Abstract
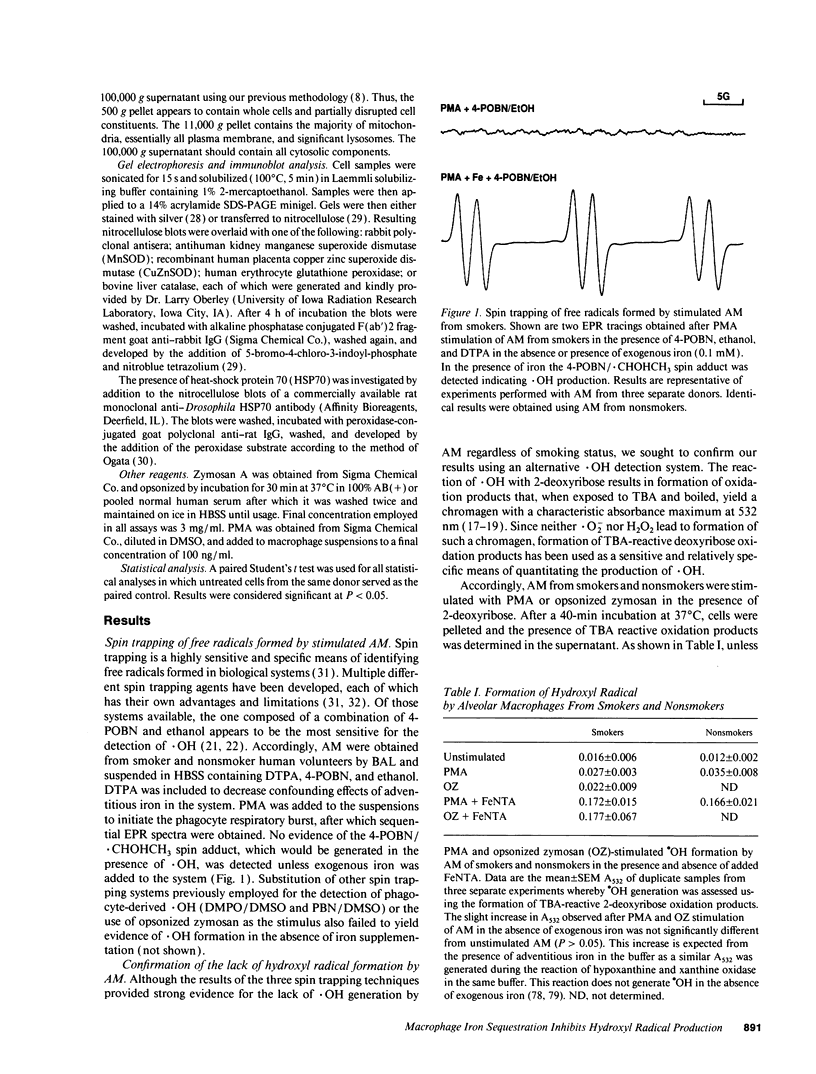
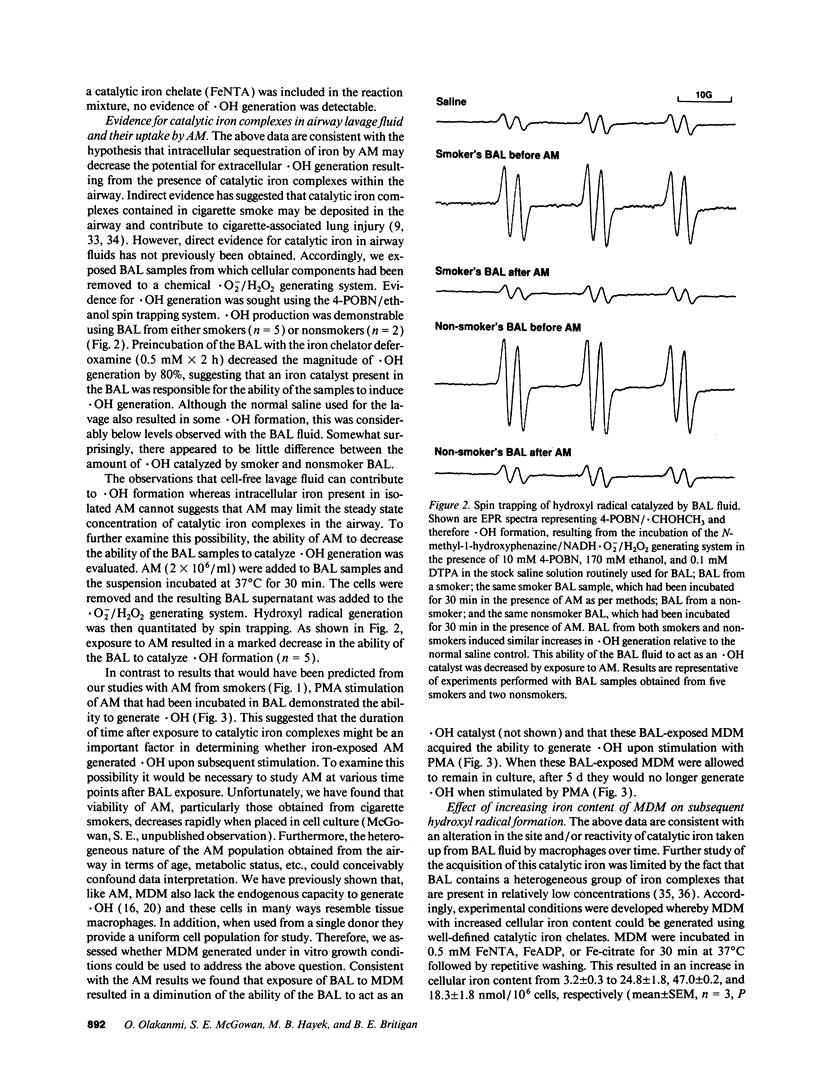
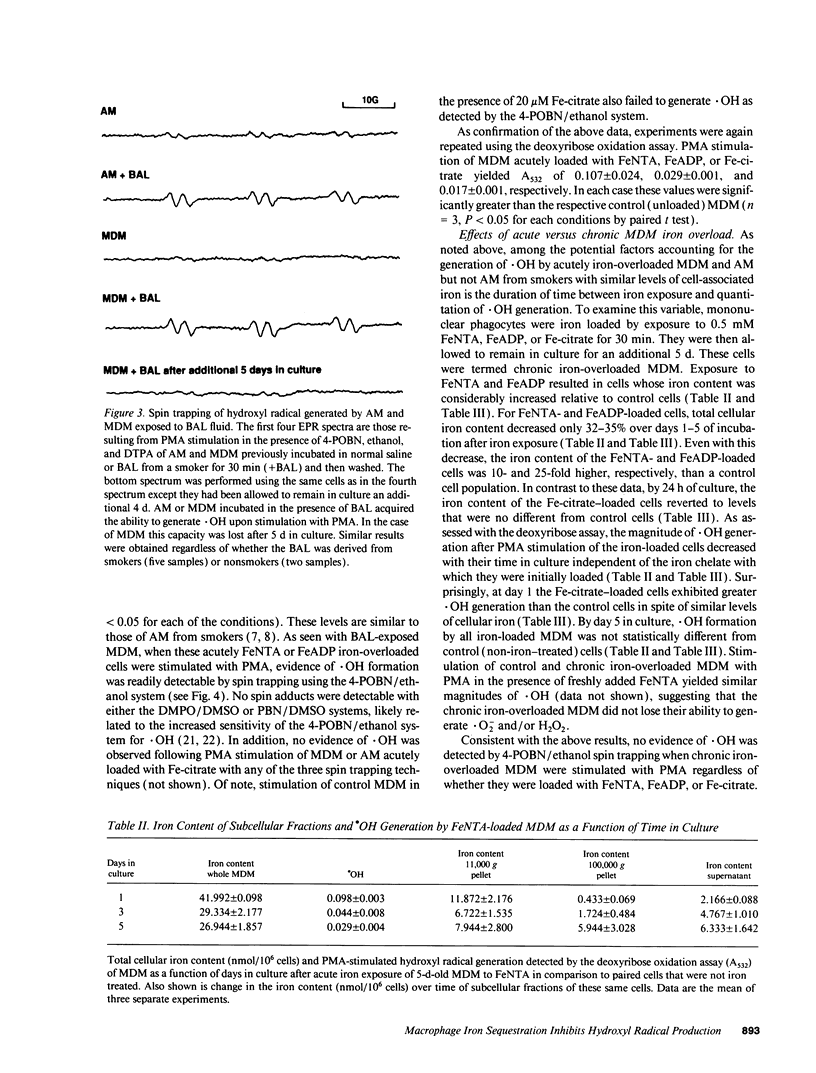
Alveolar macrophages (AM) from smokers contain a much higher quantity of intracellular iron than AM from nonsmokers. Since some forms of iron will catalyze the formation of hydroxyl radical (.OH) from superoxide and hydrogen peroxide, the ability of AM derived from smokers and nonsmokers to generate .OH was assessed. No detectable .OH was produced by AM from either source, suggesting that iron sequestration by AM may limit the potential for .OH-mediated lung injury. Consistent with this hypothesis, the ability of bronchoalveolar lavage fluid (BAL) from smokers and nonsmokers to act as an .OH catalyst decreased after exposure to AM. We found that, like AM, human monocyte-derived macrophages (MDM) have the ability to acquire large quantities of iron from small low molecular weight iron chelates as well as decrease the ability of BAL to act as a .OH catalyst. When MDM or AM were exposed to the iron chelates or BAL they were then able to generate .OH after phorbol myristate acetate stimulation. However, when acutely iron-loaded or BAL-exposed MDM were placed in culture, their ability to produce .OH decreased with time to the level of non-iron-exposed controls. This process correlated with iron translocation from the plasma membrane to the cytosol as well as a 3-9-fold increase in cellular ferritin. No increase in antioxidant enzyme levels or induction of the heat shock response was observed. Iron sequestration by macrophages may protect nearby cells from exposure to potentially cytotoxic iron-catalyzed oxidants such as .OH.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. E., King T. E., Jr, Campbell P. A. Role of transferrin, transferrin receptors, and iron in macrophage listericidal activity. J Exp Med. 1991 Aug 1;174(2):459–466. doi: 10.1084/jem.174.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Ballart I. J., Estevez M. E., Sen L., Diez R. A., Giuntoli J., de Miani S. A., Peñalver J. Progressive dysfunction of monocytes associated with iron overload and age in patients with thalassemia major. Blood. 1986 Jan;67(1):105–109. [PubMed] [Google Scholar]
- Barton J. C., Huster W. J., Parmley R. T. Iron-binding reactivity in mature neutrophils: relative cell content quantification by cytochemical scoring. J Histochem Cytochem. 1988 Jun;36(6):649–658. doi: 10.1177/36.6.3367050. [DOI] [PubMed] [Google Scholar]
- Baughman R. P., Corser B. C., Strohofer S., Hendricks D. Spontaneous hydrogen peroxide release from alveolar macrophages of some cigarette smokers. J Lab Clin Med. 1986 Mar;107(3):233–237. [PubMed] [Google Scholar]
- Britigan B. E., Coffman T. J., Adelberg D. R., Cohen M. S. Mononuclear phagocytes have the potential for sustained hydroxyl radical production. Use of spin-trapping techniques to investigate mononuclear phagocyte free radical production. J Exp Med. 1988 Dec 1;168(6):2367–2372. doi: 10.1084/jem.168.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Coffman T. J., Buettner G. R. Spin trapping evidence for the lack of significant hydroxyl radical production during the respiration burst of human phagocytes using a spin adduct resistant to superoxide-mediated destruction. J Biol Chem. 1990 Feb 15;265(5):2650–2656. [PubMed] [Google Scholar]
- Britigan B. E., Pou S., Rosen G. M., Lilleg D. M., Buettner G. R. Hydroxyl radical is not a product of the reaction of xanthine oxidase and xanthine. The confounding problem of adventitious iron bound to xanthine oxidase. J Biol Chem. 1990 Oct 15;265(29):17533–17538. [PubMed] [Google Scholar]
- Buettner G. R. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988 May;16(1):27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- Bynoe L. A., Pou S., Gottsch J. D., Rosen G. M. Light-dependent spin trapping of hydroxyl radical from human erythrocytes. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1305–1310. doi: 10.1016/0006-291x(91)91715-o. [DOI] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Invest. 1991 Oct;88(4):1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., Hassett D. J., Rosen G. M. Do humans neutrophils form hydroxyl radical? Evaluation of an unresolved controversy. Free Radic Biol Med. 1988;5(2):81–88. doi: 10.1016/0891-5849(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Cosgrove J. P., Borish E. T., Church D. F., Pryor W. A. The metal-mediated formation of hydroxyl radical by aqueous extracts of cigarette tar. Biochem Biophys Res Commun. 1985 Oct 15;132(1):390–396. doi: 10.1016/0006-291x(85)91034-4. [DOI] [PubMed] [Google Scholar]
- Cozzi A., Santambrogio P., Levi S., Arosio P. Iron detoxifying activity of ferritin. Effects of H and L human apoferritins on lipid peroxidation in vitro. FEBS Lett. 1990 Dec 17;277(1-2):119–122. doi: 10.1016/0014-5793(90)80823-2. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Davis W. B., Pacht E. R., Spatafora M., Martin W. J., 2nd Enhanced cytotoxic potential of alveolar macrophages from cigarette smokers. J Lab Clin Med. 1988 Mar;111(3):293–298. [PubMed] [Google Scholar]
- Diguiseppi J., Fridovich I. Ethylene from 2-keto-4-thiomethyl butyric acid: the Haber-Weiss reaction. Arch Biochem Biophys. 1980 Dec;205(2):323–329. doi: 10.1016/0003-9861(80)90114-9. [DOI] [PubMed] [Google Scholar]
- Fox R. B. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984 Oct;74(4):1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon D. E., Varani J., Phan S. H., Ward J. H., Kaplan J., Till G. O., Simon R. H., Ryan U. S., Ward P. A. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987 Jul;57(1):37–44. [PubMed] [Google Scholar]
- Greening A. P., Lowrie D. B. Extracellular release of hydrogen peroxide by human alveolar macrophages: the relationship to cigarette smoking and lower respiratory tract infections. Clin Sci (Lond) 1983 Dec;65(6):661–664. doi: 10.1042/cs0650661. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Rush S. W., Moak S. A., Weitz Z. Conversion of superoxide generated by polymorphonuclear leukocytes to hydroxyl radical: a direct spectrophotometric detection system based on degradation of deoxyribose. Free Radic Biol Med. 1989;6(4):385–392. doi: 10.1016/0891-5849(89)90084-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Aruoma O. I. The deoxyribose method: a simple "test-tube" assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987 Aug 15;165(1):215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Hannan S. E., Harris J. O., Sheridan N. P., Patel J. M. Cigarette smoke alters plasma membrane fluidity of rat alveolar macrophages. Am Rev Respir Dis. 1989 Dec;140(6):1668–1673. doi: 10.1164/ajrccm/140.6.1668. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Mechanism of the antibiotic action pyocyanine. J Bacteriol. 1980 Jan;141(1):156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., Beall G. D., Repine J. E. Production of hydroxyl radical by human alveolar macrophages. Infect Immun. 1979 Dec;26(3):1088–1092. doi: 10.1128/iai.26.3.1088-1092.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Gordon S., Thornalley P. J., Bannister J. V. The production of oxygen-centered radicals by bacillus-Calmette-Guerin-activated macrophages. An electron paramagnetic resonance study of the response to phorbol myristate acetate. Biochim Biophys Acta. 1983 Oct 25;763(3):245–250. doi: 10.1016/0167-4889(83)90131-3. [DOI] [PubMed] [Google Scholar]
- Ito M., Karmali R., Krim M. Effect of interferon on chemiluminescence and hydroxyl radical production in murine macrophages stimulated by PMA. Immunology. 1985 Nov;56(3):533–541. [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. Iron overload--clinical and pathologic aspects. Semin Hematol. 1977 Jan;14(1):89–113. [PubMed] [Google Scholar]
- Janco R. L., English D. Regulation of monocyte oxidative metabolism: chemotactic factor enhancement of superoxide release, hydroxyl radical generation, and chemiluminescence. J Lab Clin Med. 1983 Dec;102(6):890–898. [PubMed] [Google Scholar]
- Janoff A., Pryor W. A., Bengali Z. H. NHLBI workshop summary. Effects of tobacco smoke components on cellular and biochemical processes in the lung. Am Rev Respir Dis. 1987 Oct;136(4):1058–1064. doi: 10.1164/ajrccm/136.4.1058. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Jordan I., Sturrock A. Regulation of the transferrin-independent iron transport system in cultured cells. J Biol Chem. 1991 Feb 15;266(5):2997–3004. [PubMed] [Google Scholar]
- Karnovsky M. L., Badwey J. A. Determinants of the production of active oxygen species by granulocytes and macrophages. J Clin Chem Clin Biochem. 1983 Sep;21(9):545–553. [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura S. Effects of cigarette smoking on metabolic events in the lung. Environ Health Perspect. 1987 Jun;72:283–296. doi: 10.1289/ehp.8772283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M., Murakami K., Ishikawa Y. Role of hydroxyl radicals derived from granulocytes in lung injury induced by phorbol myristate acetate. Am Rev Respir Dis. 1987 Dec;136(6):1435–1444. doi: 10.1164/ajrccm/136.6.1435. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Gaffield L., Patino M. M., Walden W. E., Thach R. E. Specificity of the induction of ferritin synthesis by hemin. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):146–150. doi: 10.1016/0167-4781(90)90156-v. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Patino M. M., Gaffield L., Walden W. E., Thach R. E. Derepression of ferritin messenger RNA translation by hemin in vitro. Science. 1990 Jan 5;247(4938):74–77. doi: 10.1126/science.2294594. [DOI] [PubMed] [Google Scholar]
- McGowan S. E., Henley S. A. Iron and ferritin contents and distribution in human alveolar macrophages. J Lab Clin Med. 1988 Jun;111(6):611–617. [PubMed] [Google Scholar]
- McGowan S. E., Murray J. J., Parrish M. G. Iron binding, internalization, and fate in human alveolar macrophages. J Lab Clin Med. 1986 Dec;108(6):587–595. [PubMed] [Google Scholar]
- Mussalo-Rauhamaa H., Leppänen A., Salmela S. S., Pyysalo H. Cigarettes as a source of some trace and heavy metals and pesticides in man. Arch Environ Health. 1986 Jan-Feb;41(1):49–55. doi: 10.1080/00039896.1986.9935765. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner D. E., Kleinerman J., Rice D. B. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974 Oct 10;291(15):755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- Niwa Y., Sakane T., Miyachi Y., Ozaki M. Oxygen metabolism in phagocytes of leprotic patients: enhanced endogenous superoxide dismutase activity and hydroxyl radical generation by clofazimine. J Clin Microbiol. 1984 Nov;20(5):837–842. doi: 10.1128/jcm.20.5.837-842.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M., Halliwell B., Moorhouse C. P., Aruoma O. I., Baum H., Peters T. J. Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Is haemosiderin formation a biological protective mechanism? Biochem J. 1986 Mar 15;234(3):727–731. doi: 10.1042/bj2340727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley L. W., St Clair D. K., Autor A. P., Oberley T. D. Increase in manganese superoxide dismutase activity in the mouse heart after X-irradiation. Arch Biochem Biophys. 1987 Apr;254(1):69–80. doi: 10.1016/0003-9861(87)90082-8. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Kawabata T., Awai M. Iron release from haemosiderin and production of iron-catalysed hydroxyl radicals in vitro. Biochem J. 1988 Mar 1;250(2):589–595. doi: 10.1042/bj2500589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou S., Hassett D. J., Britigan B. E., Cohen M. S., Rosen G. M. Problems associated with spin trapping oxygen-centered free radicals in biological systems. Anal Biochem. 1989 Feb 15;177(1):1–6. doi: 10.1016/0003-2697(89)90002-x. [DOI] [PubMed] [Google Scholar]
- Qian M. W., Eaton J. W. Tobacco-borne siderophoric activity. Arch Biochem Biophys. 1989 Nov 15;275(1):280–288. doi: 10.1016/0003-9861(89)90374-3. [DOI] [PubMed] [Google Scholar]
- Ramos C. L., Pou S., Britigan B. E., Cohen M. S., Rosen G. M. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem. 1992 Apr 25;267(12):8307–8312. [PubMed] [Google Scholar]
- Richardson D., Baker E. The uptake of inorganic iron complexes by human melanoma cells. Biochim Biophys Acta. 1991 Jun 7;1093(1):20–28. doi: 10.1016/0167-4889(91)90133-i. [DOI] [PubMed] [Google Scholar]
- Speer C. P., Ambruso D. R., Grimsley J., Johnston R. B., Jr Oxidative metabolism in cord blood monocytes and monocyte-derived macrophages. Infect Immun. 1985 Dec;50(3):919–921. doi: 10.1128/iai.50.3.919-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson K. B., Nauseef W. M., Clark R. A. The neutrophil glycoprotein Mo1 is an integral membrane protein of plasma membranes and specific granules. J Immunol. 1987 Dec 1;139(11):3759–3763. [PubMed] [Google Scholar]
- Stocker R. Induction of haem oxygenase as a defence against oxidative stress. Free Radic Res Commun. 1990;9(2):101–112. doi: 10.3109/10715769009148577. [DOI] [PubMed] [Google Scholar]
- Sturrock A., Alexander J., Lamb J., Craven C. M., Kaplan J. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J Biol Chem. 1990 Feb 25;265(6):3139–3145. [PubMed] [Google Scholar]
- Sutton H. C. Efficiency of chelated iron compounds as catalysts for the Haber-Weiss reaction. J Free Radic Biol Med. 1985;1(3):195–202. doi: 10.1016/0748-5514(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Thompson A. B., Bohling T., Heires A., Linder J., Rennard S. I. Lower respiratory tract iron burden is increased in association with cigarette smoking. J Lab Clin Med. 1991 Jun;117(6):493–499. [PubMed] [Google Scholar]
- Thompson A. B., Bohling T., Payvandi F., Rennard S. I. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med. 1990 Feb;115(2):148–158. [PubMed] [Google Scholar]
- Thorstensen K., Romslo I. The role of transferrin in the mechanism of cellular iron uptake. Biochem J. 1990 Oct 1;271(1):1–9. doi: 10.1042/bj2710001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Hatherill J. R., Tourtellotte W. W., Lutz M. J., Ward P. A. Lipid peroxidation and acute lung injury after thermal trauma to skin. Evidence of a role for hydroxyl radical. Am J Pathol. 1985 Jun;119(3):376–384. [PMC free article] [PubMed] [Google Scholar]
- Valberg P. A., Jensen W. A., Rose R. M. Cell organelle motions in bronchoalveolar lavage macrophages from smokers and nonsmokers. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1272–1279. doi: 10.1164/ajrccm/141.5_Pt_1.1272. [DOI] [PubMed] [Google Scholar]
- Wannemuehler Y., Johansen K., Rosenbusch R. Identification of Moraxella bovis by using a monoclonal antibody to a lipopolysaccharide epitope. J Clin Microbiol. 1989 Dec;27(12):2881–2883. doi: 10.1128/jcm.27.12.2881-2883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Evidence for hydroxyl radical generation by human Monocytes. J Clin Invest. 1977 Aug;60(2):370–373. doi: 10.1172/JCI108785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Wesselius L. J., Flowers C. H., Skikne B. S. Alveolar macrophage content of isoferritins and transferrin. Comparison of nonsmokers and smokers with and without chronic airflow obstruction. Am Rev Respir Dis. 1992 Feb;145(2 Pt 1):311–316. doi: 10.1164/ajrccm/145.2_Pt_1.311. [DOI] [PubMed] [Google Scholar]
- White G. P., Jacobs A. Iron uptake by Chang cells from transferrin, nitriloacetate and citrate complexes: the effects of iron-loading and chelation with desferrioxamine. Biochim Biophys Acta. 1978 Oct 3;543(2):217–225. doi: 10.1016/0304-4165(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. The ability of scavengers to distinguish OH. production in the iron-catalyzed Haber-Weiss reaction: comparison of four assays for OH. Free Radic Biol Med. 1987;3(1):33–39. doi: 10.1016/0891-5849(87)90037-2. [DOI] [PubMed] [Google Scholar]
- Worwood M., Hourahane D., Jones B. M. Accumulation and release of isoferritins during incubation in vitro of human peripheral blood mononuclear cells. Br J Haematol. 1984 Jan;56(1):31–43. doi: 10.1111/j.1365-2141.1984.tb01269.x. [DOI] [PubMed] [Google Scholar]
- de Jong G., van Dijk J. P., van Eijk H. G. The biology of transferrin. Clin Chim Acta. 1990 Sep;190(1-2):1–46. doi: 10.1016/0009-8981(90)90278-z. [DOI] [PubMed] [Google Scholar]