Introduction
Obesity
Obesity is a major medical problem whose prevalence is increasing at an alarming rate1. It remains a major risk factor for cardiovascular disease, metabolic diseases like Type 2 Diabetes Mellitus and certain cancers. There is also an established link between obesity and hypertension. The normative aging study found adiposity is significantly positively associated with both systolic and diastolic blood pressure and that up to two-thirds of hypertension are associated with excess adiposity2. In addition, evidence from the Framingham Offspring Study suggests that obesity may account for up to 78% of hypertension in males and 64% in females3. Obesity is also responsible for increased morbidity from reproductive, skeletal and gastrointestinal disorders4 as well as being negatively associated with psychological well-being and social functioning5.
The simple principle that obesity can only arise when energy intake exceeds energy expenditure remains undisputed. Furthermore, rapid changes in the availability, composition and consumption of energy dense food coupled with a downturn in physical activity levels in all aspects of daily life have undoubtedly contributed to the recent rises in the prevalence of obesity worldwide.
However, in the midst of these dramatic societal changes, many people remain lean6. Indeed, the fact that most healthy adults maintain a steady body weight over many years despite huge variations in daily energy intake and expenditure is powerful testament to a system that is under tight homeostatic control. The last 15 years have seen a huge increase in our knowledge of the molecular players which underpin these regulatory systems. Many of the seminal observations in this field have come from obese animal models, both naturally occurring and genetically modified, but have combined synergistically with data from human genetic and physiological studies. We now understand that contained within the brain there are a number of signalling systems which are crucial to allow the central nervous system to sense peripherally-derived signals of both long term energy stores and more acute changes in energy expenditure.
One of the most critical of these central pathways is the central melanocortin system, a pathway primarily within the hypothalamus and based around the actions of a family of small peptides (the melanocortins). In this review we highlight the basic anatomical and functional architecture of the central melanocortin system and review some of the data surrounding the potential physiological roles of the melanocortin peptides. We also describe the convergent data from animal and human studies which have recently given some tantalising hints into the link between obesity and hypertension. Finally, we discuss evolving, primarily pre-clinical data, which indicate that melanocortin signalling through the central nervous system may play a part in limiting tissue damage from inflammation and ischaemia.
Architecture of the melanocortin system
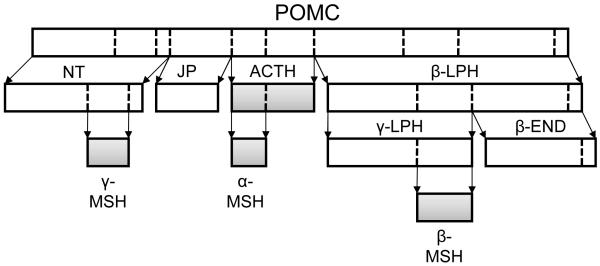
POMC
It seems likely that pro-opiomelanocortin (POMC) appeared early on in vertebrate evolution with primitive jawless fish having a POMC coding sequence very similar to that seen in higher mammals7. In humans, the POMC gene consists of three exons and although POMC mRNA can be detected in a number of tissues, the gene is expressed at physiologically significant levels in a limited range of tissues8. These include the skin9, corticotrophs of the anterior pituitary, the hypothalamus and the brainstem.
POMC is an archetypal polypeptide precursor with the 241 amino acid pro-peptide being functionally inert. It is extensively post-translationally processed, undergoing a series of proteolytic cleavages and chemical transformations to generate a series of smaller biologically active peptides (Figure 1). The precise repertoire of POMC derived products from any particular tissue is largely dependent on the range of processing enzymes expressed in that tissue10. POMC is cleaved by a family of serine proteases called prohormone convertases (PCs) with PC1 and PC2 understood to be particularly important in POMC processing. PC1 is ubiquitously expressed in POMC containing tissues and cleaves n-terminal fragment (NT), joining peptide (JP), adrenocorticotropin (ACTH) and β-lipotropin (β-LPH); while PC2 is selectively expressed in the hypothalamus but not the pituitary and further cleaves NT, ACTH and β-LPH to form γ-, α-, and β-melanocyte stimulating hormone (MSH) respectively11. α-, β- and γ-MSH together with ACTH are collectively known as the melanocortins. The name “melanocortin” derives from the early studies of peptides extracted from pituitary glands which demonstrated that these peptides were able to bring about dramatic change in melanin pigmentation and stimulate glucocorticoid production12. This family of peptides possess structural similarity with a characteristic invariant tetrapeptide motif (His-Phe-Arg-Trp) at their core13.
Figure 1.
POMC is a precursor peptide. Cleavage of this large prohormone by the action of prohormone convertases at basic amino acid cleavage sites (dotted) produces a range of smaller peptides, including the melanocortins (highlighted). α-MSH is encoded entirely within ACTH. Abbreviations: POMC, pro-opiomelanocortin; NT, N-terminal fragment; JP, joining peptide; ACTH, adrenocorticotropic hormone; β-, γ-LPH, β-, γ-lipotropin; α-, β-, γ-MSH, α-, β-, γ-melanocyte stimulating hormone; β-END, β-endorphin.
Melanocortin receptors
The actions of the melanocortin peptides are mediated through a family of five melanocortin receptors (MC1-R to MC5-R)14. These receptors show considerable homology, all being G protein-coupled, seven-transmembrane domain receptors. MC1-R is expressed on a range of cell types within the skin including melanocytes, keratinocytes and cells of the immune system14, 15. MC2-R is the classical ACTH receptor, expressed in the cortex of the adrenal gland. MC2-R binds only ACTH and has no affinity for the other melanocortin peptides16, although ACTH itself is recognised by the other four melanocortin receptors. The MC3-R is expressed in the brain, chiefly within the hypothalamus, cortex, thalamus and limbic system17. It has also been detected in the gut, placenta, kidney and heart18. MC4-R is expressed widely throughout the mammalian CNS. It is highly expressed in regions of the hypothalamus known to be involved in the control of energy homeostasis 19, as well as being found within the brainstem and spinal cord20. MC5-R is more ubiquitously expressed in many peripheral tissues but appears to have a role in pheromone and sebum producing exocrine glands21.
Central melanocortin system
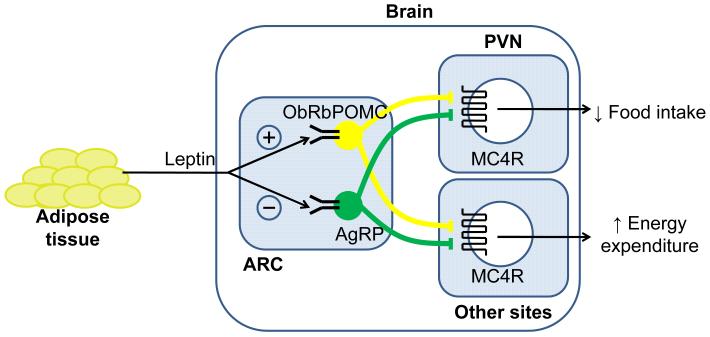
Contained within the arcuate nucleus (Arc) of the hypothalamus are two separate populations of neurons which express either POMC or both NPY and Agouti-related protein (AgRP), the latter a potent melanocortin antagonist at the MC3 and 4 receptors. From the arcuate nucleus, POMC neurons project to many other regions of the brain areas, including other hypothalamic regions such as the paraventricular nucleus that are involved in energy homeostasis (PVN) (Figure 2). Caudally, projections extend to the thalamus and the medial amygdala, while POMC neurons also send descending projections to the brainstem and spinal cord. It is these arcuate neurons together with their downstream second order neurons expressing MC3-R and MC4-R that make up the central melanocortin system. The fed state is characterised by increased POMC neuronal activity with inhibition of AgRP neurons. This increase in melanocortin tone in regions of the brain expressing MC4-R decreases food intake and increases energy expenditure. In contrast, states of negative energy balance such as fasting are characterised by inactivation of POMC neurons (therefore reducing melanocortin levels) but stimulation of AgRP activity. The resultant decrease in MC4-R signalling stimulates feeding and reduces energy expenditure.
Figure 2.
The leptin-melanocortin system and energy homeostasis. The arcuate nucleus within the hypothalamus has a critical role in integrating circulating nutritional-dependent signals from the periphery and thereby communicating energy balance status to the central nervous system. The adipose-derived cytokine leptin is produced in proportion to fat cell mass, increasing with overfeeding and decreasing with starvation. Acting via receptors expressed on POMC and AgRP neurons within the hypothalamus, leptin can modulate central melanocortin tone to effect changes in food intake and energy expenditure. Abbreviations: ObRb, leptin receptor; POMC, pro-opiomelanocortin; AgRP, agouti-related protein; ARC, arcuate nucleus; PVN, paraventricular nucleus; MC4-R, melanocortin 4 receptor.
An intact central melanocortin signalling pathway is critical for normal energy homoeostasis; with defects in synthesis, processing and action of POMC peptides resulting in obesity (reviewed in Coll et al.22). For example, genetic deletion of MC4-R in mice and humans results in severe hyperphagic obesity, with an increase in both fat and lean mass23. Indeed, MC4-R mutations are responsible for up to 5% of cases of severe childhood obesity and up to 2.5% of adult obesity24, 25. A study of the general population in the UK suggest a mutational frequency of 1/100026, making MC4-R deficiency one of the most common single-gene disorders. Of course this still means that the majority of obese people cannot ascribe their obesity to defective melanocortin receptor functioning, with many complex interactions between environment and genes yet to be untangled, but these studies have played a major role in highlighting the importance of this pathway in human physiology.
Melanocortins and the sympathetic nervous system
As befits a system which controls vital homeostatic processes in an involuntary manner, regulation of the sympathetic nervous system (SNS) is complex, with output being an integration of many inputs. There are now data emerging to indicate that one of these many inputs may arise from melanocortin signalling, with studies from rodents indicating a close functional and anatomical link between the central melanocortin system and the SNS. Many of these studies have focused on adipose depots, both thermogenic brown adipose tissue (BAT) and white adipose tissue (WAT), the principle storage depot of surplus energy. Intracerebroventricular (icv) administration of MC4-R agonist produces a dose-dependent increase in sympathetic nerve activity to BAT27, while icv AgRP can suppress sympathetic activity to BAT28. Noguieras et al.29 have also demonstrated that bidirectional modulation of central melanocortin tone can have a powerful influence on peripheral lipid metabolism quite independent of its effects on food intake. Pharmacological blockade with the melanocortin antagonist SHU9119 caused an increase in triglyceride concentration in epididymal WAT, an increase in total body fat mass and a drive towards a decreased metabolic usage of fats. Conversely, treatment with the melanocortin agonist MT-II increased expression of genes implicated in lipid catabolism. Repeating identical antagonist administration experiments in mice engineered to be deficient in β1-, β2- and β3-adrenoceptors did not bring about the increase in body weight or lipogenic activity seen in the wild-type animals, highlighting the role of the sympathetic nervous system in mediating the peripheral effects of central melanocortin activity.
Direct anatomical evidence to further support this functional link has come from studies using the pseudorabies virus as a transneuronal retrograde tracer. This virus has the ability to be taken up by the terminals of postganglionic sympathetic neurons in peripheral tissue and be transported in a retrograde fashion right back to preganglionic neurons higher in the CNS. Building upon previous studies utilising this technique to explore the CNS origins of the SNS outflow to BAT30, the Bartness laboratory combined injection of this viral tracer in WAT depots with in situ hybridisation to identify MC4-R mRNA and convincingly demonstrated that a high percentage of MC4-R bearing neurons project to fat tissue31.
In addition to these metabolic links, strands of evidence from murine and more latterly human physiology studies have now converged to give a molecular link between obesity and hypertension3. These data clearly indicate that central melanocortinergic pathways modulate blood pressure.
Early evidence to suggest a role for melanocortin peptides in autonomic cardiovascular control came from the identification of high levels of MC4-R expression in areas of the PVH that provide descending projections to autonomic preganglionic neurons20. Subsequently the ability of leptin to increase mean arterial pressure (MAP) and lumbar sympathetic nerve activity (LSNA) was convincingly shown to be mediated through melanocortin activity32, 33. Further pharmacological studies demonstrated that chronic administration of an MC3/4-R agonist increased MAP despite hypophagia34 whilst, in contrast, central infusion of the MC3/4-R antagonist SHU9119 caused a marked decrease in HR and MAP35. A similar infusion of melanocortin antagonist to spontaneously hypertensive rats caused a greater than 20 mmHg drop in mean arterial pressure, despite also causing weight gain, insulin resistance and hyperleptinaemia, indicating that, at least in this model of hypertension, an increase in endogenous melanocortin tone appears to contribute to the elevation in arterial pressure36.
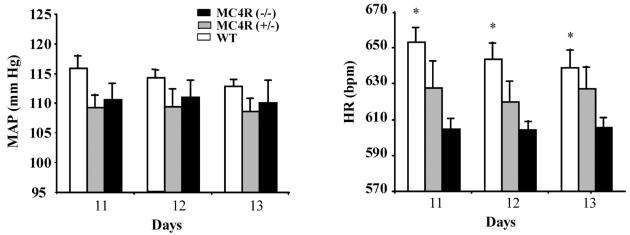
This pharmacological evidence was further supported by studies of MC4-R deficient mice (Mc4r null). These mice are not hypertensive, either on a standard or high salt diet. Their basal average heart rate is also significantly lower than wild-type mice, both during daylight hours and in the hours of darkness when, because of their reverse circadian rhythm, mice are more active (Figure 3)37. Furthermore, while icv administration of α-MSH to wild-type and Mc3r−/− mice led to a significant increase in MAP this was not seen in Mc4r null mice38.
Figure 3.
Basal 24-hour average mean arterial pressure and heart rate in male 17- to 19-week-old MC4-R (−/−), MC4-R (+/−), and wild-type mice on days 11, 12, and 13 after implantation of telemetry probe (n=5 to 7 per group). *P<0.05 compared with MC4R (−/−) mice. Reproduced from Tallam et al. 2005 with permission of the publisher. Copyright © 2005 American Heart Association. All rights reserved.
Building upon these data, Greenfield and colleagues have recently extended our knowledge of the melanocortin system in humans by a detailed phenotypic analysis of adults heterozygous for complete loss-of-function mutations in MC4-R. The prevalence of hypertension was significantly lower in MC4-R deficient subjects compared to equally overweight control subjects39. Furthermore, as compared to controls, MC4-R deficient subjects had a lower increase in heart rate on waking, a lower heart rate during euglycaemic hyperinsulinaemia and a lower 24 hour urinary norepinephrine excretion, all in keeping with reduced activity of the sympathetic nervous system. During sleep, there was no difference in the high-frequency component of heart rate variability (regarded as a measure of parasympathetic activation) between the two study groups. Of note, total cholesterol was no different between the two groups (4.9±0.8 vs 5.0±1.0 mmol/litre, MC4r deficient vs obese control, respectively).
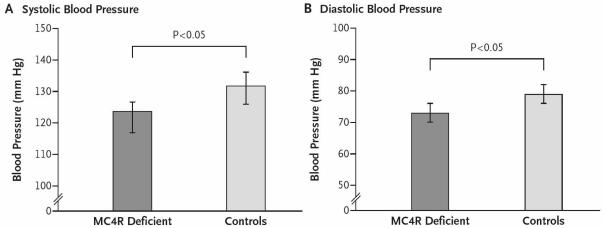
The same group also went on to examine the effects of increased central melanocortin tone by studying the effects of a subcutaneous infusion of a synthetic peptide agonist highly selective for MC4-R on 28 overweight or obese volunteers. The maximum tolerated dose led to a significant increase in both systolic and diastolic blood pressure (9.3±1.9 and 6.6±1.1 mmHg, respectively) (Figure 4). Thus by combining data from careful phenotyping of patients with congenital deficiency in melanocortin tone with data from pharmacological studies, these authors were able to show that both decreases and increases in central melanocortin signalling influence blood pressure in humans and that the effects were not explained by changes in circulating insulin levels or insulin sensitivity. This study now gives a potential mechanistic explanation to the well established, but poorly understood, effects of weight loss and obesity on human hypertension.
Figure 4.
Blood Pressure in MC4-R deficiency. The graphs show measures of systolic blood pressure (Panel A) and diastolic blood pressure (Panel B) in 46 subjects with MC4R deficiency, as compared with 30 overweight or obese control subjects. Reproduced from Greenfield et al. 2009 with permission of the publisher. Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Further studies are currently underway to determine further if MC4r deficient obese adults are protected from the adverse cardiovascular consequences that normally accompany obesity and we await the outcome of studies investigating left ventricular mass, cardiac output, arterial stiffness and endothelial function with great interest (personal communication, Dr I.S. Farooqi, Cambridge, UK).
Gamma MSH, sodium homeostasis and hypertension
γ-MSH was the last of the melanocortin peptides to be characterised and its true physiological role is somewhat less well defined when compared to its more famous “older sibling”, α-MSH. Nevertheless, it is clear from animal model systems that γ-MSH does have an effect on the cardiovascular system, albeit somewhat distinct from that of α-MSH. Administration of γ-MSH can increase MAP and HR in wild-type, Mc3r−/− and Mc4r−/− mice. The hypertensive effect of intravenous γ-MSH is not blocked by intracarotid delivery of potent melanocortin antagonists40 but can be abrogated following icv administration of the sodium channel blocker benzamil in both Mc3r+/+ and Mc3r−/− mice38. Such observations have led to speculation that γ-MSH may act upon a receptor quite distinct from one of the 5 classic melanocortin receptors.
There are also animal data to indicate that γ-MSH has a role in sodium homeostasis and can act as a natriuretic. Rats and mice given a high salt diet respond by doubling the γ-MSH content of the pituitary intermediate lobe and their circulating concentration of γ-MSH41. Mice lacking the processing enzyme PC2 (Pc2 null) are rendered γ-MSH deficient as a result of impaired secondary POMC processing and unlike their wild-type littermates, have lost the ability to respond to a high salt diet by increasing γ-MSH level42. Thus, γ-MSH deficient PC2 null mice are rendered significantly hypertensive after just a week of a high salt diet, in contrast to wild-type littermates which remain normotensive. This hypertension can be readily reversed by γ-MSH but not by α-MSH.
Despite these data, there remains much contention surrounding γ-MSH, not least of which is the determination of the predominant form in the circulation, with at least four different species recognised (γ1-, γ2-, γ3- and lys-γ3 MSH)43. Further, much of the work relating to the cardiovascular role of γ-MSH has focused on rodent models which, unlike humans, have a clearly discernible pituitary intermediate lobe from which circulating forms of this peptide are thought to arise. Thus, there remains uncertainty as to whether data from these rodent models can be faithfully inferred over to human physiology, with scant data on the role of γ-MSH in human cardiovascular physiology.
Anti-inflammatory actions of melanocortins
The immunological milieu following tissue damage is complex, with a cascade of interacting mediators influencing the pathophysiological response. Although by no means as well defined as the evidence base supporting the role of melanocortin signalling in energy homeostasis, there are now data to indicate that melanocortins can inhibit peripheral production of the chemical mediators of inflammation and by doing so potentially modify inflammatory cell migration. Interestingly, just as peripheral signals of energy balance are integrated within the brain, so central melanocortinergic systems also appear to have an important role in modulating the inflammatory response to peripheral stress44.
Melanocortins have long been known to have potent antipyretic properties, quite distinct from any role in normal thermoregulation and via a mechanism that does not involve the adrenal gland. Both α-MSH and ACTH administered centrally can significantly ameliorate the fever brought on by peripheral administration of endogenous pyrogens even in adrenalectomised animals45, 46
Direct action of α-MSH upon macrophages and fibroblasts can greatly reduce the production of pro-inflammatory cytokines and chemokines47. Further, a single icv injection of 10μg of α-MSH given to mice also receiving 200 mg of E.coli endotoxin i.p. has been reported to change the outcome from all mice dying to allowing 45% of the mice to survive48.
Preliminary data also suggest that melanocortins may be able to influence outcome following haemorrhagic shock. ACTH and α-MSH analogues have been reported to increase survival rates in rat models of hypovolaemic shock49, with one small case series describing a beneficial effect of ACTH given to patients with shock resulting from type A aortic dissection50. However, there are no clear data to determine how much of this effect is independent of steroid production from the adrenals.
Common to both septic and haemorrhagic shock is the triggering of an inflammatory cascade characterised by increased production of cytokines like TNF-α, chemokines, oxygen free radicals and other inflammatory mediators (reviewed in Schlag et al.51). Melanocortins may be able to modulate this circulating inflammatory milieu via a neuronal effector arm arising from within the CNS. Thus, this anti-shock effect can be seen with icv doses much lower than those required by iv route52, with pharmacological or physical interruption of vagal outflow negating the effect. This cholinergic anti-inflammatory pathway has been further explored by Gaurini et al. who combined precise anatomical perturbations with pharmacological interventions to show that in rats systemically shocked by haemorrhage, ACTH 1–24 can act through central MC4-R to trigger a vagal anti-inflammatory pathway, inhibit NF-κB activation, and reduce both hepatic TNF-α mRNA content and TNF-α plasma levels53. More recently, peripheral administration of 2 novel melanocortin agonists, highly selective at MC4-R have proven to be efficacious in a rat model of haemorrhagic shock, significantly reducing multiple organ damage and improving survival54.
Melanocortins, ischaemia and reperfusion injury
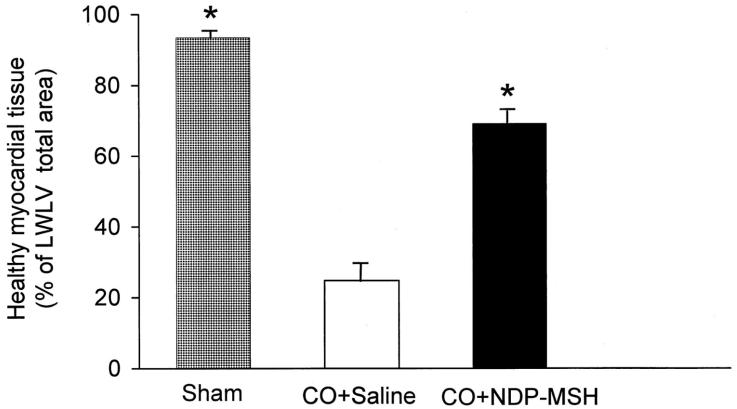
The ability of melanocortins to so substantially ameliorate the deleterious effects of severe tissue hypoxia in animal models led Bazzani et al. to study whether they may also have a beneficial effect on the tissue damage induced by coronary occlusion55. Using ligation of the left anterior descending coronary artery in rats as the model, this group were able to show that iv administration of ACTH 1–24 caused a significant dose-dependent reduction in the incidence of arrhythmia and death and effectively doubled the volume of healthy cardiac muscle remaining compared to sham treated animals (Figure 5). Further, in animals subject to temporary occlusion followed by reperfusion, ACTH treatment almost completely prevented the ischaemia/reperfusion-induced increase in circulating free radical levels. However, the authors present no data to exclude this effect being partially or wholly mediated by ACTH stimulated corticosterone production.
Figure 5.
Amount of volume of healthy myocardial tissue, as estimated 72 h after permanent coronary occlusion (CO) calculated as a percentage of the myocardial volume of the lateral wall of the left ventricle (LWLV), in sham-operated (n = 6), saline-treated (n = 8), and NDP-MSH-treated (0.27 mg/kg s.c. every 12 h; n = 8) rats (mean values ± S.E). *P < 0.001 versus saline-treated rats (Student-Newman-Keuls test). Reproduced from Bazzani et al. 2001 with permission of the publisher. Copyright © 2001 The American Society for Pharmacology and Experimental Therapeutics. All rights reserved.
Once again, the central action of melanocortins appears to be critical in bringing about this protective effect56 with the effector arm of the circuit being efferent vagal fibres57. Interestingly, this particular protective loop may, at least in part, preferentially involve MC3-R58, 59. Indeed, MC3-R may also be the critical receptor in affecting melanocortin-derived ischaemic protection in the mesenteric microcirculation too. Leoni et al. have recently shown that not only do Mc3r null mice display a more exuberant inflammatory response to mesenteric artery ischaemia/reperfusion than do wild-type mice, but that pharmacological treatment with a selective MC3-R agonist that attenuates inflammatory damage in wild-type mice fails to do so in Mc3r null littermates60.
Brain
The synthetic melanocortin analogue NDP-MSH has recently been shown to have a neuroprotective role. Giuliani et al. induced transient global cerebral ischaemia by bilateral occlusion of common carotids in gerbils61. This resulted in active inflammation and neuronal damage within the hippocampus, with peripheral administration of NDP-MSH inhibiting cytokine expression and reducing histological damage within this brain region. Further pharmacological exploration of this pathway indicated that MC4-R but not MC3-R activation was responsible. Similar results have been seen in other species62 and just as in the case of hypovolaemic and septic shock; it now appears that the vagus nerve is important in mediating the protective effects of the melanocortins against cerebral ischaemia. Ottani et al. show that focal cerebral ischaemia in rats induces a cerebral and hepatic inflammatory cascade which can both be suppressed by melanocortins63.
Therapeutic perspectives
A plethora of data has accrued over the last 15 years on the structure and function of melanocortin receptors, particularly with respect to their role in energy homeostasis. With the prevalence of obesity continuing to rise worldwide64 the ability to modulate the activity of a surface receptor like MC4-R becomes a tantalising pharmacological goal. However, getting a “clean hit” with a drug that tackles obesity by acting on central receptors is no mean feat. Consider the cannabinoid system which, just like the melanocortin system, has a central action in the regulation of food intake and is widely expressed in the CNS. The early clinical promise of rimonabant, an antagonist at the cannabinoid 1 (CB1) receptor, was dashed when unwanted off target actions led to problems which resulted in the withdrawal of the drug65. So it may be for drugs that act as melanocortin agonists.
Long before the melanocortin receptor system had been characterised, central administration of a melanocortin agonist was reported to evoke a bizarre systemic syndrome of stretching yawning66. Step forward 50 years and melanocortin agonists primarily designed as an agent to reduce food intake and decrease body weight are, perhaps unsurprisingly, reported to cause similar systemic effects39. Such findings will, at the very least, inject a note of caution into actively pursuing these agents as effective and safe weight loss agents. However, a drug effect is only an adverse effect if it is unwanted, either in its direction or magnitude. A drug that causes an increase in blood pressure is going to be hard to let pass as a long term treatment in a patient population likely to be already burdened with other cardiovascular risk factors but in an acute life-threatening emergency, this rapid-onset pressor effect may be far more helpful. Indeed the history of melanocortin pharmacology has already gone through a similar cycle of serendipity whereby an “unwanted side effect” of penile erection following melanocortin agonist administration led to this class of drugs being developed as a treatment for erectile dysfunction67.
There is still pharmaceutical industry interest in designing drugs to perturb melanocortin signalling, with high-throughput screening of compounds, in silico modelling, and the development of novel, non-peptide, low molecule weight organic compounds all on-going (see review by Wikberg & Mutulis68). However, there is still much to do before melanocortin-derived agents, be they modified peptides or chemically distinct small molecules, make it into the clinical arena. There may be a need to design an agent that goes beyond simple receptor specificity and that also has tissue-, or in the case of the brain, region-specificity.
It is also clear that the roles of melanocortins in pathways controlling energy balance have been more clearly demarcated than those relating to cardiovascular disease. The data on melanocortins and ischaemic damage are derived almost entirely from experimental animal models and there are no substantive data linking melanocortins with the pathogenesis of atherosclerosis. However, we suggest that the recent proof-of-concept studies highlighting the beneficial effects of melanocortin on limiting tissue damage in cases of ischaemia and inflammation merit further attention. We look forward to following the evolving story of how perturbations in melanocortin signalling, be it from congenital deficiency or pharmacological manipulation, influence cardiovascular pathophysiology.
Summary
The diverse roles of the melanocortin system can all be viewed as being crucial components of the body’s defence mechanisms, be this by limiting ultraviolet light damage to the skin, fuelling the drive to eat when energy stores are low or fine tuning the inflammatory response to physical trauma to minimise tissue damage.
Our knowledge of melanocortin biology continues to evolve and develop. The importance of key parts of the system in the control of energy homeostasis remain unchallenged with, for example, common variants near MC4-R now being implicated in influencing fat mass, weight and obesity risk at the population level69. Further, although not discussed in this review, there is an increasing body of work highlighting the role increased melanocortin activity has in the pathogenesis of cachexia (see review by Marks et al.70).
With this on-going expansion of knowledge, it is surely only a matter of time before pharmaceutical agents derived from these simple peptides come of age. However, whichever niche these drugs eventually occupy, clinicians need to remain mindful of this evolving sphere of influence and remain attuned to potential effects, good and bad, in tissue and organs distant from the primary target.
Figure 6.
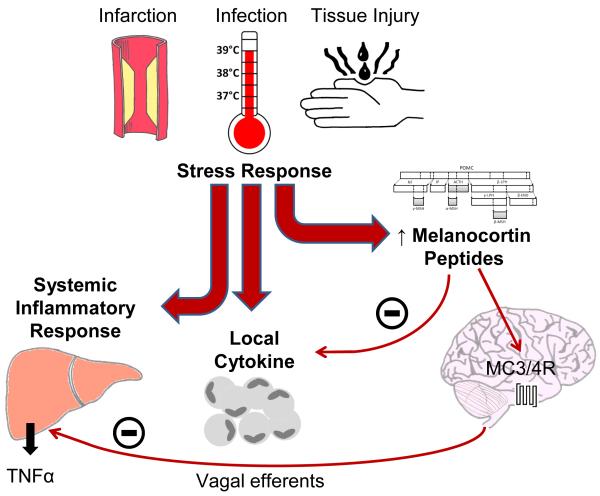
The melanocortin system and inflammatory response. Physiological insult leads to a stress response comprising systemic inflammatory and local cytokine responses. Melanocortin peptides acting both directly upon inflammatory cells, and centrally via the MC3/4-R and vagal efferents, act to suppress these cytokine and systemic responses and therefore modulate the stress response. Abbreviations: TNFα, tumor necrosis factor α; MC3/4-R, melanocortin 3/4 receptor.
Acknowledgements / Funding Sources
MPC is funded by a Medical Research Council (MRC) Centre for Obesity and Related Metabolic Disease (MRC-CORD) PhD studentship. APC is funded by the MRC (Grant number G108/617) and The Wellcome Trust (Grant number 08478/Z/08/Z).
Footnotes
Disclosures
None
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Cassano PA, Segal MR, Vokonas PS, Weiss ST. Body fat distribution, blood pressure, and hypertension. A prospective cohort study of men in the normative aging study. Ann Epidemiol. 1990;1:33–48. doi: 10.1016/1047-2797(90)90017-m. [DOI] [PubMed] [Google Scholar]
- 3.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 4.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 5.Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329:1008–1012. doi: 10.1056/NEJM199309303291406. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 7.Heinig JA, Keeley FW, Robson P, Sower SA, Youson JH. The appearance of proopiomelanocortin early in vertebrate evolution: cloning and sequencing of POMC from a Lamprey pituitary cDNA library. Gen Comp Endocrinol. 1995;99:137–144. doi: 10.1006/gcen.1995.1094. [DOI] [PubMed] [Google Scholar]
- 8.Raffin-Sanson ML, de Keyzer Y, Bertagna X. Proopiomelanocortin, a polypeptide precursor with multiple functions: from physiology to pathological conditions. Eur J Endocrinol. 2003;149:79–90. doi: 10.1530/eje.0.1490079. [DOI] [PubMed] [Google Scholar]
- 9.Wakamatsu K, Graham A, Cook D, Thody AJ. Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Res. 1997;10:288–297. doi: 10.1111/j.1600-0749.1997.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 10.Seidah NG, Chretien M. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999;848:45–62. doi: 10.1016/s0006-8993(99)01909-5. [DOI] [PubMed] [Google Scholar]
- 11.Coll AP. Effects of pro-opiomelanocortin (POMC) on food intake and body weight: mechanisms and therapeutic potential? Clin Sci (Lond) 2007;113:171–182. doi: 10.1042/CS20070105. [DOI] [PubMed] [Google Scholar]
- 12.Lerner AB, Shizume K, Fitzpatrick TB, Mason HS. MSH: the melanocyte-stimulating hormone. AMA Arch Derm Syphilol. 1954;70:669–674. doi: 10.1001/archderm.1954.01540230119017. [DOI] [PubMed] [Google Scholar]
- 13.Haskell-Luevano C, Nikiforovich G, Sharma SD, Yang YK, Dickinson C, Hruby VJ, Gantz I. Biological and conformational examination of stereochemical modifications using the template melanotropin peptide, Ac-Nle-c[Asp-His-Phe-Arg-Trp-Ala-Lys]-NH2, on human melanocortin receptors. J Med Chem. 1997;40:1738–1748. doi: 10.1021/jm960845e. [DOI] [PubMed] [Google Scholar]
- 14.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 15.Neumann Andersen G, Nagaeva O, Mandrika I, Petrovska R, Muceniece R, Mincheva Nilsson L, Wikberg JE. MC(1) receptors are constitutively expressed on leucocyte subpopulations with antigen presenting and cytotoxic functions. Clin Exp Immunol. 2001;126:441–446. doi: 10.1046/j.1365-2249.2001.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schioth HB, Chhajlani V, Muceniece R, Klusa V, Wikberg JE. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996;59:797–801. doi: 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- 17.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J Biol Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 18.Desarnaud F, Labbe O, Eggerickx D, Vassart G, Parmentier M. Molecular cloning, functional expression and pharmacological characterization of a mouse melanocortin receptor gene. Biochem J. 1994;299(Pt 2):367–373. doi: 10.1042/bj2990367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cone RD. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol Metab. 1999;10:211–216. doi: 10.1016/s1043-2760(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 20.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 22.Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 23.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 24.Hinney A, Bettecken T, Tarnow P, Brumm H, Reichwald K, Lichtner P, Scherag A, Nguyen TT, Schlumberger P, Rief W, Vollmert C, Illig T, Wichmann HE, Schafer H, Platzer M, Biebermann H, Meitinger T, Hebebrand J. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab. 2006;91:1761–1769. doi: 10.1210/jc.2005-2056. [DOI] [PubMed] [Google Scholar]
- 25.Larsen LH, Echwald SM, Sorensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab. 2005;90:219–224. doi: 10.1210/jc.2004-0497. [DOI] [PubMed] [Google Scholar]
- 26.Alharbi KK, Spanakis E, Tan K, Smith MJ, Aldahmesh MA, O’Dell SD, Sayer AA, Lawlor DA, Ebrahim S, Davey Smith G, O’Rahilly S, Farooqi S, Cooper C, Phillips DI, Day IN. Prevalence and functionality of paucimorphic and private MC4R mutations in a large, unselected European British population, scanned by meltMADGE. Hum Mutat. 2007;28:294–302. doi: 10.1002/humu.20404. [DOI] [PubMed] [Google Scholar]
- 27.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33(1 Pt 2):542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda T, Masaki T, Kakuma T, Yoshimatsu H. Hypothalamic melanocortin system regulates sympathetic nerve activity in brown adipose tissue. Exp Biol Med (Maywood) 2004;229:235–239. doi: 10.1177/153537020422900303. [DOI] [PubMed] [Google Scholar]
- 29.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schurmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O’Rahilly S, Rohner-Jeanrenaud F, Tschop MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117:3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276(6 Pt 2):R1569–1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 31.Bartness TJ, Kay Song C, Shi H, Bowers RR, Foster MT. Brain-adipose tissue cross talk. Proc Nutr Soc. 2005;64:53–64. doi: 10.1079/pns2004409. [DOI] [PubMed] [Google Scholar]
- 32.Dunbar JC, Lu H. Leptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (POMC) products. Brain Res Bull. 1999;50:215–221. doi: 10.1016/s0361-9230(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 33.Dunbar JC, Lu H. Proopiomelanocortin (POMC) products in the central regulation of sympathetic and cardiovascular dynamics: studies on melanocortin and opioid interactions. Peptides. 2000;21:211–217. doi: 10.1016/s0196-9781(99)00192-8. [DOI] [PubMed] [Google Scholar]
- 34.Kuo JJ, Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension. 2003;41(3 Pt 2):768–774. doi: 10.1161/01.HYP.0000048194.97428.1A. [DOI] [PubMed] [Google Scholar]
- 35.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension. 2004;43:1312–1317. doi: 10.1161/01.HYP.0000128421.23499.b9. [DOI] [PubMed] [Google Scholar]
- 36.da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension. 2008;51:884–890. doi: 10.1161/HYPERTENSIONAHA.107.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension. 2005;46:326–332. doi: 10.1161/01.HYP.0000175474.99326.bf. [DOI] [PubMed] [Google Scholar]
- 38.Ni XP, Butler AA, Cone RD, Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24:2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 39.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 40.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. J Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayan H, Ling KT, Lee EY, Wiedemann E, Kalinyak JE, Humphreys MH. Dietary sodium intake modulates pituitary proopiomelanocortin mRNA abundance. Hypertension. 1996;28:244–249. doi: 10.1161/01.hyp.28.2.244. [DOI] [PubMed] [Google Scholar]
- 42.Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111:1251–1258. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harmer SC, Bicknell AB. Role of gamma-MSH peptides in the regulation of adrenal steroidogenesis. Peptides. 2005;26:1944–1951. doi: 10.1016/j.peptides.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Huang QH, Entwistle ML, Alvaro JD, Duman RS, Hruby VJ, Tatro JB. Antipyretic role of endogenous melanocortins mediated by central melanocortin receptors during endotoxin-induced fever. J Neurosci. 1997;17:3343–3351. doi: 10.1523/JNEUROSCI.17-09-03343.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipton JM, Glyn JR, Zimmer JA. ACTH and alpha-melanotropin in central temperature control. Fed Proc. 1981;40:2760–2764. [PubMed] [Google Scholar]
- 46.Zimmer JA, Lipton JM. Central and peripheral injections of ACTH (1–24) reduce fever in adrenalectomized rabbits. Peptides. 1981;2:413–417. doi: 10.1016/s0196-9781(81)80097-6. [DOI] [PubMed] [Google Scholar]
- 47.Catania A. The melanocortin system in leukocyte biology. J Leukoc Biol. 2007;81:383–392. doi: 10.1189/jlb.0706426. [DOI] [PubMed] [Google Scholar]
- 48.Lipton JM, Catania A, Ichiyama T. Marshaling the Anti-Inflammatory Influence of the Neuroimmunomodulator alpha-MSH. News Physiol Sci. 2000;15:192–195. doi: 10.1152/physiologyonline.2000.15.4.192. [DOI] [PubMed] [Google Scholar]
- 49.Bertolini A, Guarini S, Rompianesi E, Ferrari W. Alpha-MSH and other ACTH fragments improve cardiovascular function and survival in experimental hemorrhagic shock. Eur J Pharmacol. 1986;130:19–26. doi: 10.1016/0014-2999(86)90179-2. [DOI] [PubMed] [Google Scholar]
- 50.Noera G, Lamarra M, Guarini S, Bertolini A. Survival rate after early treatment for acute type-A aortic dissection with ACTH-(1–24) Lancet. 2001;358:469–470. doi: 10.1016/S0140-6736(01)05631-8. [DOI] [PubMed] [Google Scholar]
- 51.Schlag G, Redl H, Hallstrom S. The cell in shock: the origin of multiple organ failure. Resuscitation. 1991;21:137–180. doi: 10.1016/0300-9572(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 52.Guarini S, Bazzani C, Cainazzo MM, Mioni C, Ferrazza G, Vergoni AV, Schioth HB, Wikberg JE, Bertolini A. Evidence that melanocortin 4 receptor mediates hemorrhagic shock reversal caused by melanocortin peptides. J Pharmacol Exp Ther. 1999;291:1023–1027. [PubMed] [Google Scholar]
- 53.Guarini S, Cainazzo MM, Giuliani D, Mioni C, Altavilla D, Marini H, Bigiani A, Ghiaroni V, Passaniti M, Leone S, Bazzani C, Caputi AP, Squadrito F, Bertolini A. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63:357–365. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 54.Giuliani D, Mioni C, Bazzani C, Zaffe D, Botticelli AR, Capolongo S, Sabba A, Galantucci M, Iannone A, Grieco P, Novellino E, Colombo G, Tomasi A, Catania A, Guarini S. Selective melanocortin MC4 receptor agonists reverse haemorrhagic shock and prevent multiple organ damage. Br J Pharmacol. 2007;150:595–603. doi: 10.1038/sj.bjp.0707115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bazzani C, Guarini S, Botticelli AR, Zaffe D, Tomasi A, Bini A, Cainazzo MM, Ferrazza G, Mioni C, Bertolini A. Protective effect of melanocortin peptides in rat myocardial ischemia. J Pharmacol Exp Ther. 2001;297:1082–1087. [PubMed] [Google Scholar]
- 56.Bazzani C, Mioni C, Ferrazza G, Cainazzo MM, Bertolini A, Guarini S. Involvement of the central nervous system in the protective effect of melanocortins in myocardial ischaemia/reperfusion injury. Resuscitation. 2002;52:109–115. doi: 10.1016/s0300-9572(01)00436-1. [DOI] [PubMed] [Google Scholar]
- 57.Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, Minutoli L, Bitto A, Marini H, Zaffe D, Botticelli AR, Iannone A, Tomasi A, Bigiani A, Bertolini A, Squadrito F, Guarini S. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- 58.Getting SJ, Di Filippo C, Christian HC, Lam CW, Rossi F, D’Amico M, Perretti M. MC-3 receptor and the inflammatory mechanisms activated in acute myocardial infarct. J Leukoc Biol. 2004;76:845–853. doi: 10.1189/jlb.0306175. [DOI] [PubMed] [Google Scholar]
- 59.Guarini S, Schioth HB, Mioni C, Cainazzo M, Ferrazza G, Giuliani D, Wikberg JE, Bertolini A, Bazzani C. MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:177–182. doi: 10.1007/s00210-002-0572-8. [DOI] [PubMed] [Google Scholar]
- 60.Leoni G, Patel HB, Sampaio AL, Gavins FN, Murray JF, Grieco P, Getting SJ, Perretti M. Inflamed phenotype of the mesenteric microcirculation of melanocortin type 3 receptor-null mice after ischemia-reperfusion. FASEB J. 2008;22:4228–4238. doi: 10.1096/fj.08-113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giuliani D, Mioni C, Altavilla D, Leone S, Bazzani C, Minutoli L, Bitto A, Cainazzo MM, Marini H, Zaffe D, Botticelli AR, Pizzala R, Savio M, Necchi D, Schioth HB, Bertolini A, Squadrito F, Guarini S. Both early and delayed treatment with melanocortin 4 receptor-stimulating melanocortins produces neuroprotection in cerebral ischemia. Endocrinology. 2006;147:1126–1135. doi: 10.1210/en.2005-0692. [DOI] [PubMed] [Google Scholar]
- 62.Forslin Aronsson S, Spulber S, Popescu LM, Winblad B, Post C, Oprica M, Schultzberg M. alpha-Melanocyte-stimulating hormone is neuroprotective in rat global cerebral ischemia. Neuropeptides. 2006;40:65–75. doi: 10.1016/j.npep.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 63.Ottani A, Giuliani D, Mioni C, Galantucci M, Minutoli L, Bitto A, Altavilla D, Zaffe D, Botticelli AR, Squadrito F, Guarini S. Vagus nerve mediates the protective effects of melanocortins against cerebral and systemic damage after ischemic stroke. J Cereb Blood Flow Metab. 2009;29:512–523. doi: 10.1038/jcbfm.2008.140. [DOI] [PubMed] [Google Scholar]
- 64.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 65.Wathion N, European Medicines Agency Public Statement On Acomplia (Rimonabant) [Accessed 20th April 2009; 2009]. EMEA/39457/2009. http://www.emea.europa.eu/humandocs/PDFs/EPAR/acomplia/3945709en.pdf.
- 66.Ferrari W. Behavioural changes in animals after intracisternal injection with adrenocorticotrophic hormone and melanocyte-stimulating hormone. Nature. 1958;181:925–926. doi: 10.1038/181925a0. [DOI] [PubMed] [Google Scholar]
- 67.Rosen RC, Diamond LE, Earle DC, Shadiack AM, Molinoff PB. Evaluation of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in patients with an inadequate response to Viagra. Int J Impot Res. 2004;16:135–142. doi: 10.1038/sj.ijir.3901200. [DOI] [PubMed] [Google Scholar]
- 68.Wikberg JE, Mutulis F. Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction. Nat Rev Drug Discov. 2008;7:307–323. doi: 10.1038/nrd2331. [DOI] [PubMed] [Google Scholar]
- 69.Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, Berndt SI, Jacobs KB, Chanock SJ, Hayes RB, Bergmann S, Bennett AJ, Bingham SA, Bochud M, Brown M, Cauchi S, Connell JM, Cooper C, Smith GD, Day I, Dina C, De S, Dermitzakis ET, Doney AS, Elliott KS, Elliott P, Evans DM, Sadaf Farooqi I, Froguel P, Ghori J, Groves CJ, Gwilliam R, Hadley D, Hall AS, Hattersley AT, Hebebrand J, Heid IM, Lamina C, Gieger C, Illig T, Meitinger T, Wichmann HE, Herrera B, Hinney A, Hunt SE, Jarvelin MR, Johnson T, Jolley JD, Karpe F, Keniry A, Khaw KT, Luben RN, Mangino M, Marchini J, McArdle WL, McGinnis R, Meyre D, Munroe PB, Morris AD, Ness AR, Neville MJ, Nica AC, Ong KK, O’Rahilly S, Owen KR, Palmer CN, Papadakis K, Potter S, Pouta A, Qi L, Randall JC, Rayner NW, Ring SM, Sandhu MS, Scherag A, Sims MA, Song K, Soranzo N, Speliotes EK, Syddall HE, Teichmann SA, Timpson NJ, Tobias JH, Uda M, Vogel CI, Wallace C, Waterworth DM, Weedon MN, Willer CJ, Wraight, Yuan X, Zeggini E, Hirschhorn JN, Strachan DP, Ouwehand WH, Caulfield MJ, Samani NJ, Frayling TM, Vollenweider P, Waeber G, Mooser V, Deloukas P, McCarthy MI, Wareham NJ, Barroso I, Kraft P, Hankinson SE, Hunter DJ, Hu FB, Lyon HN, Voight BF, Ridderstrale M, Groop L, Scheet P, Sanna S, Abecasis GR, Albai G, Nagaraja R, Schlessinger D, Jackson AU, Tuomilehto J, Collins FS, Boehnke M, Mohlke KL. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40(6):768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61:1432–1438. [PubMed] [Google Scholar]