = 12). (d) The mean time spent freezing (± s.e.m) during the acquisition phase was significantly reduced in lesioned animals (black dots, n = 10) compared to sham (n = 12). (e) Fear expression in lesioned animals was also impaired on day 2 There was no difference during baseline habituation (no tone) between groups. (f) Lesions of the rAmy-ACC did not change locomotor activity as measured by the total immobility and total distance traveled. (g) There was no difference between the two groups in the response to the six consecutive shocks given at 0.6mA.
Transient inactivation of the rAmy-ACC
To distinguish between a role of the rAmy-ACC on the initial steps of learning (acquisition) or on the expression of conditioned responses, we microinjected the GABAa receptor agonist muscimol (4.4 nmol/side, 0.20μl/side)[56,57] in the rAmy-ACC either pre-training or 24 hours later before the expression test (Fig. 2a, b). Similarly to the effect of permanent lesions, transient inactivation of the rAmy-ACC reduced the time course for the acquisition across pairings (two-way ANOVA, pairing effect F5,70 = 13.176, *P < 0.05) with pairings 4,5 and 6 being significantly different (P < 0.05). There was also a treatment effect: F1,14 =7.275, *P < 0.05, n = 8; and a significant interaction between the two: pairings × treatment F5,70 = 8.14, *P < 0.05 (Fig. 2c). The averaged time spent freezing over the acquisition phase was reduced in the lesion group (muscimol: 13% ± 4.3; n = 8; Vehicle: 35.8% ± 5.1; n = 8, *P < 0.05; Fig. 2d). Fear expression measured on day two was also reduced in the muscimol group (18% ± 2.2; n = 8; vehicle 52.3% ± 6.5; n = 8; **P < 0.05; Fig. 2e). Blocking neuronal activity in the rAmy-ACC did not change locomotor activities or the threshold for US induced movements compared to the vehicle group (Fig. 2f). We next tested whether the inactivation of the rAmy-ACC could also affect fear expression. In a separate experiment, two untreated groups (group 1 and group 2) were first fear conditioned (Fig. 2g). 24 hours later the two groups either received muscimol or vehicle injection. No differences were observed on the levels of conditioned responses (Muscimol: 52.6% ± 4.7 Vehicle: 54.1% ± 5.2; P > 0.05, Fig. 2h). Taken together, these data suggest that inactivation and lesion of the rAmy-ACC specifically impair fear acquisition but not fear expression.
Figure 2.
Fear learning is impaired by blocking neuronal activity in the rAmy-ACC. (a) Example of injections sites for the muscimol microinjected group. (b) Phase contrast image showing two toluedine dark blue dots indicating the location of the tip of the cannulae. (c) Inactivation of the rAmy-ACC also impaired fear acquisition. There was no effect of muscimol injection on baseline activity. (d) 24 hours after learning fear expression was also reduced without affecting habituation to a new context (no tone group). (e) Both groups showed no difference in the threshold for foot-shock induced behaviour. (f and g) Muscimol injection post-training did not affect freezing levels during fear acquisition and on day two during CS+ presentation.
Longer training exposure overcomes the effect of rAmy-ACC inactivation
Previous studies suggested that the rACC may facilitate the process of “learning the rule” by changing the representation associated with the CS during learning and adapting for the optimal behavioural outcome [44,52,58]. It is therefore possible that the learning deficit observed following rAmy-ACC inactivation, reflect a slower capacity for learning the CS-US associative rule. To test for this hypothesis, we doubled the number of pairings during training, subjecting both groups to 12x CS-US instead of 6x. During the acquisition, this protocol induced a ceiling effect in the vehicle group indicating that maximum learning had been reached (6x: 44 ± 5.1, n = 8 vs 12x: 55.2 ± 4.3, n = 8, Fig. 3a). The muscimol group, similar to the previous experiment (Fig. 2) showed a slower acquisition curve compared to vehicle, ANOVA analysis significant effect of pairing number (F11,154 =12.73 *P < 0.05, n = 8), treatment (F1,14 = 11.22 *P < 0.05, n = 8 both groups) and a significant interaction between pairing × treatment interaction (F11,154 = 25.8 *P < 0.05) with most of the pairings except pairing number one and the last three, being significantly different in the muscimol group. However, for the last pairing (12) the muscimol group exhibited a similar percentage of freezing as for the vehicle group following 6 pairings (6th pairing in the vehicle group: 44.5 ± 5.1 vs 12th pairing in the muscimol group 43 ± 3.9, Fig. 3a) suggesting some learning recovery. There was still a significant difference across the whole acquisition session, between the two groups mainly due to the deficits observed in the first part of the acquisition. Consistent with the idea that more pairings would overcome the effect of rAmy-ACC inactivation over learning, ear expression to the CS+ presentation was similar in both groups (vehicle: 58% ±4, muscimol: 53.5% ± 5.1 both groups n = 8). This further suggested that injection of muscimol in the rAmy-ACC during acquisition does not impair but rather delays learning.
Figure 3.
(a) A longer training protocol could overcome the impairment in fear acquisition induced by rAmy-ACC inactivation. (b) On day two, there was no difference between the lesion and vehicle group in conditioned responses to the CS+ presentation
Transient activation of the rAmy-ACC
Our results provided evidence that activating GABAA receptors in the rAmy-ACC delayed the early stages of fear learning. Thus, we next sought to examine whether inhibiting GABAA receptors in the rAmy-ACC could facilitate learning. Injections of bicuculline [59] (50pmol/0.2μl) in the rAmy-ACC pre-training resulted in a faster acquisition time course compared to vehicle (Fig. 4a, b). A two-way ANOVA showed a significant effect of pairings (F5,90 = 21,49, *P < 0.05) with pairing numbers 2, 3 and 5 being significantly different (*P < 0.05), a treatment effect (F1,18 = 6,49, *P < 0.05) and an interaction between the two (F5,90 = 7,12 *P < 0.05, Fig. 4b) were also seen. The averaged freezing time over the whole acquisition was increased in the bicuculline injected group (Lesion: 58.4% ± 3.1 vs. vehicle: 35.9% ± 4.4, P < 0.05; n = 10 Fig. 4c). On day 2, higher conditioned fear expression was also found in this group compared to vehicle (70.7% ± 5.2 vs. 48.5% ± 4.4 P < 0.05 n = 10 Fig. 4d). These effects were unlikely to be due to a bicuculline-induced hyperactivity behavior as no difference in locomotor activity (data not shown) or baseline freezing measurements were observed between groups (Fig. 4d). In addition, the thresholds for US-induced movements were similar in both groups (Fig. 4e).
Figure 4.
Activation of the rAmy-ACC facilitates and strengthens fear learning. (a) Example of bicuculline injection targeting the rAmy-ACC. (b) The time course of the fear learning was facilitated by neuronal activation of the rAmy-ACC. (c) There was a significant increase in the total amount of freezing across the conditioning phase in bicuculline treated group compare to vehicle. (c) On day two, freezing levels to the CS+ presentation were significantly increased in the bicuculline group compared to vehicle Bicuculline injection did not induce changes in baseline activity. Following three days of extinction protocol, the vehicle group showed significant reduction in freezing behavior to the CS+. In the contrary, the bicuculline group exhibited the same levels of freezing to the tone 24 hours and 4 days later suggesting that extinction was impaired. (d) No threshold difference in US-induced movements were observed between the vehicle and the bicuculline groups.
Both groups were then submitted to an extinction protocol consisting of 6x CS presentation each day for three days. The extinction of the conditioned response was assessed on day 4. While the vehicle group showed a significant reduction in freezing levels in response to the CS (before extinction 48.5% ± 4.4 vs. after extinction 34.5% ± 3.5, n = 10 Fig. 4d), the group that had learned under ramy-ACC activation expressed similar freezing levels as when tested 24hrs after learning (before extinction 70.7% ± 5.2 vs. after extinction 68.5% ± 7.3 after extinction Fig. 4d). Collectively, these results support the idea that the rAmy-ACC when recruited, allows fear to the CS+ to be acquired faster but also seems to be interfering with mechanisms underlying extinction processes.
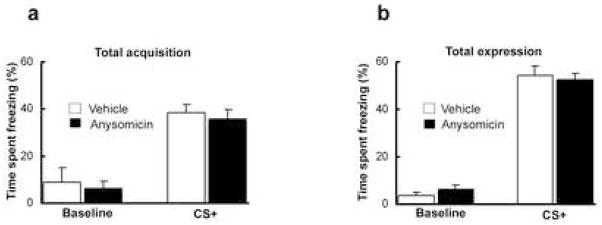
Protein synthesis is not required in the rAmy-ACC
Previous studies reported that besides the amygdala other brain areas exhibited protein synthesis dependent neuronal plasticity during the acquisition and consolidation of fear memories [60]. To investigate whether protein synthesis in the rAmy-ACC was also required during learning we infused anisomycin [61,62] (50ug/0.2 μl) or vehicle pre-training. Levels of fear acquisition and expression did not differ between the two groups (Fig. 5a and 5b) suggesting that long-term plasticity in the rAmy-ACC is not required for its modulatory action over learning.
Figure 5.
Blocking protein synthesis in the rAmy-ACC pre-training did not affect fear learning (a) or fear expression (b)
Connectivity between the rACC and the amygdala
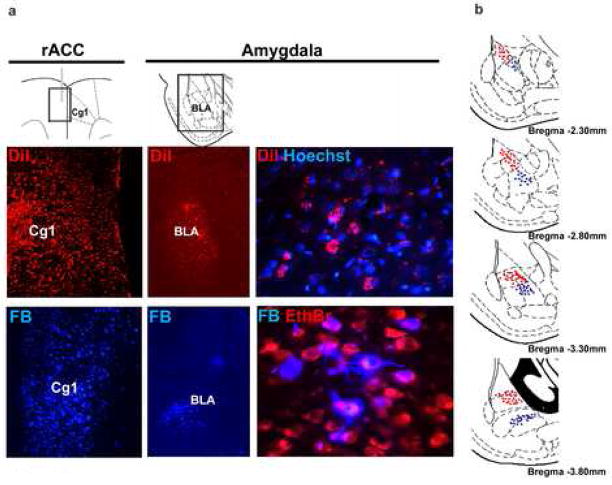
As our data indicate a key role of the rACC in the circuitry of fear, it was important to define if the area within the rACC (ramy-ACC) which we are manipulating is encompassing the entire area of connectivity with the BLA and further define the nature of the top-down connections. We injected fluorescent neuronal tracers at four different coordinates unilaterally along the anterior-posterior axis of the rACC and examined sections across the amygdala for fluorescent signals three weeks later. Injection of Fast blue (FB) performed at AP: +2.7, +1.7 coordinates within the rACC (rAmy-ACC) region, resulted in a clear labelling of neuronal cell bodies in the basolateral amygdala (BLA) (Fig. 6a and b), consistent with a retrograde tracing of axonal tracts. Injection of the tracer DiI resulted almost invariably in the labelling of disperse puncta in the BLA and unlike FB, it never labelled cell bodies, indicating that in our experiments DiI diffused in an anterograde manner [63,64] (Fig. 6a and b). No labelling was observed in the amygdala on the contraleral side of dye injections, suggesting that lateralization occurs (Fig. 6a). Injections of either tracer at AP: +1 and 0 did not show fluorescence in the BLA.
Figure 6.
Connectivity between the rACC and amygdala. (a) The fluoresecent neuronal tracers FB and DiI were stereotaxically injected at different anterior-posterior positions along the ACC. Only injections at AP:+2.7, +1.7 coordinates resulted in specific labelling in the BLA. Images show injection sites in the ACC and amygdala three weeks later. Higher magnification images reveal FB-positive neuronal cell bodies and processes and punctuated DiI-positive axonal terminals. Double labelling with the nuclear markers ethidium bromide (EthBr) or Hoechst was used to indicate the position of all nuclei. (b) Coronal sections along the anterior-posterior axis of the BLA indicate the areas of FB (blue dots) and DiI (red dots) labelling. Note that the areas occupied by the two tracers and non-overlapping and that their position changes along the anterior-posterior axis of the BLA.
Projections from the rAmy-ACC to the BLA are excitatory
We next finally examined the nature of the inputs from the rAmy-ACC and the amygdala by performing immunostaining of FB- or DiI-traced sections throughout the amygdala, with antibodies against the antigens vesicular GABA transporter (V-GAT) and the vesicular glutamate transporter (V-GLUT). These markers are expressed by GABAergic interneurons and by glutamatergic neurons respectively and they exhibit a punctuated pattern of expression along their neuronal cell body and processes. The large majority of DiI-positive puncta in the amygdala co-localized with V-GLUT-positive, but not with V-GAT-positive cells (Fig. 7a, b), suggesting that afferents from the ramy-ACC are excitatory. The majority of retrogradely labeled FB-positive neurons in the amygdala expressed V-GLUT and were mostly V-GAT-negative, indicating that these cells are also excitatory and exhibit morphological similarities to BLA pyramidal neurons [19,65] (Fig. 7d, e).
Figure 7.
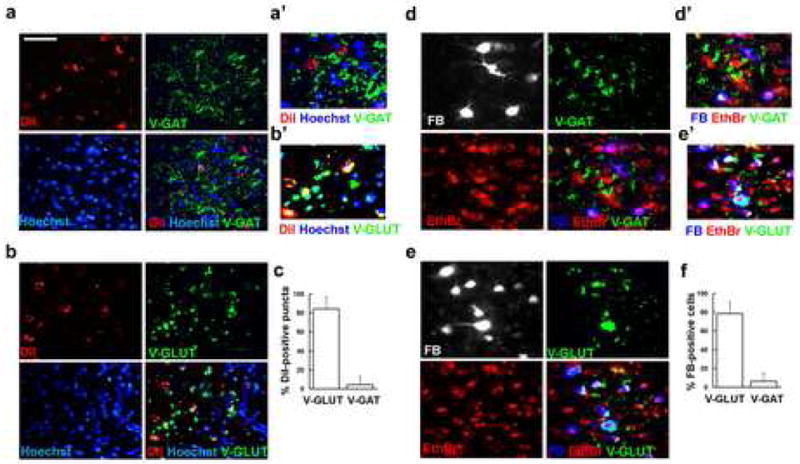
Section through the amygdala traced with either DiI or fast blue (FB) were immunolabeled with antibodies against V-GAT or V-GLUT. DiI-positive puncta do not co-localize with V-GAT (a, c), but they do with V-GLUT, co-localization is seen in yellow (b, c). a′ and b′ are higher magnification images of a and b. Most FB-positive neurons are negative for V-GAT (d, f) and express V-GLUT, co-localization in white (e, f). d′ and e′ are higher magnification images of d and e. Bar, 50 μm in a, b, d, e and 25 μm in a′, b′, d′, e′.
DISCUSSION
In this study, we provide evidence that lesion or transient inactivation of a specific sub-division of the rACC (which we referred to as ramy-ACC) can modulate the efficiency of amygdala-dependent fear conditioned learning. This effect was transient and could be reversed by increasing the number of pairings during learning. Conversely, transient activation of the ramy-ACC enhanced fear learning to the CS+ and prevented memory extinction. Since long-range ramy-ACC afferents were found to be excitatory, these results support a role for the ramy-ACC in the early steps of amygdala-dependent fear learning by possibly modulating the excitatory tone of the BLA.
Pre-training lesions as well as temporal inactivation of the ramy-ACC specifically impaired the acquisition but not the expression of auditory fear conditioning indicating specific ramy-ACC/BLA interactions during the early stages of fear learning. Since activation of the rACC has previously been shown to be involved in pain [43,50,66,67], changes in CS or US processing per se could influence the effects observed. However, this is unlikely to be the case under our experimental conditions as both pain threshold responses and contextual fear expression were unchanged following treatment. The fact that the amount of contextual fear expression was intact following lesions of the ramy-ACC is in line with recent studies demonstrating that the rACC is preferentially involved in remote contextual fear memory storage and retrieval rather than during the acquisition phase [68–71]. As we also found reciprocal connectivity between the ramy-ACC and the hippocampus (data not shown), it is possible that the ramy-ACC subserves diverse functions depending on the behavioural cue versus contextual requirements. Interestingly, whilst our data clearly demonstrate that conditioned fear responses are contingent on ramy-ACC function, our previous results indicated that the ramy-ACC does not play a role in the manifestation of unconditioned anxiety behaviours in the elevated plus maze [72]. This may be explained by reports from lesion studies which indicate that the amygdala plays a crucial role in learned fear responses [9,10,73], but not in approach-avoidance behaviour in the plus maze [74,75]. Moreover, it reinforces the concept that multiple circuits underlie the manifestation of various symptoms relevant to the diverse anxiety disorders [76].
The learning impairment induced by ramy-ACC inactivation was transient and could be overcome by doubling the number of pairings during the training session. It is unlikely reflecting a slow elimination of drug effect since muscimol was previously shown to remain stable in cortical tissue for up to two hours [77]. While we cannot exclude the possibility that increasing the number of pairings changes the essence of the task from conditioned fear learning to a repetitive chronic stress experience [42,44,53], this experiment provides evidence that in the absence of a functional ramy-ACC fear learning can be delayed and that subsequent to sustained training its recruitment is compensated by other circuits.
In mice, lesions of the entire rACC specifically impaired trace but not delay conditioning [78] however, this same study also reported c-fos expression in the rACC following delay conditioning. Whether specific ramy-ACC lesions would induce similar effects in the mouse model, have not yet been tested [51,79–81]. Considering the functional heterogeneity of this area, different observations following lesions might be accounted for by the different subregion of the rACC targeted, which differ in positions and extent. These results further support the need for carefully defining the exact lesion area of the rACC before experimentation.
Bicuculline microinjection into the ramy-ACC accelerated learning while enhancing the levels of conditioned responses. Similarly to our previous observation with muscimol activation of the ramy-ACC did not change pain sensitivity or baseline measurements compared to sham. Our results further indicated that the fear memory acquired under ramy-ACC activation was resistant to our extinction protocol. Since we did not test for longer protocols, we cannot at this point determine whether the extinction process was permanently impaired or simply delayed. A large body of evidence indicated a functional role for the medial prefrontal cortex (mPFC: infralimbic, prelimbic) in the extinction of conditioned fear [82–85]. Considering that in the rat this region is extensively connected to the rACC [48,86], activation of the ramy-ACC during learning might interfere with mPFC-mediated extinction processes. Blocking protein synthesis in the ramy-ACC had no effect over fear acquisition and expression indicating that while this region can modulate learning, it is not itself a site of protein synthesis-dependent fear memory storage. In agreement with this, it has been shown that the contribution of the auditory thalamus to the consolidation of fear memories is also independent of protein synthesis, whereas signalling via the ERK/MAPK pathway is necessary [60,87]. Whether such mechanisms are also activated at ramy-ACC-LA synapses during learning would require additional studies. While the identity of the postsynaptic targets of ramy-ACC input to the BLA remains unknown, the fact that the afferents were found to be excitatory supports the hypothesis that the excitatory tone within the BLA can be modulated as a function of ramy-ACC activity. Since fear conditioning involves the induction of long-term potentiation (LTP) at excitatory synapses of the LA [6], it would be interesting to investigate whether the ramy-ACC can modulate associative learning by interfering with such mechanisms.
Although coupling between the ramy-ACC and the BLA has previously been documented [20,22,46,88], our tracing experiments reported that the subregion of the ramy-ACC was necessary and sufficient to support the full connectivity area between the two structures. Furthermore, our study also revealed that in all cases the connectivity between the ramy-ACC and the BLA was ipsilateral. In this context, it is noteworthy that during the processing of fear memories in both rodents and humans the amygdala was functionally lateralized [89–92]. Moreover, responses in both the left amygdala and the left rACC were greater in masked affect perception studies, supporting a physiological role for the lateralization of the ramy-ACC-amygdala axis [17,93]. It would be interesting to further investigate whether such anatomical lateralization underlies hemispheric specialization, as this has been proposed to facilitate learning by reducing the redundancy of information processed by each hemisphere [94–96].
In conclusion, our data provide new insight into the physiological relevance of the ramy-ACC- amygdala axis during fear conditioning. While we found that inappropriate ramy-ACC recruitment could either disrupt or facilitate amygdala-dependent fear learning, we also found that other processes such as memory extinction were affected. Since psychiatric disorders such as anxiety or post-traumatic stress disorders are likely to result from an overall network dysregulation, it will be important to further investigate the relationship of the rACC with other structures such as the infralimbic, prelimbic and orbitofrontal cortex during the processing, integration and recall of emotional information.
Acknowledgments
The authors would like to thank Drs. Annick Vassout and Frederique Chaperon for technical assistance and advice and Prof Yves-Alain Barde (Univ. Basel) for comments on the manuscript. SB was funded through Novartis Institutes for BioMedical Research Presidential Postdoc Programme.
References
- 1.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 2.Aguado L. Neuroscience of Pavlovian conditioning: a brief review. Span J Psychol. 2003;6:155–167. doi: 10.1017/s1138741600005308. [DOI] [PubMed] [Google Scholar]
- 3.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 4.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 6.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 7.Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 8.Rescorla RA. Pavlovian conditioned fear in Sidman avoidance learning. J Comp Physiol Psychol. 1968;65:55–60. doi: 10.1037/h0025412. [DOI] [PubMed] [Google Scholar]
- 9.Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis M, Shi C. The amygdala. Curr Biol. 2000;10:R131. doi: 10.1016/s0960-9822(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 11.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki H, Adolphs R, Oya H, Kovach C, Damasio H, Kaufman O, Howard M. 3rd Analysis of single-unit responses to emotional scenes in human ventromedial prefrontal cortex. J Cogn Neurosci. 2005;17:1509–1518. doi: 10.1162/089892905774597182. [DOI] [PubMed] [Google Scholar]
- 13.Shiv B, Loewenstein G, Bechara A, Damasio H, Damasio AR. Investment behavior and the negative side of emotion. Psychol Sci. 2005;16:435–439. doi: 10.1111/j.0956-7976.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- 15.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 16.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 17.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 18.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 19.Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat (1986) Brain Res Bull. 17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- 20.Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- 21.Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Exp Brain Res. 1994;100:469–483. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- 22.Ottersen OP Connections of the amygdala of the rat. IV. Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J Comp Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- 23.McDonald AJ, Mascagni F. Projections of the lateral entorhinal cortex to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1997;77:445–459. doi: 10.1016/s0306-4522(96)00478-2. [DOI] [PubMed] [Google Scholar]
- 24.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 25.Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid projections of rat temporal cortex. J Comp Neurol. 1997;382:153–175. [PubMed] [Google Scholar]
- 26.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 27.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 28.Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L, Breiter HC, et al. A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry. 1995;52:20–28. doi: 10.1001/archpsyc.1995.03950130020003. [DOI] [PubMed] [Google Scholar]
- 29.Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 30.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 31.Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J Neurosci. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. Neuroreport. 1999;10:3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- 33.Buchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Curr Opin Neurobiol. 2000;10:219–223. doi: 10.1016/s0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 34.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 35.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 36.Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry. 1997;42:446–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- 37.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Cannistraro PA, Rauch SL. Neural circuitry of anxiety: evidence from structural and functional neuroimaging studies. Psychopharmacol Bull. 2003;37:8–25. [PubMed] [Google Scholar]
- 39.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Malin EL, McGaugh JL. Differential involvement of the hippocampus, anterior cingulate cortex, and basolateral amygdala in memory for context and footshock. Proc Natl Acad Sci USA. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala. Neurobiol Learn Mem. 2007;87:295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabriel M. Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. Prog Brain Res. 1990;85:467–482. discussion 482–463. [PubMed] [Google Scholar]
- 43.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Dissociable effects of anterior and posterior cingulate cortex lesions on the acquisition of a conditional visual discrimination: facilitation of early learning vs. impairment of late learning. Behav Brain Res. 1996;82:45–56. doi: 10.1016/s0166-4328(97)81107-2. [DOI] [PubMed] [Google Scholar]
- 45.Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- 46.Pitkanen A. connectivity of the rat amygdaloid complex. In: JPA, editor. The amygdala: A functional analysis. Oxford university press; 2000. pp. 31–113. [Google Scholar]
- 47.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 48.Fisk GD, Wyss JM. Associational projections of the anterior midline cortex in the rat: intracingulate and retrosplenial connections. Brain Res. 1999;825:1–13. doi: 10.1016/s0006-8993(99)01182-8. [DOI] [PubMed] [Google Scholar]
- 49.Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- 50.Davis KD. The neural circuitry of pain as explored with functional MRI. Neurol Res. 2000;22:313–317. doi: 10.1080/01616412.2000.11740676. [DOI] [PubMed] [Google Scholar]
- 51.Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 52.Bussey TJ, Muir JL, Everitt BJ, Robbins TW. Triple dissociation of anterior cingulate, posterior cingulate, and medial frontal cortices on visual discrimination tasks using a touchscreen testing procedure for the rat. Behav Neurosci. 1997;111:920–936. doi: 10.1037//0735-7044.111.5.920. [DOI] [PubMed] [Google Scholar]
- 53.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 54.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 55.Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–963. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- 56.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 57.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF. Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9:940–947. doi: 10.1038/nn1724. [DOI] [PubMed] [Google Scholar]
- 59.Dickinson-Anson H, Mesches MH, Coleman K, McGaugh JL. Bicuculline administered into the amygdala blocks benzodiazepine-induced amnesia. Behav Neural Biol. 1993;60:1–4. doi: 10.1016/0163-1047(93)90638-x. [DOI] [PubMed] [Google Scholar]
- 60.Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frey KA. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci. 2003;18:3080–3088. doi: 10.1111/j.1460-9568.2003.03063.x. [DOI] [PubMed] [Google Scholar]
- 61.Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 63.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 64.Godement P, Vanselow J, Thanos S, Bonhoeffer F. A study in developing visual systems with a new method of staining neurones and their processes in fixed tissue. Development. 1987;101:697–713. doi: 10.1242/dev.101.4.697. [DOI] [PubMed] [Google Scholar]
- 65.Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 67.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA. 2005;102:12950–12955. doi: 10.1073/pnas.0408576102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Frankland PW, O’Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 70.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 71.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 72.Bissiere S, McAllister KH, Olpe HR, Cryan JF. The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behav Brain Res. 2006;175:195–199. doi: 10.1016/j.bbr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 73.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 74.Moller C, Wiklund L, Sommer W, Thorsell A, Heilig M. Decreased experimental anxiety and voluntary ethanol consumption in rats following central but not basolateral amygdala lesions. Brain Res. 1997;760:94–101. doi: 10.1016/s0006-8993(97)00308-9. [DOI] [PubMed] [Google Scholar]
- 75.Decker MW, Curzon P, Brioni JD. Influence of separate and combined septal and amygdala lesions on memory, acoustic startle, anxiety, and locomotor activity in rats. Neurobiol Learn Mem. 1995;64:156–168. doi: 10.1006/nlme.1995.1055. [DOI] [PubMed] [Google Scholar]
- 76.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 77.Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- 78.Han CJ, O’Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci USA. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carter RM, O’Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 80.Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- 81.Thompson RF. In search of memory traces. Annu Rev Psychol. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- 82.Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Lebron K, Milad MR, Quirk GJ. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem. 2004;11:544–548. doi: 10.1101/lm.78604. [DOI] [PubMed] [Google Scholar]
- 85.Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones BF, Groenewegen HJ, Witter MP. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience. 2005;133:193–207. doi: 10.1016/j.neuroscience.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 87.Apergis-Schoute AM, Debiec J, Doyere V, LeDoux JE, Schafe GE. Auditory fear conditioning and long-term potentiation in the lateral amygdala require ERK/MAP kinase signaling in the auditory thalamus: a role for presynaptic plasticity in the fear system. J Neurosci. 2005;25:5730–5739. doi: 10.1523/JNEUROSCI.0096-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pitkanen A, Jolkkonen E, Kemppainen S. Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphol (Warsz) 2000;59:1–23. [PubMed] [Google Scholar]
- 89.Blair HT, Huynh VK, Vaz VT, Van J, Patel RR, Hiteshi AK, Lee JE, Tarpley JW. Unilateral storage of fear memories by the amygdala. J Neurosci. 2005;25:4198–4205. doi: 10.1523/JNEUROSCI.0674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- 91.Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 92.Coleman-Mesches K, McGaugh JL. Differential involvement of the right and left amygdalae in expression of memory for aversively motivated training. Brain Res. 1995;670:75–81. doi: 10.1016/0006-8993(94)01272-j. [DOI] [PubMed] [Google Scholar]
- 93.Killgore WD, Yurgelun-Todd DA. Activation of the amygdala and anterior cingulate during nonconscious processing of sad versus happy faces. Neuroimage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 94.Levy J. Human cognition and lateralization of cerebral function. Trends Neurosci. 1979;2:222–225. [Google Scholar]
- 95.Denenberg VH. Lateralization of function in rats. Am J Physiol. 1983;245:R505–509. doi: 10.1152/ajpregu.1983.245.4.R505. [DOI] [PubMed] [Google Scholar]
- 96.Demaree HA, Everhart DE, Youngstrom EA, Harrison DW. Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]