Abstract
It has been reported that exposure to UV light triggers DNA damage response (DDR) seen as induction of γH2AX not only in S- but also in G1- phase cells. In the present study, in addition to γH2AX, we assessed other markers of DDR, namely phosphorylation of ATM on Ser1981, of ATM/ATR substrate on Ser/Thr at SQ/TQ cluster domains and of the tumor suppressor p53 on Ser15, in human pulmonary carcinoma A549 cells irradiated with 50 J/m2 of UV-B. Phosphorylation of these proteins detected with phospho-specific Abs and measured by laser scanning cytometry in relation the cell cycle phase was found to be selective to S-phase cells. The kinetics of phosphorylation of ATM was strikingly similar to that of ATM/ATR substrate, peaking at 30 min after UV irradiation and followed by rapid dephosphorylation. The peak of H2AX phosphorylation was seen at 2 h and the peak of p53 phosphorylation at 4 h after exposure to UV light. Local high spatial density of these phospho-proteins reported by intensity of maximal pixel of immunofluorescence in the DDR nuclear foci was distinctly more pronounced in the early compared to late portion of S-phase. Exposure of cells to UV following 1 h pulse-labeling of their DNA with 5-ethynyl-2′deoxyuridine (EdU) made it possible to correlate the extent of DNA replication during the pulse with the extent of the UV-induced H2AX phosphorylation within the same cells. This correlation was very strong (R2= 0.98) and the cells that did not incorporate EdU showed no evidence of H2AX phosphorylation. The data are consistent with the mechanism in which stalling of DNA replication forks upon collision with the primary UV-induced DNA lesions and likely formation of double-strand DNA breaks triggers DDR. The prior reports (including our own) on induction of γH2AX in G1 cells by UV may have erroneously identified cells initiating DNA replication following UV exposure as G1 cells due to the fact that their DNA content did not significantly differ from that of G1 cells that had not initiated DNA replication.
Keywords: ATM activation, ATR activation, gammaH2AX, p53 phosphorylation, checkpoint, apoptosis, laser scanning cytometry, dephosphorylation, G1 phase
Introduction
Cellular DNA is the target of ultraviolet (UV) radiation. The energy of UV light when absorbed by a double bond in thymine and cytosine (pyrimidine bases) of DNA causes bond opening and reactivity with neighboring molecules. If the adjacent base is another pyrimidine, a covalent bond forms between them resulting in the four-membered cyclobutane ring of the pyrimidine dimer. In addition, a single bond may form between two carbon atoms on the cyclobutane ring resulting in the so called “6-4 (T-C) photoproduct” (1-4). These reactions are rapid and it has been estimated that 50-100 such reactions occur in a single cell in the skin during every second of exposure to sunlight (5).
The UV-induced DNA damage triggers the DNA damage response (DDR) (6-11). One of the earliest events of the DDR is remodeling of chromatin structure which involves its decondensation (relaxation) (11,12). Chromatin decondensation enhances accessibility of DNA damage sites to the repair machinery and is mediated by rapid and dynamic response to the damage by high mobility group proteins (HMGs) and histone H1 (13,14). The decondensation enables translocation of the MRN complex into the damage site and activation of the phosphatidylinositol 3-kinase like protein kinases ATM, ATR and DNA-PKcs, which phosphorylate numerous constituents of the repair machinery and checkpoint proteins (5,8,15-18). Downstream target substrates phosphorylated by these kinases include p53 (TP53), checkpoint proteins Chk2, Chk1 and histone H2AX (19). Activation of the checkpoint pathways arrests progression though the cell cycle thereby preventing transfer of the damage to progeny cells (20). Nucleotide excision repair (NER) is the mechanism by which mammalian cells repair UV-induced DNA damage. About 30 different proteins participate in NER and the sun-sensitive and cancer-prone disorders such as Xeroderma pigmentosum (XP), Cockayne's syndrome (CS) or trichotiodystrophy (TTD) are the consequence of defective genes that code for some of these proteins (1-5,21).
Phosphorylation of histone H2AX on Ser139 is one of the key events of the DDR (22). Phosphorylated H2AX, defined as γH2AX, particularly when detected immunocytochemically in the form of distinct nuclear foci, is considered to be a marker of formation of DNA double-strand breaks (DSBs) (22). We have previously reported that the induction of γH2AX in HL-60 cells following their irradiation with UV-B was maximal in S-phase cells and was abolished by suppression of DNA replication by the DNA polymerase inhibitor aphidicolin (23). The exception was a small cohort of cells in very early S-phase, presumed to have initiated DNA replication after addition of aphidicolin, that contained phosphorylated H2AX. We postulated that H2AX phosphorylation in S-phase cells reflects formation of DSBs resulting from the collapse of replication forks upon collision with the UV-induced primary DNA lesions and that cells in the early part of S phase were more sensitive to UV than cells in mid- or late-S phase (23).
Upon UV irradiation, replication of DNA is inhibited (24) and the stalled replication forks are known to attract DNA damage sensor proteins which trigger the ATR/Chk1-dependent checkpoint signaling cascade that leads to activation of a variety of proteins including p53 (25-28). Activated p53 (phosphorylated by ATR/Chk1 kinases) becomes stable and is able to arrest cell cycle progression, as well as to increase the cell's proclivity to undergo apoptosis in response to primary DNA damage as well as in the course of NER (29).
In our prior study, we observed that in addition to S-phase cells, the induction of γH2AX by UV was seen in a fraction of cells having a G1 and G2 DNA content (23). We speculated that their response was due to formation of the primary DSBs generated by UV and/or during DNA repair (23). In subsequent reports several authors also described H2AX phosphorylation in G1 cells, which was explained as triggered by nucleotide excision repair factors that exposed H2AX-Ser139 to kinase activity (30,31). It was also proposed that the UV light-induced phosphorylation of H2AX in G1 cells is in response to accumulation of DNA repair intermediates (32).
There is strong evidence that ATR rather than ATM or DNA PKcs initially mediate H2AX phosphorylation upon DNA damage by UV (33-36). However, DSB formation resulting from the collapse of replication forks after exposure to UV may also be responsible for the activation of ATM and DNA-PKcs which in turn also phosphorylate H2AX (37). Furthermore, ATM and ATR can be concomitantly activated and redundantly collaborate as part of the response elicited by DSBs and/or replication fork-collapsing lesions (38,39).
To reveal more details of the DDR process following cell exposure to UV, in the present study we have examined the kinetics of phosphorylation of H2AX on Ser139, ATM on Ser1981, tumor suppressor protein p53 on Ser 15 and the ATM/ATR protein substrate on Ser/Thr at SQ/TQ motifs (3,40). To detect possible differences in the time/sequence of phosphorylation and dephosphorylation of the respective proteins, the cells were examined at several time points after exposure to UV. Particular attention was focused on detection of possible differences between the DNA replicating and non-replicating cells in their response to UV. Towards this end the cells were pulse-labeled with the DNA precursor 5-ethynyl-2′deoxyuridine (EdU), an alkyne-conjugated thymidine analogue (41), and the amount of EdU incorporation assumed to reflect the extent of DNA replication occurring during exposure of cells to the precursor, was correlated with the H2AX phosphorylation. Unlike the BrdU-based DNA labeling assay (42) the incorporation of EdU and its subsequent detection by a fluorescent azide through a Cu(I)-catalyzed [3 + 2] cycloaddition reaction (“click” chemistry) (43) does not require treatment of cells with strong acid or heat that generally destroys the secondary structure of proteins (42). It was possible therefore to concurrently reveal DNA replication and expression of γH2AX, the latter detected immunocytochemically, in the same cells.
Materials and Methods
Cells, cell treatment
Human lung carcinoma A549 cells were purchased from American Type Culture Collection (ATCC #CCL-185, Manassas, VA). The cells were cultured in Ham's F12K medium with 2mM L-glutamine adjusted to contain 1.5g/L sodium bicarbonate (ATCC) and supplemented with 10% fetal bovine serum (ATCC). Dual-chambered slides (Nunc Lab-Tek II) were seeded with 1 ml of 105 cells/ml in each chamber 48 hours before exposure. All incubations were carried out at 37 °C in a humidified atmosphere of 5% CO2 in air. Cells were grown to 50% confluency, at which time they were exposed to UV light. The slide-chambers bearing the cultures of A549 cells were placed onto a UV-light gel illuminator (Fotodyne; West Berlin, WI) and exposed to a UV dose of 50 J/m2. The cultures were then rinsed with fresh medium and transferred into the incubator at 37.5° C for an additional 10 min to 6 h. Some cultures were treated with 10 μM EdU (Invitrogen/Molecular Probes, Eugene, OR) for 1 h. A549 cells growing on slides were fixed by transferring slides into Coplin jars containing 1% methanol-free formaldehyde (Polysciences, Inc., Warrington, PA) in PBS for 15 min on ice. The slides were then rinsed with PBS and transferred to 70% ethanol where they were stored at −20° C for 2-24 h. Slides with EdU-labeled cells after fixation in formaldehyde were stored in 1% BSA in PBS, at 4° C for up to 24h.
Immunocytochemical detection of phosphorylated histone H2AX (γH2AX), activated ATM, ATM/ATR substrate, and p53
After fixation the cells were washed twice in PBS and treated on slides with 0.1% Triton X-100 (Sigma) in PBS for 15 min, and then in a 1% (w/v) solution of bovine serum albumin (BSA; Sigma) in PBS for 30 min to suppress nonspecific antibody (Ab) binding. The cells were then incubated in a 100 μl volume of 1% BSA containing 1:200 dilution of phospho-specific (Ser139) γH2AX mAb (Biolegend, San Diego, CA) or 1:100 dilution of phospho-specific (Ser1981) ATM mAb (Cell Signaling, Danvers, MA), or 1:100 diluted phospho-(Ser/Thr) ATM/ATR substrate rabbit polyclonal antibody (Cell Signaling) or 1:100 diluted phospho-specific (Ser15) p53 rabbit polyclonal Ab (Cell Signaling) for 1.5 h at room temperature or overnight at 4 °C. Phospho-(Ser/Thr) ATM/ATR substrate antibody detects endogenous levels of proteins containing the ATM/ATR substrate motif. This antibody preferentially binds peptides and proteins that contain phospho-Ser/Thr preceded by Leu or similar hydrophobic amino acids at the −1 position and followed by Gln at the +1 position (40). The antibody does not cross-react with corresponding non-phosphorylated sequences or with other phospho-Ser/Thr-containing motifs. The secondary fluorochrome-tagged Abs were either Alexa Fluor 488 tagged Ab (Invitrogen/Molecular Probes, at 1;100 dilution) or Alexa Fluor 633 tagged Ab (Invitrogen/Molecular Probes, at 1:100 dilution). The Click-iT™ EdU Alexa Fluor 488 imaging kit (Invitrogen/Molecular Probes) was used to detect EdU incorporation. Prior to measurement by LSC, the cells were counterstained with 2.8 μg/ml 4,6-diamidino-2-phenylindole (DAPI; Sigma) in PBS for 15 min. Each experiment was performed with an IgG control in which cells were labeled only with the secondary antibody, Alexa Fluor 488 goat anti-mouse IgG (H+L) or Alexa Fluor 633 goat anti-rabbit IgG (H+L) without primary antibody incubation to estimate the extent of nonspecific binding of the secondary antibody to the cells. Other details of cell incubations with the primary and secondary Ab were presented before (23,44).
Measurement of cell fluorescence by LSC
Cellular green or far red IF representing the binding of the respective phospho-specific Abs and the blue emission of DAPI stained DNA was measured using an LSC (iCys; CompuCyte, Cambridge, MA) utilizing standard filter settings; fluorescence was excited with 488-nm argon, helium-neon (633 nm) and violet (405 nm) lasers (45). The intensities of maximal pixel and integrated fluorescence were measured and recorded for each cell. At least 3,000 cells were measured per sample. Gating analysis was carried out to obtain mean values (± SE) of immunofluorescence reporting the studied phosphoproteins for G1 (DNA Index; DI = 0.8-1.2), S (DI = 1.2 – 1.8) and G2M (DI = 1.8 – 2.2) cell populations in each experiment. The standard deviation was estimated based on Poisson distribution of cell populations. Each experiment was run at least in triplicate, some experiments were additionally repeated. The inter-sample variation in the triplicates did not exceed the value of three standard deviations of individual samples. Statistical correlation between the incorporation of EdU and intensity of γH2AX IF was carried out by plotting the mean intensity of γH2AX IF per each channel of EdU IF, and using regression analysis of the Microsoft Excel software to estimate the value of the correlation coefficient (R2). Other details are given in the figure legends.
Results
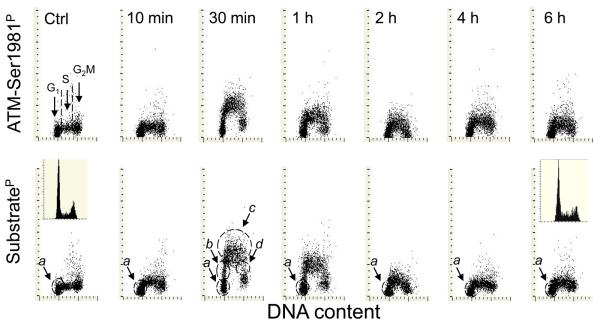
Activation of ATM and phosphorylation of the ATM/ATR substrate as detected by binding of phospho-specific Ab during 6 h following exposure to 50 J/m2 of UV light is presented in Fig. 1. The data show strikingly similar kinetics of phosphorylation and dephosphorylation of these proteins in response to UV irradiation. While a minor increase in expression of ATM-Ser1981P or ATM/ATR substrateP was already apparent 10 min after the exposure, the maximal level of phosphorylation of these proteins was seen after 30 min and was followed by a distinct decline in the level of phosphorylation 1 h after UV irradiation. The UV-induced rise in expression of ATM-Ser1981P and ATM/ATR substrateP was the most pronounced in S-phase cells, as reflected by the characteristic pattern resembling the inverted letter “U” on the bivariate distributions. Among the cells with a G1 DNA content, two subpopulations can be distinguished. One subpopulation (marked by the dashed-line outline) (a) shows no increase in expression of these phosphoproteins at any time point after exposure to UV. The second subpopulation (b) (ascending arm of the inverted letter “U”-like distribution) has an intermediate level of expression of ATM-Ser1981P or ATM/ATR substrateP, between the maximal (c) S phase and the minimal (a) G1, level of expression.
Fig. 1. Activation of ATM and phosphorylation of ATM/ATR protein substrates after irradiation of A549 cells with 50 J/m2 of UV-B light.
The cells growing on microscope slides in slide-chambers were exposed to 50 J/m2 of UV light then transferred into culture and the cultures terminated at different time points, as shown in the top panels. The data are presented as ATM-Ser1981P or ATM/ATR substrateP vs DNA content bivariate distributions. The insets in the bottom panels show the DNA content frequency histograms of cells from the culture at 0 time and 6 h after irradiation. See text for the details defining subpopulations of cells (a, b, c, d) marked by the dashed-line outlines. .
We have previously shown (46) that following a 30 min – 1 h pulse labeling with BrdU, the cells displaying such characteristics as the b cells in Fig. 1 were stochastically entering the S phase during the duration of exposure to the precursor. Conversely, the cells marked as d in Fig 1, display the characteristics of cells exiting S-phase and entering G2 during duration of the pulse-exposure (46). The cells within the c outline have an S-phase DNA content and most likely were progressing through- or blocked in- S phase during the time interval between UV irradiation and culture termination. Thus, the data shown in Fig 1 indicate that the cells with near-G1 DNA content and expressing phosphorylated ATM or the ATM/ATR substrate (cells in b) most likely were not genuine G1 but in transition between G1 and S i.e. were initiating DNA replication during the 30 min – 1 h time interval after UV irradiation. Likewise, the d subpopulation represents the cohort of cells that after exposure to UV while in S were in fact terminating DNA replication and entering G2 during the subsequent 30 min - 1 h of culturing.
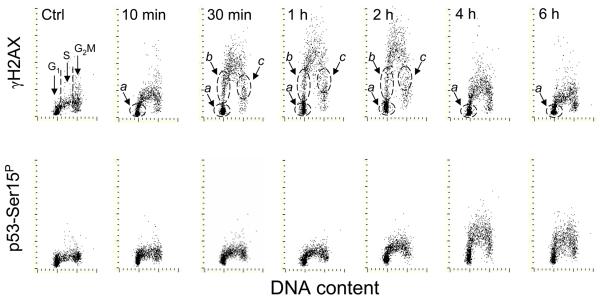
Fig. 2 illustrates the kinetics of phosphorylation of H2AX on Ser139 and p53 on Ser15 after irradiation with UV. The increase in expression of γH2AX was already seen after 10 min but unlike in the case of ATM activation, the maximal level of expression of γH2AX was 2 h after UV irradiation. The decline in expression of γH2AX was apparent after 4 h and was more pronounced 6 h after the exposure. Phosphorylation of p53 occurred with even an even greater delay, reaching its maximum at 4 h. As with ATM activation and ATM/ATR substrate phosphorylation (Fig. 1), the phosphorylation of H2AX and p53 was essentially limited to S-phase cells. Likewise, subpopulations of cells residing in G1 (a) entering S (b), progressing through- or blocked in- S (c) or exiting S (d) during the time interval between the exposure to UV and culture termination could be identified as shown in Fig. 2 (top panels). Since progression through S and consequently from S to G2 was slowed down upon exposure to UV, the cells with increased expression of γH2AX or ATM-Ser1981P during S could not effectively exit S during the short time of the post-exposure; hence the G2M cells do not show elevated levels of these phosphoproteins.
Fig. 2. Phosphorylation of H2AX on Ser139 and p53 on Ser15 after exposure of A549 cells to 50 J/m2 of UV-B light.
The cells growing on slides were irradiated with UV light, as described in the legend to Fig 1, and then were cultured for different time intervals as marked. As in Fig. 1, subpopulations a, b, c and d were identified.
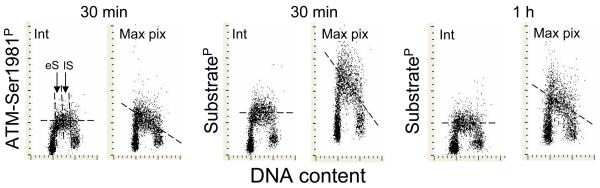
During analysis of the recorded data we observed that the pattern of intensity of IF of ATM-Ser1981P, ATM/ATR substrateP or γH2AX was strikingly different when the intensity was expressed either as the integral of IF over the nucleus or as maximal pixel (Fig. 3). It was quite evident that the intensity of maximal pixel of IF of the cells in early-S phase was higher than that of the cells in late-S phase. At the same time the integrated intensity of IF was similar across S-phase. This observation reveals that while there was no change in the total expression of these phosphoproteins during progression of cells through S phase their local density within the nuclear foci, reflecting higher compactness of foci (45,47), was increased in the cells progressing through the early portion of the S phase compared with the late-S phase cells.
Fig. 3. Different patterns of ATM-Ser1981P and ATM/ATR substrateP expression across the S-phase cells depending upon whether IF is expressed as intensity of fluorescence integrated over the nucleus (Int) or as maximal pixel of IF in the nucleus (Max pix).
The dashed horizontal or skewed lines are drawn to show the modal values of IF of S-phase cells. Note the increased intensity of IF when presented as maximal pixel in early S cells (eS) as compared with the late S (lS) cells, and no differences between eS and lS in the intensity of the integrated IF.
Comparison of the kinetics of ATM, H2AX, ATM/ATR substrate and p53 phosphorylation after irradiation of cells with UV is presented in Fig. 4. The rates of phosphorylation and dephosphorylation of ATM and ATM/ATR substrate were strikingly similar, with the peaks approaching 3.5-fold increase above the level of their constitutive level of phosphorylation (44, 48,49) at 30 min after irradiation and a steep decline almost back to the constitutive level, by 2 h. Phosphorylation of H2AX increased rapidly during the initial 30 min continued and continued to rise reaching maximum of 4.3 fold above its constitutive level of phosphorylation. Compared with ATM, ATM/ATR substrate or H2AX, the rate of phosphorylation of p53 on Ser15 was distinctly slower and the maximal level of phosphorylation, seen at 4 h, was only 2.5 –fold above its constitutive level of expression.
Fig. 4. Kinetics of ATM, ATR/ATM substrate, H2AX and p53 phosphorylation after exposure of A549 cells to 50 J/m2 of UV-B.
The data show mean values of IF of the detected phosphorylated proteins of the S-phase cells (gated between DI = 1.2 and DI = 1.8) measured at different time intervals after irradiation with UV.
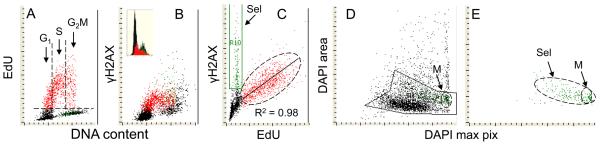
The data shown in Figs 1-3 demonstrated that in response to UV irradiation the induction of ATM activation and phosphorylation of ATM/ATR substrate, H2AX and p53 was essentially limited to cells in S phase of the cell cycle, including the cells entering S- and exiting S-phase during the time interval between UV exposure and culture termination. This data is consistent with the notion advanced by us before (23) that the DNA damage response, at least as reflected by H2AX phosphorylation, is induced by formation of secondary DNA lesions, likely DSBs, as a result of collapse of DNA replication forks upon collision with the primary UV-induced lesions (50,51). To provide more detailed evidence to this account, experiments were designed to correlate the rate of DNA replication with H2AX phosphorylation that can be assessed by multivariate analysis within the same cells (Fig. 5). The data shown in this figure reveal that essentially all cells incorporating EdU expressed γH2AX. Conversely, the cells with a G1 DNA content that did not incorporate EdU did not show any significant increase in expression of γH2AX. Most importantly, within a population of EdU incorporating cells, a very strong statistical correlation between the extent of EdU incorporation and the expression of γH2AX was evident, with the correlation coefficient as shown in this experiment R2 = 0.98 (Fig. 5). In repeated experiments (n = 4) the correlation index R2 varied between 0.98 and 0.94, with the mean 0.96.
Fig. 5. Correlation between DNA replication and the UV-induced H2AX phosphorylation in A549 cells.
Exponentially growing cells were pulse-labeled with EdU for 60 min then were exposed to UV light and subsequently incubated for 30 min before culture termination. The incorporated EdU was detected by the “click-iT™” methodology using Alexa Fluor 488 azide (green fluorescence), γH2AX was detected using Alexa Fluor 633 secondary Ab (far red fluorescence), DNA was counterstained with DAPI. Using paint-a-gate analysis, the EdU incorporating cells (above the skewed dashed line, panel A) were colored red. As shown in panel B the red colored cells demonstrate expression of γH2AX. Panel C shows the bivariate analysis of γH2AX expression vs EdU incorporation. A strong correlation (R2=0.98) between the extent of H2AX phosphorylation (intensity of γH2AX IF) and the level of EdU incorporation is seen among the EdU incorporating cells outlined with the oval dashed line (the regression plot is shown by the solid line). The subpopulation of cells with increased expression of γH2AX but not incorporating EdU (Sel) was secondarily gated and electronically painted green. These cells were characterized by high intensity of maximal pixel of DAPI fluorescence revealing their highly condensed chromatin. Imaging of these cells by iCys (“Compu-sort”) revealed that they were pre-mitotic or mitotic (M) cells (panels D,E).
A subpopulation of cells expressing increased level of γH2AX but not incorporating EdU (Sel), with a G2M DNA content and highly condensed chromatin, was apparent on the bivariate frequency distributions (Fig. 5; C, D,E). As shown before 44,52), these are the features of pre-mitotic and mitotic cells. The relocation of these cells and imaging by iCys (45) indeed confirmed that they were mitotic cells, including cells in prophase and other phases of mitosis. This observation was consistent with our earlier findings showing that the pre-mitotic and mitotic cells do express elevated level of constitutive H2AX phosphorylation (53) and ATM activation (44,48) without induction of DNA damage by exogenous factors.
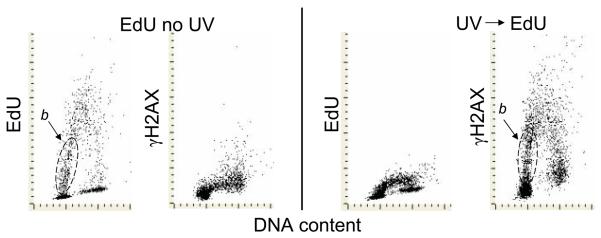
We have also tested the effect of cells exposure to UV on incorporation of EdU in parallel with the induction of γH2AX. Consistent with the earlier reports (24,543) we observed that incorporation of EdU was severely suppressed by pre-exposure to UV (Fig. 6). The subpopulation of cells stochastically entering S phase (initiating DNA replication) during 1 h of incubation with EdU is marked (b) in this figure. Their progression through the early portion of S-phase is reflected by somewhat skewed position on the EdU vs DNA content coordinates due to the fact that the cells incorporating more EdU (progressing for longer period of time through S) have higher DNA content. Expression of γH2AX induced by UV very much resembles that of EdU incorporation, with the presence of subpopulation b. The position of this subpopulation, however, is more vertical due to the fact that because of suppression of DNA replication by UV, the advancement of cells through S phase was impeded. Because these cells (b) have DNA content very close to that of G1 cells, the gating strategy based on DNA content alone can erroneously classify them as the genuine G1 cells.
Fig. 6. Suppression of EdU incorporation by exposure of cells to UV light.
The two left panels present control cells untreated with UV and incubated with EdU for 60 min. The two right panels present the cells that were treated with 50 J/m2 of UV, then transferred to culture and incubated with EdU for 1 h. The subpopulation of cells entering S phase (initiating DNA replication) during incubation with EdU is outlined (b).
Discussion
The present data provide for the first time the assessment of the kinetics of DNA damage response in terms of phosphorylation and dephosphorylation of ATM, ATM/ATR substrate, H2AX and p53Ser15 in individual cells in relation to the cell cycle phase, upon exposure to UV light. As can be seen, the activation of ATM was rapid and concurrent with phosphorylation of ATM/ATR substrate and with phosphorylation of H2AX (Fig. 4). ATM/ATR substrates contain closely spaced SQ/TQ motifs in SQ/TQ domains (SCDs) and their phosphorylation on Ser/Thr is considered to be the hallmark of DNA damage response (40). While phosphorylation of H2AX after induction of DNA damage by UV is primarily mediated by ATR (32-36), activation of ATM and DNA PKcs can be redundant, occur concurrently and contribute to phosphorylation of H2AX and other downstream protein substrates (37-40). It is not surprising therefore to see concurrent and remarkably synchronous phosphorylation of ATM and the ATM/ATR substrate. Of interest is the observation that dephosphorylation of ATM and ATM/ATR substrates was rapid, essentially completed 1 h after the irradiation. Although delayed in comparison with ATM and ATM/ATR substrate, dephosphorylation of H2AX, seen after 4 h, also occurred a relatively short time after irradiation (Fig. 5). The decline in expression of γH2AX induced by UV reported by others (32,55) likely reflecting its dephosphorylation, was seen to occur after longer time intervals compared with the present data. The apparent contradiction may be due to different cell types, higher energy of the UV light photons (UV-C) and different UV doses used in these studies (32,55).
Although the relatively rapid dephosphorylation of ATM may suggest that the primary UV lesions were responsible for its activation, in light of the evidence that the activation was selective to DNA replicating cells (Fig. 1) and was attenuated by aphidicolin (23) in all probability it was triggered by stalling replication forks. It was reported that a DNA lesion located on the lagging strand completely blocked the synthesis of the Okazaki fragment extending toward the lesion site while it did not affect the progression of the replication fork or leading-strand DNA synthesis (50). However, a DNA lesion on the leading strand was shown to stall the replication fork concurrently with inhibiting leading-strand synthesis. Thus, a ssDNA lesion affects DNA replication differently depending on which strand, leading or lagging, is located (50).
The rapid dephosphorylation of H2AX may be due to the fact that A549 cells bear wild-type p53 which was shown to be associated with decreased level of constitutive (endogenous) H2AX phosphorylation (56). This however may not be universally applicable to all cell types (57).
Phosphorylation of p53 on Ser15 was a relatively late event, clearly trailing ATM activation and the phosphorylation of ATM/ATR substrate and H2AX (Fig. 5). In analogy, we observed that p53 phosphorylation induced in A549 cells by exposure to tobacco smoke (58), after treatment with DNA topoisomerase inhibitors (59) or with supravital DNA probes Hoechst 33342 and DRAQ5 (60) also occurred subsequent to ATM and Chk2 activation and H2AX phosphorylation. Because DNA replication, as detected by reduced EdU incorporation (Fig. 6), was suppressed after UV irradiation well prior to induction of p53Ser15P the latter event can be excluded as the one that mediates inhibition of DNA replication.
It should be stressed that the induction of apoptosis and apoptosis-associated fragmentation of DNA (33,51,61) could not provide a trigger for phosphorylation of ATM, ATM/ATR substrate, H2AX or p53 in UV-irradiated A549 cells because there was no evidence of caspase-3 activation or TUNEL-positivity (62) up to 6 h after cells exposure to UV (data not shown). The apoptotic response of A549 cells which occurs late is unlike that of HL-60 cells which undergo apoptosis 3 – h after UV irradiation (23). Furthermore, because the intensity of expression of γH2AX in apoptotic cells is an order of magnitude greater compared with its induction by genotoxic agents (33,61) apoptotic cells can easily be identified and excluded from analysis.
The cells that resided in G1 phase of the cell cycle throughout the experiment, namely the cells with a G1 DNA content that did not incorporate EdU (Figs. 1 and 2, subpopulation a) did not respond at all to UV irradiation either by ATM activation, ATM/ATR substrate-, H2AX- or by p53- phosphorylation. This is in contradiction to several reports describing induction of H2AX phosphorylation by UV in G1 cells (23,30-32). The contradiction, however, is more apparent than real. In these studies, including also our prior report (23), the G1 cell population was identified by gating based on DNA content (e.g. DI = 1.0 ± 0.2). Such “G1” population included cells that at the time of irradiation with UV and subsequent incubation initiated DNA replication (entered S phase). Because the rate of DNA replication in UV irradiated cells was suppressed they did not progress far through S phase (increased their DNA content) to be identified as S-phase cells based on DNA content alone. These cells are marked in Figs. 1 and 2 as subpopulation b. Thus, among the cells gated as “G1“ were the cells initiating DNA replication as well as the cells progressing at a greatly reduced rate through the very early portion of S phase.
The strong correlation between induction of γH2AX and the extent of DNA replication as reflected by EdU incorporation (Fig. 5; R2 = 0.98) offers a convincing argument that the UV- induced H2AX phosphorylation as presently assessed by the integral of γH2AX IF over the cell nucleus is the consequence of interaction of DNA replication forks with the primary lesions caused by UV. This argument is also buttressed by the evidence that inhibition of DNA replication by the DNA polymerase inhibitor aphidicolin prevented H2AX phosphorylation triggered by UV-irradiation (23). Explanation of these earlier findings (23), however, was complicated by the induction of replicative stress by aphidicolin (63,64). The lack of response of non-proliferating peripheral blood lymphocytes or mouse fibroblasts to UV in terms of activation of stress-activated protein kinases (65) provides additional evidence that DDR or stress response to UV irradiation is a consequence of the DNA replication blockade by the UV-induced primary lesions and does not occur in the cells that do not replicate DNA. Likewise, we were also unable to detect any increase in expression of γH2AX or ATM activation in non-proliferating human peripheral blood lymphocytes after exposure to 50 J/m2 of UV (data not shown). Most likely, as is the case with DNA damage caused by DNA topoisomerase inhibitors (66), the observed UV-induced DDR in DNA replicating cells is triggered by collapse of replication forks upon collision with the primary lesions, thymidine dimers and 6-4 (T-C) photoproducts, induced by UV.
Different pattern of ATM activation, ATM/ATR substrate phosphorylation (Fig. 3) and H2AX phosphorylation (not shown) in early-S versus late-S phase cells was observed depending whether expression of these proteins was assessed as an integral or maximal pixel of IF. High maximal pixel intensity is a reporter of high spatial density of the fluorochrome while a decrease in intensity of maximal pixel indicates a more diffuse spatial localization of the fluorochrome (45,47,52). Considering that the integral of IF was relatively constant across the S-phase cells our data indicate that the foci representing location of activated ATM, phosphorylated ATM/ATR substrate or γH2AX in chromatin of UV-irradiated cells were more compact in the early- as compared with the late-S phase cells. It was recently reported that the compactness of foci (densities of γH2AX) is greater in close proximity to DSBs and less pronounced at some distance (67). Also, cell cycle phase associated differences in the composition of different DDR-associated proteins including Rad9 within nuclear foci induced by ionizing radiation and UV were observed (68). The density of foci may also be related to whether DDR is associated with the initiation of replication or with the elongation step. Replication initiation was reported to be more sensitive to UV than elongation (24,46,69). There are significant differences in the molecular machinery of initiation of DNA replication compared to that of the elongation process (70) as well as different spatial localization of these two DNA replication steps within the nucleus (71).
Since repair of DSBs is error-prone (72) their carcinogenic potential is well recognized. The carcinogenic consequences of exposure to UV light (73), including UV-phototherapy (74), are most likely mediated by the induction of DSBs. Thus, the ability to monitor the UV-induced DDR by flow- or image assisted- cytometry provides a useful means to assess these consequences and also to study mechanisms leading to cancer development.
Acknowledgments
Grant sponsor: NCI; Grant number CA 28 704
References
- 1.Sinha RP, Häder D-P. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 2.Sancar A, Lindsey-Bolz LA, Ŭnsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 3.Dunkern TR, Kaina B. Cell proliferation and DNA breaks are involved in ultraviolet light-induced apoptosis in nucleotide excision repair-deficient hamster cells. Mol Biol Cell. 2002;13:348–361. doi: 10.1091/mbc.01-05-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 5.Goodsell DS. The molecular perspective: Ultraviolet light and pyrimidine dimers. The Oncologist. 2001;6:289–299. doi: 10.1634/theoncologist.6-3-298. [DOI] [PubMed] [Google Scholar]
- 6.Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, Yang V, Beausoleil SA, Gygi SP, Livingstone M, Zhang H, Polakiewicz RD, Comb MJ. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci USA. 2007;104:19855–19860. doi: 10.1073/pnas.0707579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kastan MB. DNA damage responses: Mechanisms and roles in human disease. 2007 G.H.A. Cloves Memorial Award Lecture. Mol Cancer Res. 2008;6:517–524. doi: 10.1158/1541-7786.MCR-08-0020. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa R, Kastan MB. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb Symp Quant Biol. 2005;70:99–109. doi: 10.1101/sqb.2005.70.002. [DOI] [PubMed] [Google Scholar]
- 9.Chastain PD, 2nd, Heffernan TP, Nevis KR, Lin L, Kaufmann WK, Kaufman DG, Cordeiro-Stone M. Checkpoint regulation of replication dynamics in UV-irradiated human cells. Cell Cycle. 2006;5:2160–2167. doi: 10.4161/cc.5.18.3236. [DOI] [PubMed] [Google Scholar]
- 10.Stokes MP, Comb MJ. A wide-ranging cellular response to UV damage of DNA. Cell Cycle. 2008;7:2097–2099. doi: 10.4161/cc.7.14.6326. [DOI] [PubMed] [Google Scholar]
- 11.Murga M, Jaco I, Soria R, Martinez-Pastor B, Cuadrado M, Yang S-M, Blasco MA, Skoultchi AI, Fernandez-Capetillo O. Global chromatin compaction limits the strength of the DNA damage response. J Cell Biol. 2007;178:1101–1108. doi: 10.1083/jcb.200704140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halicka HD, Zhao H, Podhorecka M, Traganos F, Darzynkiewicz Z. Cytometric detection of chromatin relaxation, an early reporter of DNA damage response. Cell Cycle. 2009;8:2233–2237. doi: 10.4161/cc.8.14.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertlitz G, Bustin M. Nucleosome binding proteins potentiate ATM activation and DNA damage response by modifying chromatin. Cell Cycle. 2009;8:1641–1642. doi: 10.4161/cc.8.11.8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Wang Y. High mobility group proteins and their post-transcriptional modifications. Biochim Biophys Acta. 2008;1794:1159–1166. doi: 10.1016/j.bbapap.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs JA, Cote J. Dynamics of chromatin during the repair of DNA double-strand breaks. Cell Cycle. 2005;4:1373–1376. doi: 10.4161/cc.4.10.2108. [DOI] [PubMed] [Google Scholar]
- 16.Paull TT, Lee JH. The Mre11/Rad50/Nbs1 complex and its role as a DNA-double strand break sensor for ATM. Cell Cycle. 2005;4:737–740. doi: 10.4161/cc.4.6.1715. [DOI] [PubMed] [Google Scholar]
- 17.Shiloh Y. The ATM-mediated DNA-damage response taking shape. Trends Biochem Sci. 2006;31:402–410. doi: 10.1016/j.tibs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell. 2004;118:9–17. doi: 10.1016/j.cell.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Ahn J, Urist M, Prives C. The Chk2 protein kinase. DNA Repair. 2004;3:1039–1047. doi: 10.1016/j.dnarep.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Thoma F. Light, dark in chromatin repair: repair of UV-induced DNA lesions by photolyase and nucleotide excision repair. EMBO J. 1999;18:6585–6598. doi: 10.1093/emboj/18.23.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 23.Halicka HD, Huang X, Traganos F, King MA, Dai W, Darzynkiewicz Z. Histone H2AX phosphorylation after cell irradiation with UV-B: Relationship to cell cycle phase and induction of apoptosis. Cell Cycle. 2005;4:339–345. [PubMed] [Google Scholar]
- 24.Cleaver JE, Laposa RR, Limoli CL. DNA replication in face of (in)surmountable odds. Cell Cycle. 2003;2:310–315. [PubMed] [Google Scholar]
- 25.Squires S, Coates JA, Goldberg M, Toji LH, Jackson SP, Clarke DJ, Johnson RT. p53 prevents the accumulation of double-strand DNA breaks at stalled-replication forks induced by UV in human cells. Cell Cycle. 2004;3:11543–11557. doi: 10.4161/cc.3.12.1272. [DOI] [PubMed] [Google Scholar]
- 26.Zou I, Cortez D, Elledge SJ. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes Dev. 2002;16:198–208. doi: 10.1101/gad.950302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupardus PJ, Byun T, Yee MC, Kekmat-Nejad M, Cimprich KA. A requirement for replication in activation of the ATR-dependent DNA damage checkpoints. Genes Dev. 2002;16:2327–2332. doi: 10.1101/gad.1013502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, Yang V, Beausoleil SA, Gygi SP, Livingstone M, Zhang H, Polakiewicz RD, Comb MJ. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci USA. 2007;104:19855–19860. doi: 10.1073/pnas.0707579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adimoolan S, Ford JM. p53 and regulation of DNA damage recognition during nucleotide excision repair. DNA repair. 2003;18:947–954. doi: 10.1016/s1568-7864(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 30.Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE. H2AX phosphorylation within the G1 phase after UV irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci USA. 2006;103:9891–9896. doi: 10.1073/pnas.0603779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto M, Yaginuma K, Igarashi A, Imura M, Hasegawa M, Iwabuchi K, Date T, Mori T, Ishizaki K, Yamashita K, Inobe M, Matsunaga T. Perturbed gap-filling synthesis in nucleotide excision repair causes histone H2AX phosphorylation in human quiescent cells. J Cell Sci. 2007;120:1104–1112. doi: 10.1242/jcs.03391. [DOI] [PubMed] [Google Scholar]
- 32.Hanasoge S, Ljungman M. H2AX phosphorylation after UV irradiation is triggered by DNA repair intermediates and is mediated by the ATR kinase. Carcinogenesis. 2007;28:2298–2304. doi: 10.1093/carcin/bgm157. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Huang X, Halicka HD, Zhao H, Traganos F, Albino AP, Dai W, Darzynkiewicz Z. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry A. 2007;71A:648–661. doi: 10.1002/cyto.a.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yajima H, Lee KJ, Chen BP. ATR-dependent phosphorylation of DNA-dependent protein kinase catalytic subunit in response to UV-induced replication stress. Mol Cell Biol. 2006;26:7520–7528. doi: 10.1128/MCB.00048-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward IM, Minn K, Chen J. UV-induced ataxia-telangiectasia-mutated and Rad3-related (ATR) activation requires replication stress. J Biol Chem. 2004;279:9677–9680. doi: 10.1074/jbc.C300554200. [DOI] [PubMed] [Google Scholar]
- 36.Callegari AJ, Kelly TJ. Shedding light on the DNA damage checkpoint. Cell Cycle. 2007;6:660–666. doi: 10.4161/cc.6.6.3984. [DOI] [PubMed] [Google Scholar]
- 37.Yajima H, Lee KJ, Zhang S, Kobayashi J, Chen BP. DNA double-strand break formation upon UV-induced replication stress activates ATM and DNA-PKcs kinases. J Mol Biol. 2009;385:800–810. doi: 10.1016/j.jmb.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auclair Y, Rouget R, Drobetsky EA. ATR kinase as master regulator of nucleotide excision repair during S phase of the cell cycle. Cell Cycle. 2009;8:1865–1871. doi: 10.4161/cc.8.12.8800. [DOI] [PubMed] [Google Scholar]
- 39.Myers JS, Cortez D. Rapid activation of ATR by ionizing radiation requires ATM and MRE11. J Biol Chem. 2006;282:9346–9350. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Traven A, Heierhorst J. SQ/TQ cluster domains: concentrated ATM/ATR kinase phosphorylation site regions in DNA-damage-response proteins. Bioessays. 2005;27:397–407. doi: 10.1002/bies.20204. [DOI] [PubMed] [Google Scholar]
- 41.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolbeare F, Selden JR. Immunocytochemical quantitation of bromodeoxyuridine; application for cell-cycle kinetics. Meth Cell Biol. 1994;41:297–316. [PubMed] [Google Scholar]
- 43.Rostovtsev W, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Zhao H, Tanaka T, Halicka HD, Traganos F, Zarebski M, Dobrucki J, Darzynkiewicz Z. Cytometric assessment of DNA damage by exogenous and endogenous oxidants reports the aging-related processes. Cytometry A. 2007;71A:905–914. doi: 10.1002/cyto.a.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pozarowski P, Holden E, Darzynkiewicz Z. Laser scanning cytometry: Principles and applications. Meth Molec Biol. 2006;319:165–192. doi: 10.1007/978-1-59259-993-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juan G, Li X, Darzynkiewicz Z. Correlation between DNA replication and expression of cyclins A and B1 in individual MOLT-4 cells. Cancer Res. 1997;57:803–807. [PubMed] [Google Scholar]
- 47.Bedner E, Li X, Kunicki J, Darzynkiewicz Z. Translocation of Bax to mitochondria during apoptosis measured by laser scanning cytometry. Cytometry. 2000;41:83–88. [PubMed] [Google Scholar]
- 48.Tanaka T, Halicka HD, Huang X, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle. 2006;5:1940–1945. doi: 10.4161/cc.5.17.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka T, Kurose A, Huang X, Traganos F, Dai W, Darzynkiewicz Z. Extent of constitutive histone H2AX phosphorylation on Ser-139 varies in cells with different TP53 status. Cell Prolif. 2006;39:313–323. doi: 10.1111/j.1365-2184.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higuchi K, Katayama T, Iwai S, Hidaka M, Horiuchi T, Maki H. Fate of DNA replication fork encountering a single DNA lesion during oriC plasmid DNA replication in vitro. Genes Cells. 2003;8:437–449. doi: 10.1046/j.1365-2443.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 51.Battista LF, Kaina B, Meneghini R, Menck CF. How DNA lesions are turned into powerful killing structures: insights from UV-induced apoptosis. Mutat Res. 2009;681:197–208. doi: 10.1016/j.mrrev.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Luther E, Kamentsky LA. Resolution of mitotic cells using laser scanning cytometry. Cytometry. 1996;23:272–278. doi: 10.1002/(SICI)1097-0320(19960401)23:4<272::AID-CYTO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 53.Huang X, Tanaka T, Kurose A, Traganos F, Darzynkiewicz Z. Constitutive histone H2AX phosphorylation on Ser-139 in cells untreated by genotoxic agents is cell-cycle phase specific and attenuated by scavenging reactive oxygen species. Int J Oncol. 2006;29:495–501. [PubMed] [Google Scholar]
- 54.Kaufmann WK, Cleaver JE. Mechanisms of inhibition of DNA replication by ultraviolet radiation in normal human and xeroderma pigmentosum fibroblasts. J Mol Biol. 1981;149:171–187. doi: 10.1016/0022-2836(81)90297-7. [DOI] [PubMed] [Google Scholar]
- 55.Staszewski O, Nikolova T, Kaina B. Kinetics of gamma-H2AX focus formation upon treatment of cells with UV light and alkylating agents. Environ Mol Mutagen. 2008;49:734–740. doi: 10.1002/em.20430. [DOI] [PubMed] [Google Scholar]
- 56.Yu T, MacPhail SH, Banath JP, Klokov D, Olive PL. Endogenous expression of phosphorylated histone H2AX in tumors in relation to DNA double-strand breaks and genomic instability. DNA repair. 2006;5:935–946. doi: 10.1016/j.dnarep.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T, Kurose A, Huang X, Traganos F, Dai W, Darzynkiewicz Z. Extent of constitutive histone H2AX phosphorylation on Ser-139 varies in cells with different TP53 status. Cell Prolif. 2006;39:313–323. doi: 10.1111/j.1365-2184.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao H, Albino AP, Jorgensen E, Traganos F, Darzynkiewicz Z. DNA damage response induced by tobacco smoke in normal human bronchial epithelial and A549 pulmonary adenocarcinoma cells assessed by laser scanning cytometry. Cytometry A. 2009;75A:840–847. doi: 10.1002/cyto.a.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao H, Traganos F, Darzynkiewicz Z. Phosphorylation of p53 on Ser15 during cell cycle caused by Topo I and Topo II inhibitors in relation to ATM and Chk2 activation. Cell Cycle. 2008;7:3048–3055. doi: 10.4161/cc.7.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao H, Traganos F, Dobrucki J, Wlodkowic D, Darzynkiewicz Z. Induction of DNA damage response by the supravital probes of nucleic acids. Cytometry A. 2009;75A:510–519. doi: 10.1002/cyto.a.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurose A, Tanaka T, Huang X, Halicka HD, Traganos F, Dai W, Darzynkiewicz Z. Assessment of ATM phosphorylation on Ser-1981 induced by DNA topoisomerase I and II inhibitors in relation to Ser-139-histone H2AX phosphorylation, cell cycle phase and apoptosis. Cytometry A. 2005;68A:1–9. doi: 10.1002/cyto.a.20186. [DOI] [PubMed] [Google Scholar]
- 62.Darzynkiewicz Z, Galkowski D, Zhao H. Analysis of apoptosis by cytometry using TUNEL assay. Methods. 2008;44:250–254. doi: 10.1016/j.ymeth.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurose A, Tanaka T, Huang X, Traganos F, Darzynkiewicz Z. Synchronization in the cell cycle by inhibitors of DNA replication induces histone H2AX phosphorylation, an indication of DNA damage. Cell Prolif. 2006;39:231–240. doi: 10.1111/j.1365-2184.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurose A, Tanaka T, Huang X, Traganos F, Dai W, Darzynkiewicz Z. Effects of hydroxyurea and aphidicolin on phosphorylation of ATM on Ser 1981 and histone H2AX on Ser 139 in relation to cell cycle phase and induction of apoptosis. Cytometry A. 2006;69A:212–221. doi: 10.1002/cyto.a.20241. [DOI] [PubMed] [Google Scholar]
- 65.Damrot J, Helbig L, Roos WP, Barrantes SQ, Kaina B, Fritz G. DNA replication arrest in response to genotoxic stress provokes early activation of stress-activated protein kinases (SAPK/JNK) J Mol Biol. 2009;385:1409–1421. doi: 10.1016/j.jmb.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Hsiang YH, Lihou MG, Liu LF. Arrest of replication forks by drug stabilized topoisomeraseI-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res. 1989;49:5077–5082. [PubMed] [Google Scholar]
- 67.Savic V, Sanborn KB, Orange JS, Bassing CH. Chipping away at gamma-H2AX foci. Cell Cycle. 2009;8:3285–3290. doi: 10.4161/cc.8.20.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warmerdam DO, Freire R, Kanaar R, Sits VA. Cell Cycle-dependent processing of DNA lesions controls localization of Rad9 to sites of genotoxic stress. Cell Cycle. 2009;8:1165–1174. doi: 10.4161/cc.8.11.8721. [DOI] [PubMed] [Google Scholar]
- 69.Drissi R, Lee SH. In vitro analysis of UV-damage-induced inhibition of replication. Biochem J. 1998;330:181–187. doi: 10.1042/bj3300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutta A, Pell SP. Initiation of DNA replication in eukaryotic cells. Annu Rev Cell Dev Biol. 1997;13:293–332. doi: 10.1146/annurev.cellbio.13.1.293. [DOI] [PubMed] [Google Scholar]
- 71.Kennedy BK, Barbie DA, Classon M, Dyson N, Harlow E. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 2000;22:2855–2868. doi: 10.1101/gad.842600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rünger TM. How different wavelengths of the ultraviolet spectrum contribute to skin carcinogenesis: the role of cellular damage responses. J Invest Dermatol. 2007;127:2103–2105. doi: 10.1038/sj.jid.5700988. [DOI] [PubMed] [Google Scholar]
- 74.Meduri NB, Vandergriff T, Rasmussen H, Jacobe H. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. 2007;23:106–112. doi: 10.1111/j.1600-0781.2007.00291.x. [DOI] [PubMed] [Google Scholar]