Abstract
Neuregulin 1 (NRG1) is a pleiotropic growth factor involved in diverse aspects of brain development and function. In schizophrenia, expression of the NRG1 type I isoform is selectively increased. However, virtually nothing is known about the roles of this isoform in brain. We have studied transgenic mice over-expressing type I NRG1 (NRG1type 1-tg) using a series of behavioural tests. NRG1type 1-tg mice have a tremor, are impaired on the accelerating rotarod, and have reduced prepulse inhibition in the context of an increased baseline startle response. There is no overall anxiety or activity phenotype, although female NRG1type 1-tg mice show mild increases in anxiety on some measures. The pattern of results shows both similarities and differences to those reported in hypomorphic NRG1 mice, and may be relevant for interpreting the increased NRG1 type I expression seen in schizophrenia.
Keywords: neuregulin, schizophrenia, transgenic, behavioural phenotype
Introduction
Neuregulin 1 (NRG1) is a pleiotropic growth factor of the epidermal growth factor family. It has been investigated extensively with regard to its roles in neuronal and glial development [1]. Most of these studies have focused on the peripheral nervous system, and on early developmental events, but more recently its roles in the brain, and in ongoing aspects of plasticity and function, have come under scrutiny. One reason for this is the evidence implicating NRG1 as a schizophrenia susceptibility gene [2-4], and its involvement in the pathobiology of the disorder [1,5,6].
NRG1 is expressed as multiple isoforms due to different promoters and alternative splicing. A key categorisation is into types I-VI NRG1, defined by their 5′ exon usage [1]. These isoforms differ in their expression and function, for example, with regard to the roles of types I and III NRG1 in myelination [7,8] and in cell migration [9]. In schizophrenia, types I and IV NRG1 appear of most relevance. Type IV NRG1 expression is linked to genetic risk for the disorder [10] and type I NRG1, but not other isoforms, is elevated in the hippocampus [10] and prefrontal cortex [11] of subjects with schizophrenia.
Identification of NRG1 as a possible schizophrenia susceptibility gene has encouraged behavioural and ethological testing of several NRG1 genetic mouse models. The results of these studies show a number of alterations which, broadly speaking, support a role for NRG1 in the schizophrenia phenotype (see Discussion). However, all existing data concern hypomorphs, in which one or more isoform of NRG1 is decreased. Given findings in schizophrenia, a mouse which over-expresses NRG1, especially the type I isoform, is of interest. Such a mouse exists, and exhibits hypermyelination of small diameter axons in the central but not peripheral nervous system [7,8]. Here we report their initial behavioural characterisation.
Materials and Methods
The generation of NRG1type 1-tg mice has been described [7]. The mice used were heterozygous transgenic mice and their wildtype (wt) littermates obtained from the 6th and 7th generation of backcrossing of heterozygous male mice with C57/BL6 females. Animals were genotyped by PCR with primers specific for the transgene insert [7]. Same-sex littermates were group-housed in standard housing in a temperature-controlled room (21±1°C) on a 12hr light:dark cycle (off 19:00, on 07:00). Young adult mice were tested between 09:00-17:00 unless otherwise stated, with order of testing for genotypes and sexes counterbalanced where possible to avoid time of day effects. Animals were tested between 2 and 5 months of age. All experiments were performed in accordance with the UK Animals (Scientific Procedures) Act, 1986. See Supplemental digital content 1 for detailed methods of the behavioural tests.
In situ hybridization
Over-expression in the NRG1type 1-tg mice was measured and confirmed using in situ hybridization with a 35S-labelled riboprobe (Riboprobe system, SP6/T7, Promega), specific to the NRG1 type I transgene on brain sections of mice aged 3 months, with standard procedures (probe sequence available on request).
Motor Tests
Locomotor activity
Locomotor activity was monitored in transparent plastic cages (26 × 16 × 17cm), containing a thin layer of bedding with two photocell beams crossing the bottom of the cage. The total number of beam breaks was recorded in a 2 hour testing period (09:00-11:00). The following animals were tested: wt male, n=9, wt female, n=17, NRG1type 1-tg male, n=12, NRG1type 1-tg female, n=17.
Rotarod
The accelerating rotarod test was performed as described previously [12]. The speed at which the mouse fell from the rod was recorded. Data were analysed with ANCOVA, covarying for testing day body mass.
Anxiety Tests
Light-dark box (start in dark), anxiogenic open field, elevated plus maze, successive alleys
Several ethological, unconditioned tests of anxiety were performed as described [12-14].
Species-typical tests
Nesting and burrowing were assessed as described previously [15,16]. Both procedures began around 2 hours before the dark cycle commenced. Nest construction was scored with established criteria the following morning (~16 hours later; see Supplemental digital content 1). The mass of food pellets burrowed out of a tube was measured after 2 hours.
Prepulse inhibition
Mice were tested for prepulse inhibition of the acoustic startle response. First, there were 10 presentations of a startle tone (105dB, 12kHz). Next there were 10 repetitions of four types of stimulus: (i) startle pulse alone, and a startle pulse preceded by 100ms by a prepulse of either (ii) 64dB, (iii) 68dB or (iv) 76dB (12kHz). The different types of trial were interleaved in a pseudorandom order with a variable inter-trial interval of 20-30s. Baseline acoustic startle response was calculated as the mean response of the 10 trials with no prepulse during the interleaved test trials, and the percentage prepulse inhibition calculated for each prepulse volume.
Statistics
All data were inspected for normality (Kolmogrov-Smirnoff test) and homogeneity of variance (Levene's test). Data meeting these criteria were analysed with ANOVA, with between-subjects factors of genotype and sex. α was set at 0.05. Significant sex*genotype interactions were investigated by simple main effects, with “least significant difference” (LSD) correction. Non-spherical (Mauchly's test) repeated-measures within-subjects effects are reported with Huyn-Feldt correction. The nesting scores for each genotype were compared by a Mann Whitney U-test.
Results
The NRG1type 1-tg mice were viable and bred normally producing offspring at Mendelian ratios, and had comparable body mass to wt littermates between 1-5 months of age (data not shown). The only overt abnormality was a movement-related tremor (as reported previously [7,8]), which was visible from weaning, was similar in both sexes, and did not change in prominence or characteristics with age. The NRG1type 1-tg mice showed robust over-expression of NRG1 type I in the brain, notably in the hippocampus and cerebral cortex, particularly the deeper laminae (Fig. 1A,B). There was also over-expression in some brainstem nuclei, but not in the cerebellum (data not shown). The distribution of signal (i.e. the enhancement over grey versus white matter, and over the principal cell layer of the hippocampus) is indicative of a predominantly neuronal expression of the transgene, as would be predicted given the Thy-1 promoter construct [17].
Figure 1.
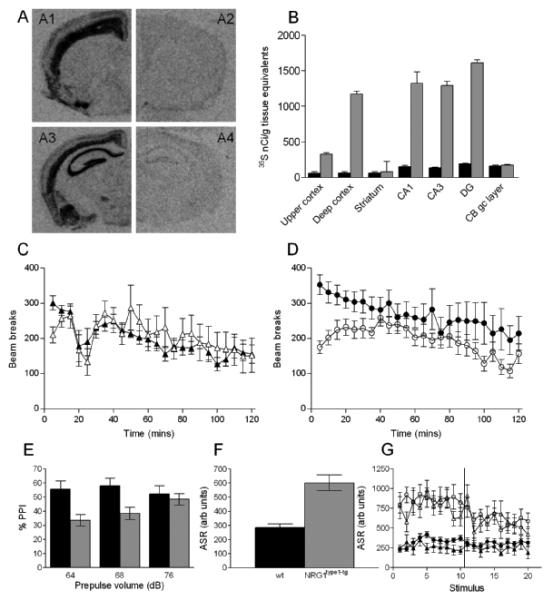
A: In situ hybridization showing expression of type I NRG1 mRNA in NRG1type 1-tg mice (A1, A3) and wildtype mice (A2, A4).
B: Quantitation of the autoradiograms showing relative expression of type I NRG1 mRNA in NRG1type 1-tg mice (grey bars) and wild type mice (black bars). DG: dentate gyrus. CB gc layer: granule cell layer of the cerebellum.
C and D: Locomotor activity monitoring, mean number of beam breaks ± S.E.M. (C) Male wildtype, filled triangles (n=9) and NRG1tyI mice, open triangles (n=12). (D) Female wildtype, filled circles (n=17) and NRG1tyI mice, open circles (n=17).
E - G: Acoustic startle response and prepulse inhibition. E: Mean (±s.e.m.) percent prepulse inhibition (PPI) of the acoustic startle response (total wt mice, black bars, n=24; total NRG1 type 1-tg mice, grey bars n=18). F: Mean (± s.e.m.) acoustic startle response (ASR) from 10 “no prepulse” trials interleaved during the prepulse inhibition testing. G: Baseline startle response shown trial-by-trial, including the first ten trials, which preceded the prepulse inhibition trials. Symbols as in Fig. 1C and D.
Motor tests
Locomotor activity
NRG1type 1-tg mice were not hyperactive (F<1; Fig.1C, D). Mouse activity decreased across the session as the animals habituated to the test environment (block, F(23,1173)=11.49, p<0.001). The change in activity during the test was different between genotypes (genotype*block, F(23,1173)=2.45, p=0.016). NRG1type 1-tg mice were less active than wt mice in the first 25 minutes of the test (blocks 1-3 and 5; all, F(1,51)>3.5, p<0.05), an effect that was more obvious in the female mice, although this did not reach significance (Fig. 1C and 1D).
Rotarod
NRG1type 1-tg mice showed a marked impairment on the rotarod, falling from the rod at a lower speed (Table 1).
Table 1. Results of the basic behavioural screen.
Data are mean ± s.e.m unless otherwise stated. Genotype and sex main effects or interactions only shown if significant (p<0.05). Time in seconds.
| Test and measure | Male wt |
Female wt |
Male NRG1type 1-tg |
Female NRG1type 1- tg |
Statistics |
|---|---|---|---|---|---|
| Rotarod | |||||
| Speed at fall (rpm) | 11.2±1.4 | 13.0±1.0 | 6.4±0.4 | 8.3±0.5 | genotype, F(1,56)=30.19, p<0.001 |
|
| |||||
| Open field | |||||
| Latency to centre | 68.2±7.3 | 39.0±7.9 | 45.9±7.9 | 63.1±14.5 | sex*genotype, F(1,59)=4.23, p=0.044 |
|
| |||||
| Time in central area | 21.5±3.2 | 27.0±5.5 | 21.6±2.2 | 16.6±1.9 | |
|
| |||||
| Total activity (m) | 3.97±0.22 | 4.33±0.35 | 4.50±0.26 | 3.04±0.17 | sex, F(1,59)=6.18, p=0.016 sex*genotype, F(1,59)=9.67, p=0.003 |
|
| |||||
| Light-dark box (start in dark) | |||||
| Latency to cross | 7.9±1.7 | 35.9±6.9 | 14.0±2.1 | 53.2±17.5 | sex, F(1,59)=26.55, p<0.001 |
|
| |||||
| Time in dark | 169.8±9.1 | 203.6±8.1 | 162.7±7.0 | 215.9±10.9 | sex, F(1,59)=20.07, p<0.001 |
|
| |||||
| Total crossings | 21.9±1.5 | 17.5±1.6 | 21.3±1.8 | 13.1±1.7 | sex, F(1,59)=13.35, p=0.001 |
|
| |||||
| Elevated plus maze | |||||
| Time in open | 58±7.6 | 49±4.3 | 41±5.3 | 34±4.4 | |
|
| |||||
| % open entries | 32.5±3.0 | 31.7±2.4 | 27.5±3.1 | 28.7±2.6 | |
|
| |||||
| Total entries | 59.8±4.6 | 65.3±4.9 | 67.2±3.3 | 47.6±5.0 | sex*genotype, F(1,59)=6.32, p=0.015 |
|
| |||||
| Successive Alleys | |||||
| Alley 2 latency | 15±2.6 | 31±4.3 | 15±1.6 | 36±5.6 | sex, F(1,59)=26.95, p<0.001 |
| Alley 3 latency | 44±9.4 | 102±20 | 53±21 | 169±26 | sex, F(1,59)=20.43, p<0.001 |
| Alley 4 latency | 69±20 | 154±21 | 97±28 | 243±5 | genotype, F(1,59)=6.07, p=0.017 sex, F(1,59)=23.60, p<0.001 |
|
| |||||
| Time | |||||
| Alley 1 | 194±7.3 | 214±8.5 | 190±8.9 | 243±4.6 | alley, F(2,177)=920.71, p<0.001 |
| Alley 2 | 69±5.0 | 67±7.7 | 69±5.3 | 46±3.0 | alley*sex, F(2,177)=16.13, p<0.001 |
| Alley 3 | 27±2.4 | 16±1.9 | 27±3.6 | 12±2.1 | |
| Alley 4 | 17±1.9 | 9.4±1.8 | 21±3.6 | 4.2±1.9 | |
|
| |||||
| Total entries | |||||
| Alley 1 | 9.7±1.0 | 9.0±0.8 | 8.6±0.7 | 5.1±0.6 | genotype, F(1,59)=17.86, p<0.001 |
| Alley 2 | 14.9±1.5 | 12.4±1.0 | 12.4±1.2 | 6.4±0.8 | sex, F(1,59)=17.87, p<0.001 |
| Alley 3 | 8.0±0.9 | 4.9±0.7 | 5.5±0.8 | 1.7±0.4 | alley, F(3,177)=274.69, p<0.001 |
| Alley 4 | 3.3±0.4 | 1.9±0.4 | 1.7±0.3 | 0.5±0.2 | alley*genotype, F(3,177)=5.31, p=0.008 alley*sex, F(3,177)=6.55, p=0.003 |
|
| |||||
| Species typical | |||||
| Nesting (median score) |
4 | 4 | 2 | 1 | genotype,U=9.5, p<0.001 |
|
| |||||
| Burrowing (mass burrowed) |
124±24 | 58±29 | 15±14 | 13±7 | genotype, F(1,24)=14.30, p=0.001 |
Anxiety tests
See Supplemental digital content 2 for detailed results of anxiety and motor tests.
Light-dark box (start in dark)
There was no effect of genotype, nor genotype*sex interaction for any measure. Female mice had a greater latency to leave the dark section, spent more time in the dark section, and made fewer crossings between the dark and light sections than male mice, irrespective of genotype (Table 1).
Open field
The latency to enter the central, most anxiogenic region was not different according to genotype or sex, but there was a sex*genotype interaction (Table 1), due to the transgene having opposite effects in males and females. However, investigation with simple main effects showed no significant difference between male wt and male NRG1type 1-tg mice (F(1,59)=1.56, p=0.216) and only a trend towards increased latency in female NRG1type 1-tg compared to female wt mice (F(1,59)=3.02, p=0.087). There were no significant differences between genotypes or sexes in the time spent in the central region. Total activity was lower in female than male mice, and there was a sex*genotype interaction (Table 1) which arose because female NRG1type 1-tg mice were less active than both female wt (F(1,59)=13.62, p<0.001) and male NRG1type 1-tg mice (F(1,59)=16.34, p<0.001).
Elevated plus maze
The time in the open arms and percentage of entries made into the open arms was not different according to genotype or sex (Table 1). Total activity (measured by the total number of entries into any section) showed no main effect of genotype or sex, but a sex*genotype interaction (Table 1). Investigation with simple main effects showed that female NRG1type 1-tg mice made fewer arm entries than female wt mice (F(1,59)=8.25, p=0.006), and compared to male NRG1type 1-tg mice (F(1,59)=8.09, p=0.006).
Successive Alleys
The latency to enter the most aversive alley (Alley 4) was greater in NRG1type 1-tg mice than wt mice (Table 1). Female mice had greater latencies to enter the more aversive alleys than male mice (Table 1). All mice spent more time in the less aversive alleys (Table 1). There was an alley*sex interaction, which was because female mice spent more time in the first alley (F(1,59)=21.43, p<0.001) and less time in the third and fourth alleys (both, F(1,59)>26, p<0.001).
The total number of entries into each alley was greater in wt mice than NRG1type 1-tg mice, and in male mice than female mice (Table 1). There were fewer entries into the more anxiogenic alleys and a significant alley*genotype interaction (Table 1). Wt mice made more entries into all of the arms than NRG1type 1-tg mice (all alleys, F(1,59)>10, p<0.002). Both wt and NRG1type 1-tg mice made more entries into the less aversive alleys than the more aversive alleys (effect of alley, both genotypes, F(3,57)>55, p<0.001).
Species-typical tests
NRG1type 1-tg mice built less structured nests than wt mice and burrowed out a lower mass of pellets from a tunnel (Table 1).
Prepulse inhibition
Prepulse inhibition was impaired in NRG1type 1-tg mice (Fig. 1E; main effect of genotype, F(1,38)=4.98, p=0.032). The impairment was present with prepulse volumes of 64dB and 68dB, but not at 76dB (genotype × prepulse volume, F(2,76)=5.09, p=0.011; investigated by simple main effects: 64dB, F(1,38)=9.09, p=0.005; 68dB, F(1,38)=7.20, p=0.011; 76dB, F(1,38)=0.06, p=0.810). However, the baseline acoustic startle response was greater in NRG1type 1-tg mice (Fig. 1F; F(1,38)=28.58, p<0.001), evident from the first exposure to the startle stimulus (Fig. 1G).
Discussion
We studied the behaviour of mice over-expressing the type I isoform of NRG1 and found several differences from their wt littermates. We confirmed the presence of a tremor [7], which we assume may be a manifestation of their altered central myelination [8], and which in turn underlies the poor performance of the NRG1type 1-tg mice on the rotarod (Table 1). NRG1type 1-tg mice have decreased prepulse inhibition, in the context of an increased acoustic startle response (Fig. 1E-G). No consistent anxiety phenotype is seen across tests, but there is some indication of increased anxiety in female NRG1type 1-tg mice (Table 1). Table 2 summarises the present results and compares them with those found in the four NRG1 genetically modified mice studied previously.
Table 2. Behavioural phenotypes of NRG1 mouse mutants.
Heterozygous mutants with deletion of the epidermal growth factor (EGF) domain (NRG-EGF+/−), the trans-membrane domain (NRG1-TM+/−), the immunoglobulin domain of types I and II NRG1 (NRG1-Ig+/−), or with disruption of the cysteine-rich domain of type III NRG1 (NRG1-CRD+/−). OFT, open field test; EPM, elevated plus maze; LD, light-dark box; HB, holeboard; TM, RW, running wheel; CM, cross maze; SA, successive alleys; PPI, prepulse inhibition; LI, latent inhibition; ASR, acoustic startle response. See Supplemental digital content 3 for supplementary references S1-S7. Table adapted from [25] and ref. S7.
| Behaviour | NRG1-EGF+/− | NRG1-TM+/− | NRG1-Ig+/− | NRG1-CRD+/− | NRG1type 1-tg |
|---|---|---|---|---|---|
|
Locomotor activity |
Hyperactive (OFT, EPM, LD, HB). |
Hyperactive (OFT, HB, LD). |
Normal (RW). |
Normal (OFT). |
Generally normal but hypoactive initially. |
|
Motor tasks |
Normal horizontal bar. Normal rotarod, but increased learning with training. Normal marble burying. |
Normal fine motor skills (chewing, rearing to wall). |
Normal grip strength. Normal rotarod. |
- | Impaired on rotarod. Species-specific skills impaired. |
| Anxiety | Normal OFT thigmotaxis. Normal LD and EPM. |
Increased entries in all arms of EPM. Decreased anxiety in OFT. Normal LD, EPM. |
- | - | Slight increase, in females only (OFT, EPM, SA). |
| PPI | Normal PPI. | Impaired PPI [2]. Normal PPI and ASR [27,31]. |
Normal PPI. Impaired LI. |
Impaired PPI. Normal ASR. |
Impaired PPI. Increased ASR. |
| References | [2,7,25, S1] | [2,18,19,S2-S4] | [S5,S6] | [S7] |
In the four anxiety tests used, there were no main effects of genotype in any of the anxiety-related measures (latency to enter, and time spent in, anxiogenic areas). However, there were genotype*sex interactions, in the anxiogenic open field and successive alleys, for latency to enter the more anxiogenic sections, because female NRG1type 1-tg mice were slower than the other groups. Hence there was some suggestion of increased anxiety in female NRG1type 1-tg mice, but this was not consistently found in all tests, and may be secondary to changes in locomotor activity or initiation of movement. That is, the NRG1type 1-tg mice, especially females, were less active during the initial period of testing in the photocell activity cages, and in the successive alleys test (in terms of total crossings). There were also genotype*sex interactions in activity measures in the anxiogenic open field and elevated plus maze, largely driven by decreased activity in female NRG1type 1-tg mice. Notably, sex differences were reported in NRG1-TM+/− mice (Table 2) in terms of their habituation of exploratory behaviour [18], but not in the elevated plus maze or light-dark box [19].
The prepulse inhibition deficit in the NRG1type 1-tg mice is potentially of interest (Fig. 1E), as decreased prepulse inhibition is a frequent finding in rodent models of schizophrenia – including some NRG1 hypomorphs (Table 2) – and in schizophrenia itself. As such, the present finding is in keeping with the proposed involvement of type I NRG1 in the disorder. However, the marked baseline difference in acoustic startle response (Fig. 1F, G) makes the difference difficult to interpret [20].
Finally, comparing under- with over-expressing mice might be expected to show either a gene dosage effect, such that reciprocal changes from wt mice are seen, or an ‘inverted U’, such that similar changes are seen in both types of mutant compared to wt. An inverted U has been hypothesised for NRG1 effects on synaptic plasticity [21]. Table 2 suggests that neither simple model fits the behavioural data. For example, the hypoactive phenotype in female NRG1type 1-tg mice contrasts with the hyperactive female NRG1-TM+/− mice, whereas both lines, as well as NRG1-CRD+/− mice, show impaired prepulse inhibition. Clearly, such comparisons are limited since the different isoforms affected in the hypomorphs are confounded with the direction of the genetic manipulation. Nevertheless, it is notable that there is a similarly complex pattern of results for multiple types of mutant DISC-1 mice [22], DISC-1 being another schizophrenia susceptibility gene. These data emphasise the unpredictability of phenotypes observed in mice wherein different modifications of a gene have been made, and hinder extrapolation back to complex human phenotypes such as schizophrenia, especially when the mouse models are only crude approximations to the genetic alteration being modelled in the first place [23,24].
Conclusions
NRG1type 1-tg mice are impaired on the rotarod, and show decreased prepulse inhibition against a background of an increased startle response. There is no consistent anxiety or activity phenotype, though there is some evidence that female NRG1type 1-tg mice exhibit increased anxiety, accompanied by reduced activity. The results indicate that there are behavioural consequences of over-expressing type I NRG1, which may be relevant to the role that NRG1 plays in brain disorders such as schizophrenia.
Supplementary Material
Acknowledgements
We thank Phil Burnet, Rob Deacon, Sharon Eastwood, Amy Taylor and Mary Walker for their contributions. IHD and DMB are supported by the Wellcome Trust. AJL is supported by the UK Medical Research Council. MHS and KAN acknowledge grant support from the National Multiple Sclerosis Society and the Deutsche Forschungsgemeinschaft (CMPB).
References
- 1.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li DW, Collier DA, He L. Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet. 2006;15:1995–2002. doi: 10.1093/hmg/ddl122. [DOI] [PubMed] [Google Scholar]
- 4.Keri S, Kiss I, Kelemen O. Effects of a neuregulin 1 variant on conversion to schizophrenia and schizophreniform disorder in people at high risk for psychosis. Mol Psychiatry. 2009;14:118–119. doi: 10.1038/mp.2008.1. [DOI] [PubMed] [Google Scholar]
- 5.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: Genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nature neuroscience. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- 7.Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Mueller T, Wende H, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, et al. Short- and long-range attraction of cortical GABAergic interneurons by Neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- 12.Contet C, Rawlins JN, Deacon RM. A comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: implications for the study of genetically modified mice. Behav Brain Res. 2001;124:33–46. doi: 10.1016/s0166-4328(01)00231-5. [DOI] [PubMed] [Google Scholar]
- 13.Deacon RM, Rawlins JN. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav Brain Res. 2005;156:241–249. doi: 10.1016/j.bbr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Solberg LC, Valdar W, Gauguier D, Nunez G, Taylor A, Burnett S, et al. A protocol for high-throughput phenotyping, suitable for quantitative trait analysis in mice. Mamm Genome. 2006;17:129–146. doi: 10.1007/s00335-005-0112-1. [DOI] [PubMed] [Google Scholar]
- 15.Deacon RMJ. Assessing nest building in mice. Nat Protocols. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 16.Deacon RMJ. Burrowing in rodents: a sensitive method for detecting behavioral dysfunction. Nat Protocols. 2006;1:118–121. doi: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- 17.Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Meth. 1997;71:3–9. doi: 10.1016/s0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- 18.O'Tuathaigh CM, O'Sullivan GJ, Kinsella A, Harvey RP, Tighe O, Croke WT, et al. Sexually dimorphic changes in the exploratory and habituation profiles of heterozygous neuregulin-1 knockout mice. NeuroReport. 2006;17:79–83. doi: 10.1097/01.wnr.0000192738.31029.0a. [DOI] [PubMed] [Google Scholar]
- 19.O'Tuathaigh CM, O'Connor AM, O'Sullivan GJ, Lai D, Harvey R, Croke DT, et al. Disruption to social dyadic interactions but not emotional/anxiety-related behaviour in mice with heterozygous ‘knockout’ of the schizophrenia risk gene neuregulin-1. Prog Neuropsychopharm Biol Psychiatry. 2008;32:462–466. doi: 10.1016/j.pnpbp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Role LW, Talmage DA. Neurobiology - New order for thought disorders. Nature. 2007;448:263–265. doi: 10.1038/448263a. [DOI] [PubMed] [Google Scholar]
- 22.Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: from hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Duffy L, Cappas E, Schofield PR, Scimone A, Karl T. Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci. 2008;122:748–759. doi: 10.1037/0735-7044.122.4.748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


