Abstract
NK cells have potential therapeutic impact in suppressing graft-versus-host disease (GVHD) and enhancing antitumor effects as a cellular therapy for hematologic malignancies. However, few studies have addressed the trafficking and in vivo behavior of NK cells in murine models of bone marrow transplantation (BMT). We investigated NK cell trafficking and survival following allogeneic and syngeneic BMT using a novel bioluminescence-based imaging strategy. Transplantation of luciferase-expressing NK cells revealed CD62L-mediated trafficking to lymphoid organs and trafficking to GVHD target tissues, as evidenced by in vivo and ex vivo bioluminescence imaging. The NK cells persisted for ~4 wk after transplantation in allogeneic recipients, but were not detectable in syngeneic recipients. CFSE-labeling studies showed extensive NK cell proliferation in vivo. Transplanted NK cells up-regulated molecules necessary for homing to the lymph nodes, gastrointestinal tract, and skin, yet did not cause clinical GVHD. This expansion and tissue-specific homing was not solely due to the conditioning regimen, as NK cells proliferated and reached lymphoid and GVHD target tissue in unconditioned allogeneic RAG2−/− γ-chain−/− recipients. IL-2 enhanced expansion and antitumor activity of NK cells. These results provide significant insight into the behavior and potential therapeutic impact of NK cells in BMT.
Natural killer cells are lymphocytes that play critical roles in both innate and adaptive immune responses and provide a natural defense against virally infected cells (1), intracellular microbes (2), and certain tumors (3). NK cells exert their effector functions by causing direct cellular lysis through the release of perforin and granzymes and by secreting proinflammatory cytokines, such as IFN-γ, GM-CSF, and TNF-α (4, 5). Cytokines, including IL-15, IL-2, IL-18, IL-12, and IFN-α/IFN-β, are involved in activating NK cells (6 – 8).
Trafficking to sites where cells exert their functions is critical for an effective immune response. Much work has focused on characterizing the homing mechanisms used by T cells in an adaptive immune response. These studies have demonstrated the importance of T cell migration to key anatomical sites where interaction with APCs and other immune reactive cells is required for the generation of an effective immune reaction (9, 10). Comparatively less is known about the capability of NK cells to traffic to specific tissues, the regulation of NK cell homing, and their survival after adoptive transfer.
Under steady-state conditions, NK cells develop in the bone marrow and mature NK cells reside in the periphery in the spleen, liver, lung, thymus, lymph nodes, and peripheral blood (11, 12). NK cells can be signaled to migrate in response to inflammatory stimuli because they express several inflammatory chemokine receptors (12). During an immune response to an inflammatory stimulus, NK cells can enter local draining lymph nodes as well as nonlymphoid tissues, such as the liver or lung (13, 14). Martin-Fontecha et al. (13) have shown that NK cells are recruited from the blood to draining lymph nodes in a CD62L- and CXCR3-dependent manner and play a functional role by producing IFN-γ to induce a TH1 adaptive immune response. Unlike T cells, mature NK cells localized in the periphery do not proliferate at significant levels (15, 16). However, it has been shown that NK cells can undergo homeostatic proliferation in a syngeneic, lymphopenic environment (17, 18).
NK cells have shown promise as a cellular therapy in bone marrow transplantation (BMT)3 because they themselves do not cause graft-versus-host disease (GVHD) (19), yet can prevent this complication while maintaining the graft-versus-tumor (GVT) effect (19, 20). Studies done in an allogeneic mouse model of BMT demonstrated the ability of IL-2-activated NK cells to suppress GVHD while providing a GVT effect against a murine colon adenocarcinoma (20). The GVT effects were shown to be due in part to the cytokine TGF-β. Studies in a haploidentical murine transplant model demonstrated that the administration of alloreactive NK cells before BMT prevented GVHD by elimination of host APCs (19). In human studies, killer Ig receptor ligand incompatibility in the graft-versus-host direction led to improved outcomes in haploidentical hematopoietic stem cell transplants (19, 21). These studies indicate a promising role for the use of NK cells as a cellular therapy in allogeneic BMT. The clinical use of NK cells has been applied to the treatment of patients with solid tumors and leukemia with early evidence of efficacy in some patients (22). However, little is known about the trafficking and in vivo behavior of NK cells themselves in murine models of BMT. Understanding NK cell trafficking in the setting of BMT will provide crucial information to help understand the function of these cells and the biology governing these processes, as well as insights that could be developed into more effective therapeutic interventions in BMT and other settings.
We used a noninvasive in vivo method of bioluminescence imaging (BLI) to monitor NK cell trafficking and survival following allogeneic and syngeneic BMT (23). Using a major MHC-mismatch model, we showed NK cell trafficking to and proliferation in lymphoid organs, mediated by CD62L, as well as nonlymphoid organs. Phenotypic analysis correlated the up-regulation of tissue-specific homing receptors with the tissue localization of the NK cells. We demonstrated that the trafficking pattern is not solely a result of the BMT conditioning regimen, but that NK cell expansion and antitumor function is enhanced by addition of exogenous IL-2.
Materials and Methods
Mice
FVB/N (H-2q), BALB/c (H-2d) and C57BL/6 (H-2b, CD45.2) mice were obtained from either The Jackson Laboratory or Charles River Laboratories. Mice used were between 7 and 16 wk of age. All experiments were done with gender-matched animals. Luciferase-expressing (luc+) FVB/N L2G85 mice were created as previously described (24). C57BL/6 (H-2b, CD45.1, luc+) animals were bred by backcrossing FVB/N L2G85 animals into the C57BL/6 strain for more than 10 generations. RAG2−/− γ-chain (γC) −/− BALB/c mice were obtained from I. L. Weissman (Stanford University). Animal protocols were approved by the Institutional Animal Care and Use Committee at Stanford University.
Flow cytometric analysis
The following Abs used for flow cytometric analysis were obtained from BD Pharmingen and eBioscience: CD3 (17A2), CD49b (DX5), CD45.1 (A20), CD3 (145-2C11), CD62L (MEL-4), α4β7 (DATK32), and P-selectin-IgG fusion protein. F(ab′)2 goat anti-human IgG-PE was obtained from Jackson ImmunoResearch Laboratories. Propidium iodide was used to exclude dead cells.
Cell isolation and sorting
Splenocytes were harvested from donor mice and processed in 1× PBS (Invitrogen) supplemented with 2% FCS (Invitrogen) into single-cell suspensions. After RBC lysis, cells were blocked with normal rat serum (Caltag Laboratories/Invitrogen), stained with anti-DX5 microbeads (Miltenyi Biotec), and positive selection for the DX5+ population was done either by the AutoMACS system or by manual MACS column selection. The enriched DX5+ fraction was stained with CD3-FITC, DX5-PE, and propidium iodide and sorted for DX5+CD3− cells on a FACStar or MoFlow system. This purification protocol yielded >95% purity. For FACS reisolation experiments, NK cells were enriched with anti-DX5 microbeads by AutoMACS and transplanted. Bone marrow was flushed from the femurs and tibiae of donor mice and processed into single-cell suspensions, which were then stained with anti-CD4 and anti-CD8 beads (Miltenyi Biotec). T cell-depleted bone marrow was isolated using manual MACS negative selection.
Bone marrow transplantation
Recipient mice were irradiated with 8 Gy (BALB/c) or 9 Gy (FVB/N and C57BL/6) split into two equal doses (4 and 4.5 Gy, respectively) 4 h apart. Irradiated recipients were subsequently given 5 × 106 donor T cell-depleted bone marrow (TCD-BM) cells and 5 × 105 highly purified donor NK cells a minimum of 2 h after the second dose. Mice were kept in autoclaved cages and on antibiotic water (sulfamethoxazole and tri-methoprim; Hi-Tech Pharmacal) for a minimum of 30 days after transplant.
In vivo and ex vivo BLI
In vivo BLI was performed as previously described (25). Briefly, 10 min after i.p. injection of luciferin, mice were imaged for 5 min with an IVIS 100 charge-coupled device imaging system (Xenogen). Images were analyzed with Living Image Software 2.5 (Xenogen) and Igor Pro Carbon (Wavemetrics). Ex vivo imaging was performed as previously described (9).
CFSE proliferation analysis
Donor NK cells were labeled with a 5 μM Vybrant CFSE Tracer Kit (Molecular Probes) in PBS with 2% FCS for 6 min at 37°C. The labeling reaction was quenched by addition of cold RPMI 1640 (Invitrogen) with 10% FCS, and cells were washed twice with PBS with 2% FCS to remove excess CFSE. FACS analysis allowed gating on individual CFSE generations, and the percentage of transplanted donor NK cells dividing was calculated (18).
In vivo Ab administration
Transplanted recipients received the following dose of either anti-L-selectin (anti-CD62L, MEL14) or isotype control (Hermes-1, 9B5) Abs, both rat IgG2a, i.v.: 150 μg on day 0 immediately before cell injection, 100 μg on days 1– 4, and 50 μg on day 5. Abs were purified from hybridomas at Stanford University and were a gift from E. Butcher (Stanford University).
Tissue reisolation
Spleen, mesenteric lymph nodes (mLN), and liver were removed from transplanted animals and processed into a single-cell suspension for flow cytometric analysis. Livers were perfused with PBS, processed into single-cell suspensions, and lymphocytes were separated on a Percoll (GE Health-care) gradient. RBC lysis was performed on lymphocytes from liver and spleen. For lamina propria lymphocyte (LPL) isolation, the small bowel was removed and flushed with complete RPMI 1640 with 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (all from Invitrogen), and 5 μg/ml 2-ME (Sigma-Aldrich). Bowel was cut longitudinally and then into small pieces and washed with complete RPMI 1640. For intraepithelial lymphocyte (IEL) isolation, gut segments were incubated with RPMI 1640 containing 5 mM EDTA (Fisher Scientific) and 1 mM DTT (Invitrogen) for 20 min three times. Supernatants were pooled and centrifuged. Remaining gut pieces were incubated with 0.2 mg/ml collagenase (Sigma-Aldrich) in RPMI 1640 at 200 rpm at 37°C twice for 1 h each. Remaining gut pieces were processed and filtered into supernatants, which were pooled and centrifuged. IEL and LPL were separated on a 44%/66% Percoll gradient and washed.
In vivo IL-2 treatment
Mice were injected i.p. with 5 × 104 U of recombinant human IL-2 (Chiron) in sterile PBS daily for 2 wk. Control mice received an equal volume of sterile PBS daily.
Tumor model
Mice were lethally irradiated and transplanted i.v. with 5 × 106 FVB TCD-BM cells and 1 × 104 A20 luc+/yfp tumor cells created as previously described (25). Two days later, animals received 2.5 × 105 FVB NK cells and either 5 × 104 U of exogenous recombinant human IL-2 or sterile PBS daily. Tumor burden was assessed by in vivo BLI on successive days posttransplant.
Results
Trafficking of luc+ NK cells in allogeneic and syngeneic BMT
NK cells have been demonstrated to not cause GVHD and, in some models, are capable of preventing it (20, 26). To explore the biological basis of these observations, we studied NK cell homing and the mechanisms that govern their in vivo trafficking, particularly in the setting of BMT. To assess the in vivo trafficking and survival of adoptively transferred NK cells, we transplanted highly purified luc+ NK cells into lethally irradiated allogeneic and syngeneic recipients along with TCD-BM. Donor luc+DX5+CD3+ NK cells were isolated from splenocytes of FVB/N L2G85 donor mice and sorted to high purity, >95% (Fig. 1A). In the allogeneic recipients, in vivo BLI posttransplant revealed NK cell trafficking to the spleen and lymph nodes (Fig. 1B). The NK signal appeared first in the spleen, cervical lymph nodes (cLN), and inguinal lymph nodes (iLN) on day 3 and then the abdomen by day 5. At day 6 after transplant, the NK cell signal was visible in the spleen, cLN, iLN, axillary lymph nodes (aLN), and mLN as well as the abdomen (Fig. 1B). The BLI signal persisted for 3– 4 wk after transplant, whereas in the syngeneic recipients, the BLI signal was not detectable above background levels. A visible increase in the allogeneic BLI signal in the first 2 wk after transplantation indicated NK cell expansion in vivo. Quantification of the ventral whole-body images confirmed a significantly greater BLI signal in the allogeneic recipients compared with the syngeneic recipients (Fig. 1C). In addition to an increasing bioluminescent signal in the lymphoid organs, a remarkable accumulation of NK cells in the abdomen was visible by in vivo BLI within the first 2 wk after infusion. Ex vivo imaging of the gastrointestinal tract was performed to determine the origin of this signal. In the allogeneic recipients, NK cells were visualized in the mLN as well as in the gastrointestinal tissue, a GVHD target organ (Fig. 1D). Weaker NK cell infiltration was visible in the mLN and spleen of syngeneic recipients. However, no signal was visible in the gastrointestinal tract. Allogeneic BMT recipients not receiving luc+ cells are shown as controls. These ex vivo images demonstrate that NK cells can traffic to lymphoid as well as nonlymphoid tissues after allogeneic BMT. Similar to alloreactive T cells, NK cells traffic to the gut; however, they do not cause GVHD at this site. Mice transplanted with allogeneic TCD-BM and this dose of NK cells showed no clinical signs of GVHD such as hunched posture, diarrhea or ruffled fur, and gained weight similarly to TCD-BM control animals (data not shown).
FIGURE 1.
NK cells traffic to priming sites and target organs without causing GVHD. A, Donor NK cells (H-2q, DX5+CD3−) are sorted to >95% purity and transplanted into lethally irradiated allogeneic (BALB/c, H-2d) and syngeneic (FVB/N, H-2q) recipients. B, BLI demonstrated that NK cells in allogeneic recipients traffic to the lymphoid organs (spleen and cLN, iLN, aLN, and mLN) and abdomen and proliferate in vivo. No visible NK cell signal is observed in syngeneic recipients. The signal persists in allogeneic recipients for ~4 wk. One representative animal for each group is shown over time. Number of mice per group is three and is representative of more than three independent experiments. Days posttransplant are indicated along the top of the panels. C, Expansion of NK cells in vivo was quantified at time points after transplant in allogeneic and syngeneic recipients. The average luc+ NK signal emitted from allogeneic recipients was significantly greater than that from syngeneic recipients, as measured by total photons emitted per whole mouse per second. Animals receiving only TCD-BM are shown as controls. Error bars represent SE. D, Ex vivo imaging of the gastrointestinal tract of recipient mice revealed NK cell trafficking to and proliferation in the mLN and the gut tissue of allogeneic recipients (ALLO NK). NK cells traffic to the mLN in syngeneic recipients (SYN NK), but no signal was visible in the gastrointestinal tract. Allogeneic TCD-BM transplant animals are shown as controls (BM).
NK cells proliferate in lymphoid and nonlymphoid tissues
The increase in BLI signal indicated an in vivo expansion of NK cells in allogeneic recipients. We sought to investigate whether this increase was due to proliferation or accumulation of cells in these tissues. To address this, we labeled donor NK cells with CFSE before allogeneic and syngeneic transplantation to perform proliferation analysis of the dividing donor NK cells isolated from tissues after transplant. To distinguish donor NK cells from donor TCD-BM and recipient NK cells, a congenic CD45.1-expressing NK cell donor was used with the TCD-BM and recipient NK cells both expressing CD45.2. By gating on the DX5+CD3−CD45.1+ population on day 5 after BMT (Fig. 2A), we calculated the percentage of transplanted NK cells that underwent division in the recipient tissues (18). We observed extensive in vivo proliferation of NK cells in allogeneic recipients in the spleen, mLN, liver, and gastrointestinal tract (LPL and IEL; Fig. 2B). In all tissues, NK cell proliferation was greater in the allogeneic than syngeneic recipients (Fig. 2B), as measured by percentage of donor NK cells proliferating (18) and as evidenced by the CFSE histograms. This indicates that the increase in BLI signal shown in Fig. 1B was due to in vivo proliferation of donor NK cells in specific tissue sites. Interestingly, donor NK cells were reisolated from the gastrointestinal tract of syngeneic recipients, regardless of the fact that they were not visible in the ex vivo BLI in Fig. 1D. This indicates that NK cells are not visible in syngeneic recipient gastrointestinal tissue not because they do not reach the tissues or proliferate in situ, but rather because they do not proliferate at high enough levels to be detectable by BLI.
FIGURE 2.
CFSE-based in vivo proliferation analysis of donor NK cells. Donor NK cells (CD45.1, H-2b) were CFSE labeled and transplanted into allogeneic (CD45.2, H-2d) and syngeneic (CD45.2, H-2b) recipients. A, The congenic markers CD45.1 and CD45.2 were used to distinguish between donor NK cells and recipient cells, respectively. On day 5 after transplant, spleen, mLN, liver, and gut tissue was isolated and analyzed by FACS by gating on NK cells, which are DX5+CD3−, and successively on the donor marker CD45.1 to determine the proliferation of CFSE-labeled NK cells in each recipient tissue. B, Histograms depict the FACS profile of CFSE-labeled NK cells in recipient spleen, mLN, liver, and gut (LPL and IEL). Proliferation was more extensive in allogeneic recipients. Percents shown in the panels indicate percentage of transplanted NK cells that have divided in vivo.
NK cells up-regulate homing receptors for tissue-specific trafficking
We next determined what factors influenced the observed NK cell trafficking patterns. We first evaluated the phenotype of the cells pre-and posttransplant to determine whether NK cells up-regulate the receptors required for homing to specific tissues. L-selectin (CD62L), a lymph node-homing molecule, is down-regulated upon T cell activation (27). Most NK cells express CD62L, which has also been shown to be down-regulated upon IL-2 activation (12, 28, 29). Additionally, expression of CD62L is required for homing to lymph nodes (30). We observed that 77% of wild-type C57BL/6 NK cells express CD62L (Fig. 3A). NK cells reisolated from the spleens of syngeneic recipients on day 5 after transplant had no reduction in expression of CD62L (75%, p = 0.44), but there was a substantial reduction on NK cells recovered from allogeneic recipients (54%, p = 0.01). Findings were similar on NK cells reisolated from peripheral lymph nodes (pLN, including cLN, iLN, and aLN): 75% expression on NK cells from syngeneic recipients and 57% on NK cells from allogeneic recipients. This finding indicates that NK cells transplanted into an allogeneic recipient are activated in vivo in contrast to those in syngeneic recipients. We measured expression of the P-selectin ligand, the ligand for the skin-homing receptor P-selectin, expression of which has been found on NK cells (12, 28, 31). Although we observed that 41% of wild-type splenic NK cells express P-selectin ligand, expression of the molecule was up-regulated in both allogeneic (70%, p = 0.04) and syngeneic recipients (61%, p = 0.01). Again, findings were similar on pLN-reisolated NK: 69% expression from syngeneic recipients and 84% from allogeneic recipients. The gut-homing molecule α4β7 has been reported to be expressed on a subset of NK cells (28, 32). We found α4β7 to be expressed by 9% of wild-type NK cells and it was significantly up-regulated on splenic NK cells in allogeneic (25%, p = 0.002) and syngeneic recipients (18%, p = 0.008). NK cells reisolated from allogeneic and syngeneic mLN both up-regulated this homing receptor, although to a lesser extent (13% for both). Thus, NK cells do express and up-regulate homing molecules required for migration into lymphoid organs, gut, and skin. The allogeneic and syngeneic reisolated NK cell populations differed significantly from each other only in CD62L expression ( p = 0.03). Interestingly, the results from syngeneic recipients imply that down-regulation of CD62L is not a requirement for up-regulation of tissue-specific homing receptors. In allogeneic recipients, we demonstrate that each of the homing markers α4β7 and P-selectin ligand are up-regulated with successive divisions of NK cells, as measured by co-CFSE staining (Fig. 3B). In contrast, CD62L is successively down-regulated in each generation. This is also evident when comparing the mean fluorescence intensity of the homing receptor staining between each successive division of the NK cells (Fig. 3C).
FIGURE 3.
Dividing NK cells up-regulate markers required for tissue-specific homing. A, Transplanted donor NK cells (H-2b, CD45.1) were reisolated from spleen, pLN, and mLN on day 5 after transplant from allogeneic (open histograms) and syngeneic recipients (gray histograms) and stained for expression of CD62L, α4β7, and P-selectin ligand. In comparison to expression levels on freshly isolated, nontransplanted NK cells (black histograms), reisolated splenic NK cells showed up-regulation of α4β7 (p = 0.002 and 0.008 for allogeneic and syngeneic, respectively) and P-selectin ligand (p = 0.04 and 0.01 for allogeneic and syngeneic, respectively). Allogeneic reisolated NK cells down-regulated CD62L (p = 0.01), in contrast to syngeneic reisolated NK cells which did not. Histograms are also shown for pLN and mLN. Data are representative of three independent experiments. B, Splenic reisolated NK cells costained for CFSE and homing receptors. Allogeneic NK cells up-regulate α4β7 and P-selectin ligand as they divide; CD62L is down-regulated on proliferating NK cells. Each color represents a successive CFSE generation. C, Histograms were gated on each CFSE generation on NK cells costained with CD62L, α4β7, and P-selectin ligand, and the mean fluorescence intensity of each homing marker is plotted for each CFSE generation for spleen-reisolated (▼), pLN-reisolated (◆), and mLN-reisolated (■) NK cells.
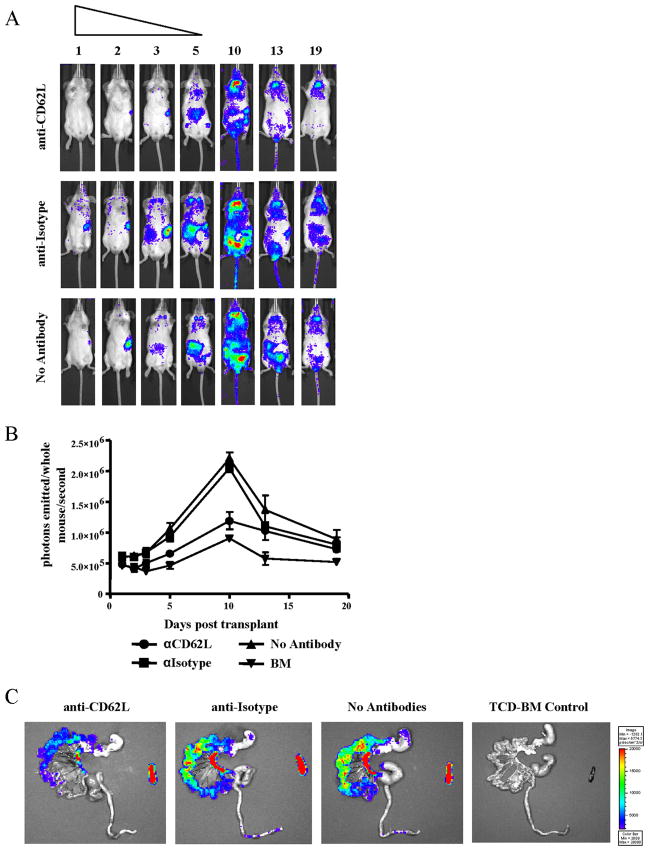
Blocking CD62L reduces NK cell entry into pLN
Martin-Fontecha et al. (13) previously demonstrated the requirement for CD62L expression for NK cell recruitment to dendritic cell-draining lymph nodes. Having shown the trafficking of donor NK cells into lymph nodes and high levels of CD62L on these cells, we asked whether blocking this tissue-specific homing receptor would alter NK cell trafficking into lymphoid organs or target tissues after allogeneic BMT. Transplanted animals receiving allogeneic NK cells were treated with either anti-CD62L or isotype control Abs on day 0 immediately before cell injection and for 5 successive days after transplant. BLI revealed that anti-CD62L Ab treatment blocked NK cell trafficking to the lymphoid organs compared with isotype control Ab-treated animals and animals receiving no Abs (Fig. 4A). This is particularly evident when comparing the signal arising from the cLN in each of the groups. Cell entry to the lymph nodes was reduced only throughout the time period of blocking Ab administration. Quantification of the bioluminescent images revealed a lower overall luc+ donor NK cell bioluminescent signal in animals receiving anti-CD62L Ab (Fig. 4B). Ex vivo BLI revealed a reduced, but not absent, population of donor NK cells in the mLN in the presence of anti-CD62L Ab (Fig. 4C). NK cells trafficked normally to the spleen in both treatment groups. Additionally, NK cells were still visible in the gastrointestinal tract in anti-CD62L-treated animals, however, at a reduced intensity, correlating with the reduced NK cell signal in the mLN. Therefore, CD62L is required for NK cell homing to the lymph nodes after allogeneic BMT.
FIGURE 4.
NK cell homing to LN is reduced with CD62L blockade. A, Bioluminescent images of groups receiving donor luc+ NK cells with either anti-CD62L Ab (top panel), isotype control Ab (middle panel), or no Ab treatment (bottom panel) showed effective blocking of NK cell homing to LN during the first 5 days after transplant in anti-CD62L-receiving animals. One representative animal from a group of three to six is shown for each time point. B, Total photons emitted per mouse per second are reduced in anti-CD62L-receiving groups (●) compared with isotype control (■) or animals receiving no Ab treatment (▲). Animals receiving TCD-BM are shown as controls (▼). C, Ex vivo imaging of gastrointestinal tract and spleen at day 5 after transplant revealed decreased NK cell homing to the mLN and gastrointestinal tissue in anti-CD62L-receiving animals compared with isotype control and no Ab treatment groups.
Impact of the BMT conditioning regimen on NK cell trafficking
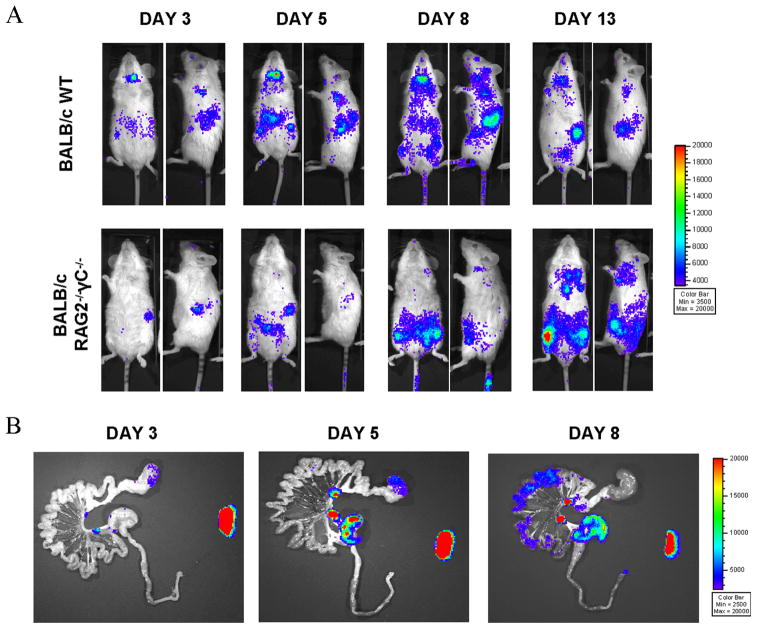
Irradiation can result in host tissue injury and cause extensive inflammation and cytokine release (33, 34), which can influence cellular trafficking. Therefore, we next investigated the extent to which the conditioning regimen contributed to the NK cell homing and proliferation observed in allogeneic recipients. To address this question, we transplanted NK cells into allogeneic, nonirradiated BALB/c RAG2−/− γC−/− recipients lacking T, B, and NK cells compared with wild-type animals prepared with total body irradiation. The RAG2−/− γC−/− animals are allogeneic to the donor-derived NK cells but do not have a functional immune system, making them incapable of rejecting the donor-derived cells, and lack the inflammatory environment created by total body irradiation. In vivo BLI revealed that NK cells still traffic to the spleen and lymphoid organs (Fig. 5A), while ex vivo imaging of the gastrointestinal tract showed that NK cells still migrate to the mLN and gastrointestinal tract tissue in the absence of irradiation (Fig. 5B). There were a few notable differences between conditioned and nonconditioned RAG2−/− γC−/− hosts: in the nonconditioned RAG2−/− γC−/− animals, the trafficking to the cLN was delayed and reduced, and there appears to be NK cell homing to the bone marrow cavity and thymus. Thus, tissue changes from the conditioning regimen are not the only factors allowing NK cells to enter and proliferate in lymphoid organs and gut tissue in vivo and thus is not solely responsible for driving the tissue-specific homing and proliferation observed; however, it does seem to impact NK cell trafficking by altering homing to different target tissues (thymus, bone marrow) and kinetics of tissue-specific proliferation.
FIGURE 5.
Impact of irradiation on NK cell proliferation and homing in allogeneic BMT. A, Donor luc+ NK cells still proliferate when transplanted into unirradiated RAG2−/− γC−/− allogeneic recipients (lacking T, B, and NK cells) as measured by bioluminescence intensity. One representative animal for each group is shown over time. Groups consisted of three animals each and data are representative of two independent experiments. Days posttransplant are indicated along the top of the panels. B, Ex vivo imaging revealed trafficking patterns in the gastrointestinal tract of RAG2−/− γC−/− mice similar to irradiated, allogeneic recipients. Donor luc+ NK cells traffic to the mLN, spleen, and gastrointestinal tissue.
IL-2 enhances in vivo NK cell proliferation
IL-2 is important for NK cell activation and effector function (35). We evaluated the impact of administering exogenous IL-2 to allogeneic and syngeneic BMT recipients having received donor NK cells. IL-2 (5 × 104 U/day) or PBS was administered i.p. every day for 14 days after transplant, and the animals were imaged with BLI on successive days. An increase in the BLI signal was observed in both allogeneic and, more dramatically, in syngeneic recipient animals, as seen in the in vivo BLI images and BLI quantification (Fig. 6A and B). Both recipient groups had a large increase in signal intensity in the abdomen, but further ex vivo imaging of the gut tissue confirmed that this was not a tissue-specific expansion in syngeneic recipients, but rather expansion at the site of IL-2 administration in the peritoneum (Fig. 6C). The amplification of the BLI signal in the gastrointestinal tissue of allogeneic recipients in the presence of exogenous IL-2 is apparent in the ex vivo bioluminescent images (Fig. 6C). Additionally, we sought to determine the impact of IL-2 administration on expression of tissue-specific homing receptors. Compared with freshly isolated NK cells, NK cells isolated from allogeneic IL-2-treated animals significantly down-regulated CD62L ( p = 0.003), and NK cells isolated from allogeneic as well as syngeneic recipients treated with IL-2 significantly down-regulated α4β7 ( p = 0.005 and 0.002, respectively). However, there was no significant change of expression of homing markers on NK cells isolated from allogeneic recipients with or without IL-2 for any of the markers examined (data not shown). Thus, exogenous administration of IL-2 does enhance NK cell expansion, but does not alter the homing patterns of NK cells during BMT.
FIGURE 6.
Exogenous IL-2 amplifies NK cells proliferation in allogeneic and syngeneic BMT recipients. A, Daily administration of exogenous IL-2 (50,000 U i.p.) in vivo increases tissue-specific proliferation of donor luc+ NK cells (FVB/N, H-2q) in allogeneic recipients (luc+NK+PBS) as measured by BLI, compared with allogeneic animals receiving luc+ NK cells and PBS as a control (luc+NK+PBS). Exogenous IL-2 increased the total BLI signal emitted from syngeneic mice receiving luc+ NK cells compared with PBS controls, but this signal arose mainly from the peritoneum, the site of IL-2 administration. One representative animal for each group of four to five mice is shown over time. Days posttransplant are indicated along the top of the panels. B, The average luc+ NK cell signal from transplanted mice was measured with BLI. Comparing total photons emitted per mouse per second, a significant increase in BLI signal occurs among allogeneic NK cells supplemented in vivo with IL-2 compared with PBS controls (left). A significant increase was also seen in animals receiving syngeneic NK cells and IL-2 compared with PBS controls (right). Allogeneic bone marrow recipients are shown as controls. Data are an average of four to five mice per group and are representative of three independent experiments. Error bars represent SE. C, Ex vivo imaging of the gastrointestinal tract of allogeneic PBS-treated and allogeneic IL-2-treated mice indicate greater tissue-specific proliferation of NK cells in the spleen and gut in the presence of IL-2. Ex vivo BLI of syngeneic transplanted, IL-2-treated animals reveals that the BLI signal coming from the peritoneum was not tissue specific in the gut or spleen, but was rather peritoneal expansion of the cells at the site of IL-2 administration.
NK cells treated with IL-2 exhibit greater antitumor ability
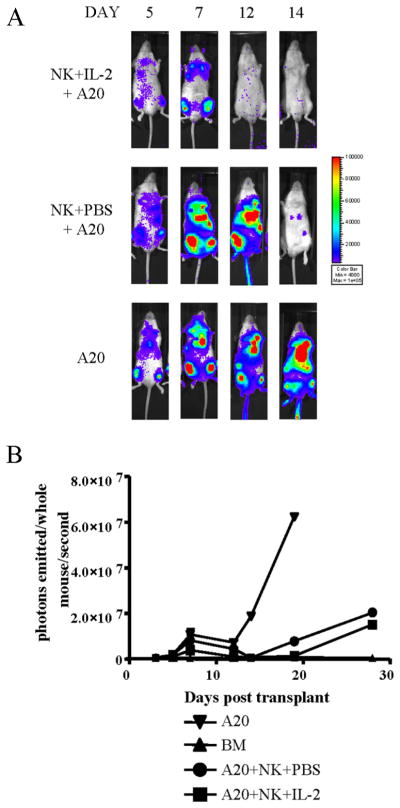
Having observed a significant increase in NK cell proliferation in the presence of exogenous IL-2, we asked whether this had any functional consequences by assessing NK cell activity in the presence or absence of exogenous IL-2 in a murine tumor model. Mice were administered A20 luc+/yfp B cell lymphoma cells, allowing us to assess tumor burden and growth by in vivo BLI (25). Tumor-bearing animals were treated with either NK cells plus daily exogenous IL-2 or NK cells plus PBS as a control. The tumor expanded initially in both treatment groups, as assessed by in vivo BLI engraftment in the bone marrow cavities (Fig. 7A). However, the NK+IL-2+A20 group showed greater tumor clearance by 2 wk after transplant, evident in both in vivo BLI and quantification of the whole body images (Fig. 7, A and B). The A20 control animals receiving no treatment exhibited greater tumor growth. Although this NK cell dose was not sufficient to completely eradicate the tumor at later time points, this study demonstrates that NK cells have greater antitumor potential in the presence of IL-2.
FIGURE 7.
Enhanced antitumor potential of NK cells treated with exogenous IL-2. A, In vivo BLI of animals treated with the A20 luc+/yfp B cell lymphoma cell line and treated with NK + IL-2 (top panel) or NK + PBS (control, middle panel) on successive days after transplant. A20 luc+/yfp controls are shown (bottom panel). One representative animal is shown of a group of four. B, Average quantification of whole-body bioluminescent images shows lower overall BLI signal in A20 + NK + IL-2 (■) compared with A20 + NK + PBS (●). Steady increases in tumor burden of A20 controls are indicated (▼) along with TCD-BM recipients (▲) as control animals. Error bars indicate SE.
Discussion
In this report, we provide the first description of NK cell trafficking in allogeneic and syngeneic BMT using an in vivo BLI strategy. This study provides critical new information regarding the behavior of NK cells in BMT, which has important clinical application as NK cells hold promise as a cellular therapy in reducing GVHD in allogeneic BMT, especially from a haploidential donor. Additionally, donor NK cells have been shown to promote bone marrow engraftment in allogeneic recipients, while not causing GVHD themselves (26). GVHD has a characteristic tissue-specific induction pattern; thus, the description of the tissue-specific homing of NK cells in BMT provides valuable insights into their therapeutic potential in reducing GVHD. Our study describes the homing and proliferative capabilities of transplanted NK cells and examines some of the factors responsible for these biological characteristics.
The duration of the luc+ NK cell BLI signal in our studies is consistent with NK cell survival time in vivo as reported in the literature. Jamieson et al. (18) have reported a half-life of NK cells of 17 days based on BrdU-labeling studies. We are able to visualize the BLI signal up to 4 wk after transplantation. This duration of the BLI signal was likely due to the limited life span of NK cells, but could also be due to the reconstitution of donor NK cells from bone marrow at this time point, limiting further expansion of the donor population of NK cells. There is also a small population of mature NK cells in the TCD-BM that could be competing for space with the adoptively transferred NK cells. Previous studies have demonstrated a correlation between the BLI signal intensity from a luc+ tumor line in vivo and the number of luc+ tumor cells reisolated from this tumor-bearing animal (25). Therefore, taken together, the increase in the BLI signal shown in Fig. 1 as well as the CFSE studies demonstrate that NK cells have a greater proliferative capacity in allogeneic recipients.
Interestingly, the striking differences in NK cell proliferation between irradiated allogeneic and syngeneic recipients does not appear to be related to Ly49/MHC class I-mediated interactions by the donor NK cells in the allogeneic environment. In preliminary studies, the Ly49 repertoire of transplanted NK cells pre- and post-transplant did not reflect an expansion of the alloreactive subset of NK cells (Ly49C/I+) in allogeneic recipients (our unpublished observations). This finding indicates that factors other than MHC interactions may be driving NK cell proliferation in allogeneic recipients.
The in vivo trafficking patterns of NK cells in allogeneic transplant recipients are remarkably similar to allogeneic T cell trafficking. In addition to homing to the same organs and tissues, spleen, lymph nodes, and gastrointestinal tract, the cells also acquire a similar phenotype regarding homing receptor up-regulation. Like allogeneic T cells, NK cells lose expression of L-selectin in vivo upon activation and proliferation; however, this is not observed among syngeneic NK cells, which up-regulate homing markers without down-regulating CD62L. Both cell populations up-regulate the gut-homing receptor α4β7 and the skin-homing molecule P-selectin ligand. However, they differ in the duration of time they persist in the tissues as measured by BLI. These findings are especially interesting when taken in combination with the knowledge that T cells cause GVHD in an allogeneic environment, whereas NK cells do not. Based upon these observations, the reason for this difference in GVHD induction potential is not because NK cells are not reaching GVHD priming sites and target tissues; however, they do not cause the tissue damage at those sites characteristic of the GVHD response.
It is interesting to note that there is an increase in bioluminescence from lymph node sites, while the NK cells in the allogeneic setting down-regulate CD62L, a lymph node-homing molecule. Based upon the fact that syngeneic NK cells also have increased bioluminescence in lymph nodes without down-regulating CD62L and our CFSE analysis of NK cells reisolated from lymph nodes (data not shown), we can conclude that this is due to cell proliferation in these sites rather solely to an accumulation of cells from the peripheral blood.
Previous work from our laboratory assessed the impact of anti-CD62L Ab on donor T cell trafficking in GVHD induction with a similar blocking regimen, and parallel observations were made with regard to the impact of this Ab on NK cell trafficking. The anti-CD62L Ab, when administered together with anti-MAdCAM-1, prevented T cell entry into cLN and other lymph nodes as well as Peyer’s patches (36). The treatment with anti-CD62L Ab in our studies reduced NK cell trafficking into lymph nodes, although some luc+ NK cells were visible at some nodal sites during Ab administration, albeit with a lower BLI signal. This is due to either insufficient blocking at the indicated Ab dose or more likely redundant molecules responsible for NK cell trafficking into these lymphoid tissues.
It has been well demonstrated that the pretransplant conditioning regimen causes tissue damage that is associated with GVHD severity (34, 37). This tissue injury activates host cells which secrete proinflammatory cytokines, such as IL-1 and TNF-α (33), which can activate host dendritic cells and contribute to GVHD pathophysiology (38). Because NK cells express receptors for many proinflammatory chemokines and migrate in response to them, we reasoned that our observations of NK cell trafficking in allogeneic BMT could be a result of inflammation induced by the irradiation. However, using RAG2−/− γC−/− recipients, which do not require conditioning since they lack endogenous T and NK cells and are incapable of rejecting the donor-derived allogeneic NK cells, demonstrates that the conditioning regimen necessary in BMT is not solely responsible for the observed NK cell trafficking patterns. NK cell trafficking to the lymph nodes is slightly delayed and lessened in the RAG2−/− γC−/− animals, possibly due to the decreased lymphoid compartment and organ size in these immunodeficient animals. We observed NK cell trafficking to the bone marrow compartment in these recipient animals, indicating that their trafficking and expansion may be driven more by homeostatic mechanisms in this transplant setting. Therefore, the irradiation does not seem to be solely responsible for NK cell trafficking, since the cells are still able to enter lymph nodes and gut tissue in the absence of irradiation.
NK cells require IL-2 for activation and survival and a number of clinical trials have been performed with IL-2 in addition to NK cells. Indeed, exogenous IL-2 augmented the proliferation of transplanted NK cells, especially in recipients of syngeneic NK cells, but did not alter or enhance the expression of homing molecules toward lymphoid or GVHD target tissues. These findings indicate that the homing receptor expression of the NK cells is not enhanced with the addition of an activating cytokine. The large increase in the BLI signal in the peritoneal cavity represented a localized accumulation of NK cells at the site of IL-2 administration. This study has functional consequences, as we observed increased tumor clearance in the presence of NK cells which was augmented with exogenous IL-2. This increased tumor clearance is presumably due to either increased proliferation of NK cells and thus greater cell numbers or enhanced functional capacity of IL-2-activated NK cells.
In conclusion, these studies provide the first visualization of NK cells following transplantation. The results demonstrate that NK cells proliferate in response to allogeneic signals and cytokines, survive for ~30 days following adoptive transfer, and infiltrate a variety of organs and tissues without GVHD induction. These results provide important insights into the biology of adoptively transferred NK cells and form the basis of future studies aimed at modulating NK cell survival and function.
Acknowledgments
We thank Ruby M. Wong for assistance with statistical analysis, Suparna Dutt for assistance with isolation of gut-infiltrating lymphocytes, and Eugene Butcher for the gift of blocking Abs against CD62L and isotype control Abs. We thank all members of the Negrin laboratory for helpful discussions and technical assistance.
Footnotes
Abbreviations used in this paper: BMT, bone marrow transplantation; GVHD, graft-versus-host disease; GVT, graft-versus-tumor; BLI, bioluminescence imaging; luc+, luciferase expressing; LPL, lamina propria lymphocyte; IEL, intraepithelial lymphocyte; TCD-BM, T-cell depleted bone marrow; cLN, cervical lymph node; iLN, inguinal lymph node; aLN, axillary lymph node; mLN, mesenteric lymph node; pLN, peripheral lymph node; γC, γ-chain.
Disclosures
The authors have no financial conflict of interest.
This work was supported by National Institutes of Health Grants 2 P01 CA049605 and 1 R01 CA125276.
References
- 1.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 2.Harshan KV, Gangadharam PR. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infect Immun. 1991;59:2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci USA. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scharton TM, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy WJ, Keller JR, Harrison CL, Young HA, Longo DL. Interleukin-2-activated natural killer cells can support hematopoiesis in vitro and promote marrow engraftment in vivo. Blood. 1992;80:670–677. [PubMed] [Google Scholar]
- 6.Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 7.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 8.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beilhack A, Schulz S, Baker J, Beilhack GF, Wieland CB, Herman EI, Baker EM, Cao YA, Contag CH, Negrin RS. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panoskaltsis-Mortari A, Price A, Hermanson JR, Taras E, Lees C, Serody JS, Blazar BR. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–3598. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- 11.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 12.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 14.Jiang D, Liang J, Hodge J, Lu B, Zhu Z, Yu S, Fan J, Gao Y, Yin Z, Homer R, Gerard C, Noble PW. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J Clin Invest. 2004;114:291–299. doi: 10.1172/JCI16861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 16.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 17.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 20.Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, Murphy WJ. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101:1835–1842. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, Maccario R, Bonetti F, Wojnar J, Martinetti M, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 22.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 23.Negrin RS, Contag CH. In vivo imaging using bioluminescence: a tool for probing graft-versus-host disease. Nat Rev Immunol. 2006;6:484–490. doi: 10.1038/nri1879. [DOI] [PubMed] [Google Scholar]
- 24.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–648. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 26.Murphy WJ, Bennett M, Kumar V, Longo DL. Donor-type activated natural killer cells promote marrow engraftment and B cell development during allogeneic bone marrow transplantation. J Immunol. 1992;148:2953–2960. [PubMed] [Google Scholar]
- 27.Ohgama J, Katoh M, Hirano M, Arase H, Arase-Fukushi N, Mishima M, Iwabuchi K, Ogasawara K, Onoe K. Functional studies on MEL-14− and MEL-14− T cells in peripheral lymphoid tissues. Immunobiology. 1994;190:225–242. doi: 10.1016/S0171-2985(11)80271-8. [DOI] [PubMed] [Google Scholar]
- 28.Morris MA, Ley K. Trafficking of natural killer cells. Curr Mol Med. 2004;4:431–438. doi: 10.2174/1566524043360609. [DOI] [PubMed] [Google Scholar]
- 29.Uksila J, Salmi M, Butcher EC, Tarkkanen J, Jalkanen S. Function of lymphocyte homing-associated adhesion molecules on human natural killer and lymphokine-activated killer cells. J Immunol. 1997;158:1610–1617. [PubMed] [Google Scholar]
- 30.Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J Exp Med. 2005;202:1679–1689. doi: 10.1084/jem.20051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andre P, Spertini O, Guia S, Rihet P, Dignat-George F, Brailly H, Sampol J, Anderson PJ, Vivier E. Modification of P-selectin glycoprotein ligand-1 with a natural killer cell-restricted sulfated lactosamine creates an alternate ligand for L-selectin. Proc Natl Acad Sci USA. 2000;97:3400–3405. doi: 10.1073/pnas.040569797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Villar JJ, Zapata JM, Melero I, Postigo A, Sanchez-Madrid E, Lopez-Botet M. Expression and function of α4/β7 integrin on human natural killer cells. Immunology. 1996;89:96–104. doi: 10.1046/j.1365-2567.1996.d01-706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xun CQ, Thompson JS, Jennings CD, Brown SA, Widmer MB. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood. 1994;83:2360–2367. [PubMed] [Google Scholar]
- 34.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 35.Henney CS, Kuribayashi K, Kern DE, Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981;291:335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- 36.Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, Landan G, Herman EI, Butcher EC, Contag CH, Negrin RS. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111:2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara JL, Levy R, Chao NJ. Pathophysiologic mechanisms of acute graft-vs.-host disease. Biol Blood Marrow Transplant. 1999;5:347–356. doi: 10.1016/s1083-8791(99)70011-x. [DOI] [PubMed] [Google Scholar]
- 38.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–161. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]