Abstract
Background
Limited information exists about effects of different highly active antiretroviral therapy (HAART) regimens and duration of regimens on mother-to-child transmission (MTCT) of HIV among women in Africa who start treatment for advanced immunosuppression.
Methods
Between January 2004 to August 2008, 1,142 women were followed at antenatal antiretroviral clinics in Johannesburg. Predictors of MTCT (positive infant HIV DNA PCR at 4-6 weeks) were assessed with multivariate logistic regression.
Results
Mean age was 30.2 years (SD=5.0) and median baseline CD4 count was 161 cells/mm3 (SD=84.3). HAART duration at time of delivery was a mean 10.7 weeks (SD=7.4) for the 85% of women who initiated treatment during pregnancy and 93.4 weeks (SD=37.7) for those who became pregnant on HAART. Overall MTCT rate was 4.9% (43/874), with no differences detected between HAART regimens. MTCT rates were lower in women who became pregnant on HAART than those initiating HAART during pregnancy (0.7% versus 5.7%; p=0.01). In the latter group, each additional week of treatment reduced odds of transmission by 8% (95% CI: 0.87-0.99, p=0.02).
Conclusion
Late initiation of HAART is associated with increased risk of MTCT. Strategies are needed to facilitate earlier identification of HIV-infected women.
Keywords: HIV/AIDS, pregnancy, highly active antiretroviral therapy, mother-to-child transmission, integration, South Africa
Introduction
Mother-to-child transmission (MTCT) rates for HIV-infected pregnant women in Africa vary widely depending on access to and type of antiretroviral therapy (ART)(1-4). Throughout resource-rich countries, and in middle-income countries such as Brazil, triple-drug combination highly active antiretroviral therapy (HAART) is the standard of care for prophylaxis in women with CD4 cell counts above current thresholds for treatment(5). In South Africa, HAART in the public sector is reserved for pregnant women with advanced immunosuppression (CD4 count < 200 cells/mm3), and evidence on MTCT among this subset of women is lacking. In a variety of settings outside of Africa, women with a range of CD4 cell counts who receive HAART have rates of infant HIV infection less than 1-2%(6-8). As HIV testing in antenatal clinics is a common entry point to care in South Africa, women with advanced HIV infection are often only identified in pregnancy and initiated on therapy late in gestation, with consequent high rates of HIV transmission to infants(9).
South Africa faces one of the most serious HIV epidemics, with an estimated 3.2 million women with HIV infection, a national antenatal prevalence of 29.3%, and slow uptake of services for the prevention of MTCT(10, 11). The South African HIV program has utilized maternal and infant single-dose nevirapine (sdNVP) and more recently transitioned to the use of short-course regimens with zidovudine and nevirapine for prophylaxis in pregnancy(12). Only those women with CD4 counts <200 cells/mm3 or World Health Organization Stage 4 classification qualify for HAART (13). For this latter group, the effect of specific HAART regimen and the duration of regimen on MTCT is not well defined.
HIV care in South Africa has traditionally been delivered separately from antenatal services, creating barriers to care for women of reproductive age, particularly those diagnosed with HIV during pregnancy. We previously reported on a novel health delivery strategy of integrated antenatal and antiretroviral clinics (ANC-ARV clinics) at Charlotte Maxeke Johannesburg Academic Hospital and Rahima Moosa Mother and Child Hospitals in Johannesburg, designed to expedite initiation of HAART in pregnant women with advanced immunosuppression, to improve maternal health in these women, and to prevent infant HIV infection(14, 15). Since publication of our experience, a number of ANC-ARV clinics have been implemented in the region. We now report on MTCT outcomes from a combined prospective cohort from these sites. In particular, we focus on effects of HAART regimen and duration of therapy on rates of MTCT.
Methods
The ANC-ARV clinics receive referrals from approximately 28 clinics in Johannesburg city and the surrounding suburbs. HIV-infected pregnant women are referred with indications for HAART including CD4 cell counts < 250 cells/ mm3 (increased from 200 cells/mm3 early in the program) or World Health Organization Stage 4 classification, poor obstetric history, or conditions requiring amniocentesis. Women attending ANC-ARV clinics constitute a prospective convenience cohort, in which HIV-infected women are enrolled and are observed in accordance with standard clinical and laboratory practice of the South African National HIV program(16). The primary outcome of interest for the cohort is infant HIV status.
Women followed at these clinics from January 2004 to August 2008 were included if they were pregnant, had a positive HIV test, and a baseline CD4 count <250 cells/mm3 or another indication for treatment with HAART. Inclusion was open to women attending the clinic between January 2004 and August 2008 who had received prior sdNVP, those who became pregnant on HAART, as well as those who initiated HAART during pregnancy. Women were followed at the ANC-ARV clinics throughout pregnancy until 4-6 weeks postpartum when infant HIV testing was performed (HIV-1 DNA Roche Amplicor). After infant HIV status was determined, women and infants were referred to HIV clinics within the community for long-term follow-up.
Data collected at each visit was entered in an ACCESS database (Microsoft 2003) and included demographics, laboratory values, HAART regimens, and pregnancy complications. Prolonged rupture of membranes (PROM) was defined by duration greater than 12 hours. Data on maternal mortality (both related and unrelated to pregnancy) have been published previously by the Charlotte Maxeke Johannesburg Academic Hospital(17). Maternal outcome data were not recorded by the Rahima Moosa Mother and Child Hospital. Caesarian section is not performed for prevention of MTCT in South Africa but is done for obstetric indications only. When available, details of mode of delivery, infant weight, and infant HIV status were recorded. CD4 cell counts were collected at baseline and per South African HIV Guidelines monitored every 6 months. In the study period, HIV viral load was not routinely monitored and is therefore not included in analysis. Data from ACCESS was transferred into STATA (version 9; StataCorp, College Station, TX).
Univariate and multivariate logistic regression were used to determine predictors of infant HIV infection among women who started HAART during pregnancy. HIV infection in one or more infants of a multiple birth was counted as a single transmission. Variables associated with outcome (p<0.15) were included in the initial model and retained if their removal markedly altered the model fit. Regimen duration and type were forced into the model. In this same group, Student's t-tests and chi square tests were performed to determine characteristics of women and infants with incomplete information. Differences were also assessed in women who became pregnant on HAART and those who started HAART during pregnancy. Fisher's exact test was utilized for analysis of categorical variables with sparse data and Wilcoxan rank sum was performed when data were skewed. The study was approved by the institutional Ethics Committee of the University of the Witwatersrand (protocol number M070438/M050445) and exemption was given by the University of California, Los Angeles, Internal Review Board. Verbal assent was given by women at the Charlotte Maxeke Johannesburg Academic Hospital, and written informed consent was obtained from women at the Rahima Moosa Mother and Child Hospital.
Results
Maternal Characteristics
Data are reported on all women referred to the ANC-ARV clinics with known HAART regimen and duration of regimen prior to infant delivery (n=1142). From these women, 873 infants (including 19 sets of twins and 1 set of triplets) had HIV status determined at 4-6 weeks of life. In the remainder of pregnancies either the mother and/or mother-infant pair was lost to follow-up or the pregnancy resulted in stillbirth (Figure 1).
Figure 1.
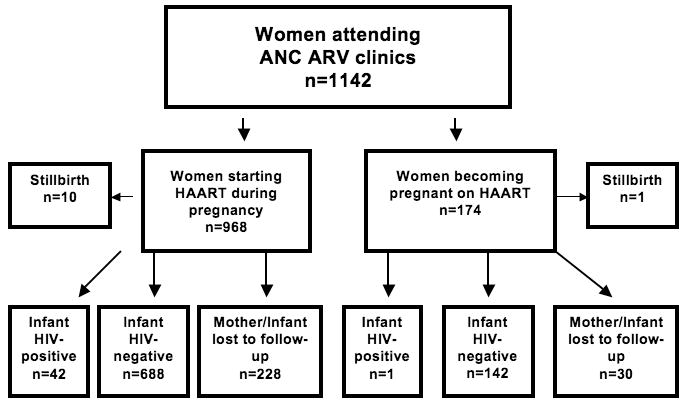
Pregnancy and infant outcomes of women attending ANC ARV clinics at the Charlotte Maxeke Johannesburg Academic Hospital and Rahima Moosa Mother and Child Hospital in Johannesburg.
The mean age of women was 30.2 years (SD=5.0) and among those with race reported (n=348) 98.3% were black African. The majority of women (85.2%) were started on HAART during pregnancy with the remainder (14.8%) conceiving while on therapy. The mean number of prior pregnancies per woman was 2.6 (SD=1.2). Pregnancy was planned in 31.4% of women starting HAART during pregnancy and 28.6% who become pregnant on HAART. The median baseline CD4+ cell count for 875 women in whom this was available was 161.0 cells/mm3 (SD=84.3), with 76.0% of women in the cohort below 200 cells/mm3. Syphilis was the only sexually transmitted infection routinely screened in this cohort, with 3.1% testing positive. The most common non-infectious medical co-morbidity was hypertension (defined a single systolic >160 mm Hg and/or diastolic >90 mm Hg on a two occasions separated by 4 hours or a single diastolic >110 mm/Hg) with a prevalence of 9.4%. Diabetes, defined as a random blood glucose >11 millimoles/liter or a positive glucose tolerance test, was rare with 0.6% of women having a diagnosis categorized as either chronic or gestational. Use of tobacco and alcohol in pregnancy were uncommon among women in whom data was available (n=769), with a rate of 3.5% for each behavior. Nine women reported both tobacco and alcohol use.
Among those who started HAART during pregnancy, the mean duration of therapy before childbirth was 10.7 weeks (SD=7.4), and the most common regimen was lopinavir/ritonavir-based (51.2%), followed by nevirapine (43.1%), and efavirenz (5.7%). Among those who became pregnant on HAART the mean number of weeks on therapy prior to delivery was 93.4 (SD=37.7, range 38.0-196.9 weeks), and the most common regimen was efavirenz-based (53.6%), followed by nevirapine (28.6%), and lopinavir/ritonavir (17.9%). The most frequently utilized nucleoside backbone in all women was stavudine and lamivudine (97.3%). Of women who conceived on efavirenz, six were switched to alternate HAART regimens for the remainder of pregnancy, and were categorized in the analysis by the new regimen. Data on prior sdNVP was only available in 294 women with 1.4% reporting exposure.
Comparisons of women who started HAART during pregnancy versus those who became pregnant on therapy were performed and showed that the latter group had higher pre-HAART baseline CD4 cell counts (155.5 versus 187.7cells/mm3 respectively), a higher proportion of women on efavirenz-based HAART, and a higher proportion of low birth weight infants. The groups were comparable in regard to age, gravidity, PROM, and proportion stating that pregnancy was planned.
Delivery Characteristics and HIV Transmission Rates
From the cohort of 1,142 women, a total of 258 had unknown infant HIV status. Among women starting HAART during pregnancy there were ten stillbirths, as compared to women on HAART prior to pregnancy, in whom a single stillbirth occurred. PROM (prolonged rupture of membranes >12 hours) was documented in 3.4% (n=20) of 596 women with data available. Among 396 women with detailed delivery information available, 75.3% (n=298) had normal vaginal deliveries; 10.1% (n=40) had scheduled cesarean sections for reasons unrelated to HIV; and 14.6% (n=58) had urgent cesarean section for preeclampsia/eclampsia, fetal distress, placental abruption, or placenta previa. The majority of infants received replacement feeding (96.9%).
The overall MTCT rate for the cohort with infant PCR data available was 4.9% (43/873). Among the 143 women who became pregnant on HAART and had infant PCR results, there was a single transmission event in a woman who delivered at 31 weeks gestational age. Women who became pregnant on HAART had significantly lower MTCT rates than women who initiated HAART during pregnancy (0.7% versus 5.7%; p=0.01; 95% CI 0.02-3.8 and 4.2-7.7, respectively). For those who started HAART during pregnancy, transmission decreased with longer duration of HAART: 9.3% (14/151) with less than 4 weeks of HAART during pregnancy, 5.5% (23/422) with 4-16 weeks of HAART, and 3.5% with >16 to 32 weeks. Of note, there were no transmissions among women who were on HAART for more than 32 weeks prior to delivery. Women with CD4 cell counts >250 cells/mm3 within the same clinics who received only sdNVP had an MTCT rate of 7.9% (121/1534; 95% CI 6.6-9.3) and women presenting to the clinic system during or just after infant delivery with no prophylactic ART had an MTCT rate of 17.4% (4/23; 95% CI 5.0-38.8). Figure 2 summarizes infant HIV transmission rates in women in the cohort as well as the sdNVP and no maternal prophylaxis groups.
Figure 2.
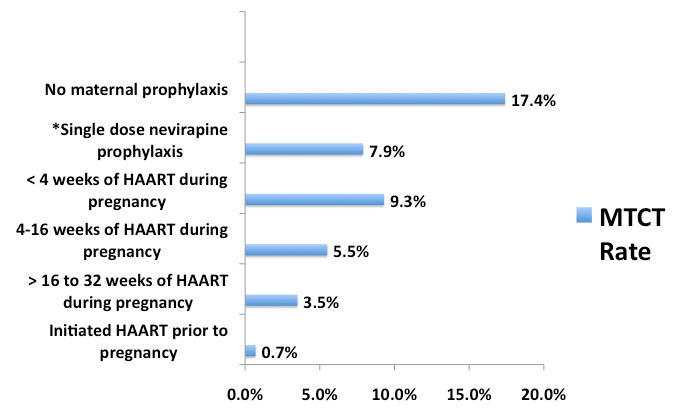
Risk of mother-to-child transmission (infant HIV DNA positive at 4-6 weeks) among women receiving HAART before or during pregnancy compared to those receiving single-dose nevirapine or no maternal prophylaxis at the Charlotte Maxeke Johannesburg Academic Hospital and Rahima Moosa Mother and Child Hospital.
* all women receiving single dose nevirapine had CD4 counts ≥ 250 cells/mm3
Characteristics of Women Whose Infants Have Unknown HIV Status
Characteristics of women who remained in follow-up compared to those who were lost to follow-up were evaluated. No differences were detected in maternal demographic and health status factors including age, baseline CD4 cell count, and gravidity. There were also no differences noted in HAART regimen, the duration of HAART during pregnancy, PROM, birthweight, or proportion of low birth weight (<2.5 kg) infants.
Predictors of Transmission in Women Initiating HAART During Pregnancy
The majority of MTCT occurred among women starting HAART during pregnancy. We performed regression analysis for this group of 730 women (Table 1). In univariate analysis, HAART duration was predictive of infant HIV status, with every additional week of therapy reducing the odds of transmission by 7% (95% CI: 0.87- 0.99, p=0.02). In a multivariate model that included baseline CD4 cell count and HAART regimen (NNRTI versus PI), duration of therapy during pregnancy remained significant (OR 0.92, 95% CI: 0.87-0.99, p=0.02). PROM was associated with MTCT in univariate analysis with an OR of 5.5 (95% CI 1.8-17.7, p = 0.002); however, in the multivariate model with HAART duration, regimen, and baseline CD4 cell count significance was not retained (OR=3.8, p=0.08). Among the subset of patients with baseline CD4 data (n=553), an increase of 50 cells/mm3 in baseline CD4 cell count was associated with 26% reduced odds of MTCT (95% CI: 0.56-0.99, p=0.045).
Table 1. Univariate analysis of factors associated with mother-to-child transmission in women starting HAART during pregnancy.
| Variable category | Variable | Women with an HIV-uninfected infant | Women with an HIV-infected infant | Univariate odds ratio (95% CI) | ˆp value |
|---|---|---|---|---|---|
| Maternal demographics and health status | Maternal age, mean y (SD) (n) | 30.3 (4.8) (n=640) | 30.8 (5.5) (n=40) | 1.02 (0.96-1.09) | 0.54 |
| CD4 count during pregnancy, median cells/uL (SD) | 156.8 (73.9) (n=523) | 130.4 (64.5) (n=30) | *0.74 (0.56-0.99) | 0.045 | |
| Gravidity, mean: SD | 2.6 1.2 (n=547) | 2.6 1.1 (n=33) | 0.97 (0.72-1.31) | 0.85 | |
| Antiretroviral treatment | ART regimen n/N (%) | ||||
| nevirapine-based | 295/688 (42.9%) | 20/42 (47.6%) | 1.0 | — | |
| efavirenz-based | 40/688 (5.8%) | 2/42 (4.8%) | 0.74 (0.17-3.27) | 0.69 | |
| lopinavir/ritonavir-based | 353/688 (51.3%) | 20/42 (47.6%) | 0.84 (0.44-1.58) | 0.58 | |
| Time from ART initiation to childbirth, mean weeks (SD) | 10.8 (7.6) (n=688) | 7.6 (6.7) (n=42) | 0.93 (0.88-0.98) | 0.01 | |
| Childbirth and newborn characteristics | Prolonged rupture of membranes > 12 hours n/N (%) | 12/411 (2.9%) | 4/28 (14.3%) | 5.5 (1.76-17.7) | 0.002 |
| Female infant: n/N (%) | 148/281 (52.7%) | 10/18 (55.6%) | 1.12 (0.43-2.93) | 0.81 | |
| Mean birth weight, kg (sd) (n) | 2.93 (0.55) (n=632) | 3.01 (0.68) (n=37) | 1.28 (0.71-2.32) | 0.41 | |
| Low birth weight: n/N<2.5 kg (sd) (%) | 112/632 (17.7%) | 7/37 (18.9%) | 1.08 (0.46-2.52) | 0.85 | |
Expressed as per 50 cells/uL CD4 change.
p-values determined by Student's t-test, Chi-square test, Fisher's exact, or Wilcoxan rank sum, as appropriate.
HAART regimen was not associated with infant HIV status. There were no HIV transmissions among the small subgroup of women who had prior single dose nevirapine exposure. No associations were detected for infant HIV status and maternal age, gravidity, hypertension, alcohol, tobacco, female infant, mean infant birth weight, and low birth weight.
Discussion
Duration of HAART and MTCT Among Women With Advanced Immunosuppression
In our cohort of women from Johannesburg with advanced immunosuppression, rates of MTCT are high relative to other countries where HAART is given to all women regardless of CD4 cell count, and are also high compared to recent African studies. In West Africa, women qualifying for HAART during pregnancy had an MTCT rate of 2.3% at 12 months, half our 4-6 week rate of 4.9%(18). The mean treatment duration was 11.7 weeks, similar to our cohort, and the majority (87%) were treated with a nevirapine-based HAART. In the DREAM cohort from Malawi, Mozambique, and Tanzania, women on HAART from 25 weeks of gestational age also had lower MTCT rates, ranging from 0.8-1.2% at one-month(4). Reasons for higher rates of transmission in our setting are unclear but may be related to the slightly lower mean duration of HAART (10.7 weeks) or higher proportion of women with very low CD4 counts (75.4%<200 cells/mm3 and 21.3%<100 cells/mm3). It is notable that women in our cohort who had ≤ 12 weeks of HAART had the same transmission rate as those within the same clinic who received only sdNVP (7.4%), and both groups had significantly lower rates of transmission compared to a group of women who delivered infants with no form of ART prophylaxis (17.4%).
In a French Perinatal cohort (ANRS), only duration of HAART was associated with transmission in women with term deliveries and viral load <400 copies/ml, with an OR for duration of 0.94/week (CI 0.90-0.99), similar to the OR found in our cohort (0.92, 95% CI: 0.87-0.99). Additionally, a subset of women in the ANRS study were noted to transmit to infants with viral load <50 copies/ml. All of these women started HAART at ≥ 32 weeks, suggesting in utero transmission or perinatal transmission due to another factor such as compartmentalized replication in the genital tract. Regardless of setting and resource availability, large benefits can be gained from each additional week of HAART during pregnancy and this finding highlights the need to screen women for HIV prior to pregnancy, and to reach women and provide a healthcare service that can expedite entry into care for both antenatal and HIV services.
In our study it is likely that duration of HAART serves as a proxy for HIV viral load at time of delivery, as viral suppression typically occurs after 10-16 weeks of HAART in non-pregnant adults, and plasma viral load has been shown to be the preeminent risk factor for MTCT(19-23). In research settings, women with viral load <50 copies/mm3 and term deliveries have MTCT rates as low as 0.4%(20). Routine viral load monitoring is not recommended in the public sector in resource-constrained settings(24). Therefore, identifying other factors that can be used to risk stratify pregnant women is critical.
In our cohort, compared to women who conceived on HAART, women starting therapy during pregnancy had markedly increased risk of MTCT (0.7% versus 5.7%). Other studies have shown low rates of MTCT in women who become pregnant on HAART including the European Collaborative study in which the transmission rate was 0.3% (1/397) in women who became pregnant on HAART versus 1.9% (10/521) among women who started HAART during pregnancy(19). Similar findings were reported from the United Kingdom where a single transmission occurred in women on HAART before pregnancy (0.1%, 1/928), significantly lower than those who started during pregnancy (1.3%, 39/2967) (25).
HAART Regimen and MTCT Among Women With Advanced Immunosuppression
Little data are available about the efficacy of PI versus NNRTI therapy for prevention of MTCT in women with advanced disease starting HAART during pregnancy, particularly in African settings where differences in viral clade and underlying host genetics may influence antiretroviral responses. In South Africa, efavirenz is utilized in the first-line HAART regimen, and both nevirapine and lopinavir/ritonavir are available for use in pregnant women who qualify for HAART(16). Since efavirenz has been used as first-line therapy in South Africa, a number of women in this cohort conceived on an efavirenz-containing regimen. Data regarding infant outcomes in this cohort of women becoming pregnant on antiretrovirals is undergoing analysis and will contribute to the growing literature on infant outcomes in women becoming pregnant on HAART(26-28). Given toxicity of nevirapine for women with higher CD4 counts, research studies comparing PI to NNRTI regimens are limited to women with advanced immunosuppression such as those in ANC-ARV clinics. In our cohort, MTCT rates were comparable regardless of HAART regimen. Our study was not specifically powered to detect differences in HAART regimen, and while the data suggest no specific regimen is superior for preventing infant transmission, small numbers limit the ability to interpret these findings.
The majority of studies have shown no difference in transmission by HAART regimen among pregnant women from a variety of settings(20, 25), however few adequately powered randomized studies have been conducted. A single publication from Europe demonstrated that pregnant women on nevirapine-based HAART had shorter time to viral suppression compared to women on PI(29). Most women in this cohort were on nelfinavir, which has been found to be less potent than lopinavir/ritonavir(30), the sole PI utilized in our cohort. Additionally, there have been concerns about sub-therapeutic PI levels in the third trimester, specifically for nelfinavir(31-33), and this issue may explain the less favorable viral kinetics seen compared to nevirapine in this trial. A number of studies have demonstrated nevirapine resistance after exposure to single dose regimens for prophylaxis(34-36) and a recent trial from Africa revealed that women exposed to sdNVP who were subsequently placed on nevirapine-based HAART had significantly higher rates of treatment failure(37). Prior sdNVP exposure was uncommon in our population due to the high rate of women diagnosed with HIV in the current pregnancy and lack of availability of sdNVP or other preventative regimens in the South African health system, particularly prior to 2005.
Beyond HAART Regimen and Duration: Other Predictors of MTCT in Settings Without Viral Load Monitoring
Many studies have shown a protective effect of elective Caesarean section in MTCT with up to an approximate 50-70% reduction in risk compared to vaginal delivery(38-40). Lack of an association between mode of delivery and infant HIV status in our study is likely the result of low numbers of women with information available since many women in our setting deliver infants outside of the hospital setting. Although research findings have informed delivery guidelines in settings where resources exist to provide this service, South Africa is unable to provide elective Caesarian section given the high number of women with HIV, the lack of viral load monitoring to guide decisions about delivery, and overall health system resource constraints; therefore decisions about Caesarian section are guided largely by obstetric necessities rather than HIV infection.
In univariate analysis PROM was associated with infant HIV infection, although this risk factor has not been well established in studies of women on HAART(41). The significance of PROM noted in our cohort may be secondary to the brief duration of HAART in many women, making them more similar to those receiving mono- or dual prophylaxis in regard to the role of other risk factors on MTCT (42-44). Baseline CD4 cell count was of borderline significance as a factor predictive of MTCT. We did not have CD4 cell counts at or near the time of labor and are therefore unable to determine if degree of immunosuppression at the time of delivery is predictive of infant HIV status. Studies have been mixed regarding the relationship between degree of immunosuppression and infant transmission with many showing no association(19, 25, 45, 46) and others finding low CD4 count to be an important risk factor(2, 20, 47). We believe that advanced immunosuppression contributes to the high rate of MTCT in our cohort as compared to rates reported from women on HAART in which the majority have CD4 counts > 200 cells/mm3. Infant female sex(48-50), low birth weight(50-52), and smoking(53, 54) have been shown in studies to be associated with HIV transmission, although none of these associations were detected in our analysis.
Loss to Follow-up and Other Limitations
The lack of maternal viral load at the time of delivery is an important limitation of this study, but reflects the reality of HIV care in resource-poor settings. Our goal was to learn about other factors that may have predictive value in clinical programs without funding for serial viral load during pregnancy. Further, the ability to distinguish between the timing of MTCT was limited by lack of availability of newborn HIV PCR testing. The majority of infants in this cohort were formula-fed, although low rates of mixed feeding cannot be excluded. HIV status at 4-6 weeks of life was considered to represent in utero or perinatal transmission.
The ANC ARV program was designed with the primary goal of clinical care, with observational research as a secondary component. Efforts are made to record all pertinent data and ensure follow-up, but data reflect the realities of our practice circumstance, with missing information on women who completed follow-up as well as a large number of women and infants lost to follow-up before infant HIV testing. Pregnant women often come to Johannesburg for antenatal care but return to their homes at remote locations for infant delivery and often remain confined to their homes in the early postpartum period. Additionally, women face stigma, poverty, and fear about infant HIV diagnosis, all of which may serve as a deterrent to returning for 4-6 week infant testing and results. Linkage of antenatal and HIV services in the ANC-ARV clinic attempts to overcome some of these barriers, but retaining women in care remains a significant challenge. In this analysis we attempted to evaluate the influence of loss to follow up by performing comparisons among women with and without infant HIV results. This analysis did not show differences in women lost to follow-up compared to those who were retained through infant HIV testing.
Conclusion
Late identification of HIV increases risks for maternal health and MTCT, and remains a challenge despite successful integration of antenatal and HIV services in the ANC-ARV clinic program. Widespread testing of women is needed to identify those with HIV infection prior to pregnancy and allow for optimal maternal health and prevention of infant transmission. Our observational data highlights the importance of duration of HAART in women initiating therapy during pregnancy and demonstrates the high efficacy of long-term HAART in preventing MTCT in women becoming pregnant on therapy. While the ANC-ARV clinics have been important in developing the necessary infrastructure, further efforts are needed to address social and health service barriers that may contribute to late identification of HIV-infected women in South Africa.
Acknowledgments
The authors thank Dr. Catherine Sugar and Lily Altstein of the University of California Los Angeles Department of Biostatistics. We also gratefully acknowledge PEPFAR for support of the ANC ARV clinics, the South African Department of Health, and the staff and patients of the ANC-ARV program at both the Charlotte Maxeke and Rahima Moosa Mother and Child hospitals.
Footnotes
Data from this manuscript were presented in poster format at the 5th International AIDS Conference on HIV Pathogenesis, Treatment and Prevention; Capetown, South Africa, July 22, 2009
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999 Mar 6;353(9155):781–5. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 2.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004 Jul 15;351(3):217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 3.Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: results from 13 perinatal studies. The Working Group on Mother-To-Child Transmission of HIV. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Apr 15;8(5):506–10. doi: 10.1097/00042560-199504120-00011. [DOI] [PubMed] [Google Scholar]
- 4.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. Aids. 2007 Jul;21 4:S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 5.Perinatal HIV Guidelines Working Group. Public Health Service Task Force. 2009. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States; pp. 1–90. [Google Scholar]
- 6.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002 Apr 15;29(5):484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Dorenbaum A, Cunningham CK, Gelber RD, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. Jama. 2002 Jul 10;288(2):189–98. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 8.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005 Feb 1;40(3):458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 9.Black V, Hoffman R, Sugar C, et al. e. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008 Nov 1;49(3):577–81. doi: 10.1097/QAI.0b013e318189a769. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollins N, Little K, Mzolo S, Horwood C, Newell ML. Surveillance of motherto-child transmission prevention programmes at immunization clinics: the case for universal screening. Aids. 2007 Jun 19;21(10):1341–7. doi: 10.1097/QAD.0b013e32814db7d4. [DOI] [PubMed] [Google Scholar]
- 11.National Antenatal Sentinel HIV & Syphilis Prevalence Survey. Department of Health Republic of South Africa; 2008. [Google Scholar]
- 12.Policy and Guidelines for the Implementation of the PMTCT Programme. Pretoria: Government of South Africa, National Department of Health; 2008. [Google Scholar]
- 13.Martinson NA, Morris L, Gray G, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007 Feb 1;44(2):148–53. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 14.Black V, Hoffman RM, Sugar CA, et al. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008 Nov 1;49(3):276–81. doi: 10.1097/QAI.0b013e318189a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Merwe K, Chersich MF, Technau K, Umurungi Y, Conradie F, Coovadia A. Integration of antiretroviral treatment within antenatal care in Gauteng Province, South Africa. J Acquir Immune Defic Syndr. 2006 Dec 15;43(5):577–81. doi: 10.1097/01.qai.0000243099.72770.d2. [DOI] [PubMed] [Google Scholar]
- 16.National Antiretroviral Treatment Guidelines. National Department of Health South Africa; 2004. [Google Scholar]
- 17.Black V, Brooke S, Chersich MF. Effect of human immunodeficiency virus treatment on maternal mortality at a tertiary center in South Africa: a 5-year audit. Obstet Gynecol. 2009 Aug;114(2 Pt 1):292–9. doi: 10.1097/AOG.0b013e3181af33e6. [DOI] [PubMed] [Google Scholar]
- 18.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Cote d'Ivoire. Aids. 2008 Sep 12;22(14):1815–20. doi: 10.1097/QAD.0b013e32830b8ab9. [DOI] [PubMed] [Google Scholar]
- 19.Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005 Feb 1;40(3):458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 20.Warszawski J, Tubiana R, Le Chenadec J, et al. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS French Perinatal Cohort. Aids. 2008 Jan 11;22(2):289–99. doi: 10.1097/QAD.0b013e3282f3d63c. [DOI] [PubMed] [Google Scholar]
- 21.Mayaux MJ, Dussaix E, Isopet J, et al. Maternal virus load during pregnancy and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohort studies. SEROGEST Cohort Group. J Infect Dis. 1997 Jan;175(1):172–5. doi: 10.1093/infdis/175.1.172. [DOI] [PubMed] [Google Scholar]
- 22.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999 Aug 5;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Krogstad P, Korber BT, et al. Maternal HIV-1 viral load and vertical transmission of infection: the Ariel Project for the prevention of HIV transmission from mother to infant. Nat Med. 1997 May;3(5):549–52. doi: 10.1038/nm0597-549. [DOI] [PubMed] [Google Scholar]
- 24.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006 Aug 5;368(9534):505–10. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 25.Townsend CL, Cortina-Borja M, Peckham CS, de Ruiter A, Lyall H, Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000-2006. Aids. 2008 May 11;22(8):973–81. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 26.Grosch-Woerner I, Puch K, Maier RF, et al. Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV-1-infected women. HIV Med. 2008 Jan;9(1):6–13. doi: 10.1111/j.1468-1293.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 27.Watts DH. Teratogenicity risk of antiretroviral therapy in pregnancy. Curr HIV/AIDS Rep. 2007 Aug;4(3):135–40. doi: 10.1007/s11904-007-0020-y. [DOI] [PubMed] [Google Scholar]
- 28.Bussmann H, Wester CW, Wester CN, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):269–73. doi: 10.1097/QAI.0b013e318050d683. [DOI] [PubMed] [Google Scholar]
- 29.Patel D, Cortina-Borja M, Thorne C, Newell ML. Time to undetectable viral load after highly active antiretroviral therapy initiation among HIV-infected pregnant women. Clin Infect Dis. 2007 Jun 15;44(12):1647–56. doi: 10.1086/518284. [DOI] [PubMed] [Google Scholar]
- 30.Walmsley S, Bernstein B, King M, et al. Lopinavir-ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002 Jun 27;346(26):2039–46. doi: 10.1056/NEJMoa012354. [DOI] [PubMed] [Google Scholar]
- 31.Villani P, Floridia M, Pirillo MF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006 Sep;62(3):309–15. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nellen JF, Schillevoort I, Wit FW, et al. Nelfinavir plasma concentrations are low during pregnancy. Clin Infect Dis. 2004 Sep 1;39(5):736–40. doi: 10.1086/422719. [DOI] [PubMed] [Google Scholar]
- 33.van Heeswijk RP, Khaliq Y, Gallicano KD, et al. The pharmacokinetics of nelfinavir and M8 during pregnancy and post partum. Clin Pharmacol Ther. 2004 Dec;76(6):588–97. doi: 10.1016/j.clpt.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Wind-Rotolo M, Durand C, Cranmer L, et al. Identification of Nevirapine-Resistant HIV-1 in the Latent Reservoir after Single-Dose Nevirapine to Prevent Mother-to-Child Transmission of HIV-1. J Infect Dis. 2009 May 1;199(9):1301–9. doi: 10.1086/597759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flys TS, Mwatha A, Guay LA, et al. Detection of K103N in Ugandan women after repeated exposure to single dose nevirapine. Aids. 2007 Oct 1;21(15):2077–82. doi: 10.1097/QAD.0b013e3282703847. [DOI] [PubMed] [Google Scholar]
- 36.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007 Oct;36(5):1009–21. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 37.Lockman SatASTO. Lopinavir/ritonavir+Tenofovir/Emtricitabine Is Superior to Nevirapine+Tenofovir/Emtricitabine for Women with prior Exposure to Single-dose Nevirapine: A5208 (“OCTANE”). Conference on Retroviruses and Opportunistic Infections; 2009; Montreal, Canada. 2009. [Google Scholar]
- 38.The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1--a meta-analysis of 15 prospective cohort studies. The International Perinatal HIV Group. N Engl J Med. 1999 Apr 1;340(13):977–87. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 39.Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: a randomised clinical trial. The European Mode of Delivery Collaboration. Lancet. 1999 Mar 27;353(9158):1035–9. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 40.Read JS, Newell MK. Efficacy and safety of cesarean delivery for prevention of mother-to-child transmission of HIV-1. Cochrane Database Syst Rev. 2005;(4):CD005479. doi: 10.1002/14651858.CD005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Tejedor A, Maiques V, Perales A, Lopez-Aldeguer J. Influence of highly active antiretroviral treatment (HAART) on risk factors for vertical HIV transmission. Acta Obstet Gynecol Scand. 2009;88(8):882–7. doi: 10.1080/00016340903062836. [DOI] [PubMed] [Google Scholar]
- 42.Duration of ruptured membranes and vertical transmission of HIV-1: a metaanalysis from 15 prospective cohort studies. Aids. 2001 Feb 16;15(3):357–68. doi: 10.1097/00002030-200102160-00009. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Tejedor A, Perales A, Maiques V. Duration of ruptured membranes and extended labor are risk factors for HIV transmission. Int J Gynaecol Obstet. 2003 Jul;82(1):17–23. doi: 10.1016/s0020-7292(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 44.Welles SL, Bauer GR, LaRussa PS, Colgrove RC, Pitt J. Time trends for HIV-1 antiretroviral resistance among antiretroviral-experienced and naive pregnant women in New York City during 1991 to early 2001. J Acquir Immune Defic Syndr. 2007 Mar 1;44(3):329–35. doi: 10.1097/QAI.0b013e31802f1296. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez JR, Bardeguez A, Iffy L, Apuzzio JJ. Preterm premature rupture of membranes in pregnancies complicated by human immunodeficiency virus infection: a single center's five-year experience. J Matern Fetal Neonatal Med. 2007 Dec;20(12):853–7. doi: 10.1080/14767050701700766. [DOI] [PubMed] [Google Scholar]
- 46.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999 Aug 5;341(6):385–93. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 47.Minkoff H, Burns DN, Landesman S, et al. The relationship of the duration of ruptured membranes to vertical transmission of human immunodeficiency virus. Am J Obstet Gynecol. 1995 Aug;173(2):585–9. doi: 10.1016/0002-9378(95)90286-4. [DOI] [PubMed] [Google Scholar]
- 48.Thorne C, Newell ML. Are girls more at risk of intrauterine-acquired HIV infection than boys? Aids. 2004 Jan 23;18(2):344–7. doi: 10.1097/00002030-200401230-00033. [DOI] [PubMed] [Google Scholar]
- 49.Piwoz EG, Humphrey JH, Marinda ET, Mutasa K, Moulton LH, Iliff PJ. Effects of infant sex on mother-to-child transmission of HIV-1 according to timing of infection in Zimbabwe. Aids. 2006 Oct 3;20(15):1981–4. doi: 10.1097/01.aids.0000247123.04703.6e. [DOI] [PubMed] [Google Scholar]
- 50.Tonwe-Gold B, Ekouevi DK, Viho I, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007 Aug;4(8):e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magder LS, Mofenson L, Paul ME, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005 Jan 1;38(1):87–95. doi: 10.1097/00126334-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 52.Charurat M, Datong P, Matawal B, Ajene A, Blattner W, Abimiku A. Timing and determinants of mother-to-child transmission of HIV in Nigeria. Int J Gynaecol Obstet. 2009 Apr 3; doi: 10.1016/j.ijgo.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns DN, Landesman S, Muenz LR, et al. Cigarette smoking, premature rupture of membranes, and vertical transmission of HIV-1 among women with low CD4+ levels. J Acquir Immune Defic Syndr. 1994 Jul;7(7):718–26. [PubMed] [Google Scholar]
- 54.Turner BJ, Hauck WW, Fanning TR, Markson LE. Cigarette smoking and maternal-child HIV transmission. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Apr 1;14(4):327–37. doi: 10.1097/00042560-199704010-00004. [DOI] [PubMed] [Google Scholar]


