Abstract
Objective
Regulation of vascular smooth muscle (VSM) proliferation and contractile differentiation are important factors in vascular development and subsequent cardiovascular diseases. Recently, microRNAs (miRNAs) have been shown to regulate fundamental cellular processes in a number of cell types but the integrated role of miRNAs in VSM in blood vessels is unknown. Here, we investigated the role of miRNAs in VSM by deleting the rate limiting enzyme in miRNA synthesis, Dicer.
Methods and Results
Deletion of Dicer in VSM results in late embryonic lethality at E16-E17 associated with extensive internal hemorrhage. The loss of VSM Dicer results in dilated, thin walled blood vessels caused by a reduction in cellular proliferation. In addition, blood vessels from VSM deleted Dicer mice exhibited impaired contractility due to a loss of contractile protein markers. We found this effect to be associated with a loss of actin stress fibers, and partly rescued by over expression of miR-145 or myocardin.
Conclusion
Dicer-dependent miRNAs are important for VSM development and function by regulating proliferation and contractile differentiation.
Keywords: vascular smooth muscle, micro RNA, cell proliferation, differentiation, and contractile function
Introduction
microRNAs (miRNAs) are short (∼22 nt) noncoding RNAs that are involved in the regulation of mRNA expression and protein synthesis 1 and each miRNA potentially targets multiple transcripts 2. Immature miRNAs are processed by the two RNase III endonucleases, Drosha and Dicer, and then incorporate into the RNA-induced silencing complex (RISC). Depending on the complementarity of the miRNA with the 3′UTR of the target mRNA, the RISC complex will mediate either translational repression/activation or degradation of the target mRNA.
One way to decipher the importance of miRNAs in development and cell biology has been by Dicer gene disruption in mice. The global loss of Dicer in knockout mice results in embryonic lethality associated with a loss of pluripotent stem cells and defective blood vessel formation 3, 4. However, Dicer is not essential for developmental angiogenesis, since endothelial specific Dicer hypomorphic mice survive, but have impaired postnatal angiogenic responses 5. Although VSM specific KO of Dicer have not been investigated to date, important functions of miRNAs in VSM are beginning to emerge. miR-21 is induced in at least one VSM type by the differentiation factors TGF-β and bone morphogenic protein 6. In contrast, platelet derived growth factor (PDGF) induces miR-221 and the dedifferentiation process in VSM 7. miR-21 and miR-221 are also upregulated in vivo after vascular injury and may regulate the extent of neointimal growth in vivo 8, 9. Recently, several groups have found that miR-145 positively regulates VSM-specific gene expression, differentiation, actin cytoskeleton and contractile function 10-13. As expected, the potential mechanisms for these effects are varied including possible effects on Kruppel-like factors 4 and 5 (KLF4 and KLF5), myocardin activity and angiotensin converting enzyme. These data suggest the essential role of miR-145 as a critical regulator of VSM differentiation and function. However, despite the role of miR-145 in key aspects of VSM differentiation and stem cell renewal 10, 14, 15, mice lacking miR-145 develop normally, but exhibit hypotension, impaired vascular contractility, reduced injury-evoked neointima or enhanced aging-dependent neointima consistent with an important, yet non-essential, role of miR-145 in VSM.
Since many miRNAs can regulate the levels of overlapping genes, we wanted to address the global integration of miRNAs in VSM development and function using mice with a floxed Dicer allele 16 bred to VSM-specific SM22α Cre-recombinase. Here we show that inactivation of Dicer in VSM results in late, embryonic lethality at E16-17 due to decreased smooth muscle cell (SMC) proliferation and differentiation resulting in thinner vessel walls, impaired contractility and hemorrhage. Mechanistically, the loss of Dicer impaired the actin cytoskeleton and defects in VSM-specific gene expression could be partially rescued by miR-145 or myocardin but not miR-221 or miR-21, suggesting that miR-145 regulates VSM differentiation upstream or at the level of myocardin:SRF. Thus, miRNAs are critical for VSM development independent of miR-145 and regulate blood vessel diameter and contractility.
Methods
For expanded Materials and Methods, please see supplemental materials (available online at http://atvb.ahajournals.org).
Mice
Constitutive inactivation of Dicer in VSM was achieved by breeding SM22αCre transgenic mice (The Jackson Laboratory) with mice harboring loxP sites flanking an RNaseIII domain (exons 20 and 21) of Dicer (Dicerfl/fl) 16. In order to generate adult mice with SMC specific deletion of Dicer, Dicerfl/fl mice were intercrossed with transgenic mice expressing the tamoxifen-dependent Cre recombinase CreERT2 under control of the SMC-specific myosin heavy chain (SM-MHC) gene Myh11 17. A comprehensive study of these animals is forthcoming. All animal procedures were approved by the Yale Animal Care Committee.
Cell culture
Aortic SMC were isolated from tamoxifen-inducible SMC-Dicer KO and Control mice as described in supplemental Materials and Methods. For miRNA over expression, SMC at passages 3-5 were transfected with 60nM miRIDIAN miRNA mimics (Dharmacon) of mmu-miR-145, mmu-miR-21, mmu-miR-221, mmu-miR-143 or negative control mimic for 24-96h. For actin depolymerization, SMC were treated with 250nM Latrunculin B 6h prior to transfection with miRNA mimics.
Force measurement
Approximately 1.7 mm long segments of umbilical arteries from E15.5-E16.5 embryos were dissected in Ca2+-free HEPES buffer composed of [(in mM) 135.5 NaCl, 5.9 KCl, 1.2 MgCl2, 11.6 glucose, and 11.6 HEPES; pH 7.4, 37°C] and mounted on a myograph (610M; Danish MyoTechnology, Aarhus, Denmark). The vessel segments were then allowed to equilibrate at 37°C at a preload of 0.5mN/mm for 1h in HEPES Buffer with 2.5mM Ca2+ (Ca2+-HEPES). Active and passive circumference-tension relationships were then generated as described in supplemental Materials and Methods.
Results
SM22α-targeted deletion of Dicer results in embryonic lethality
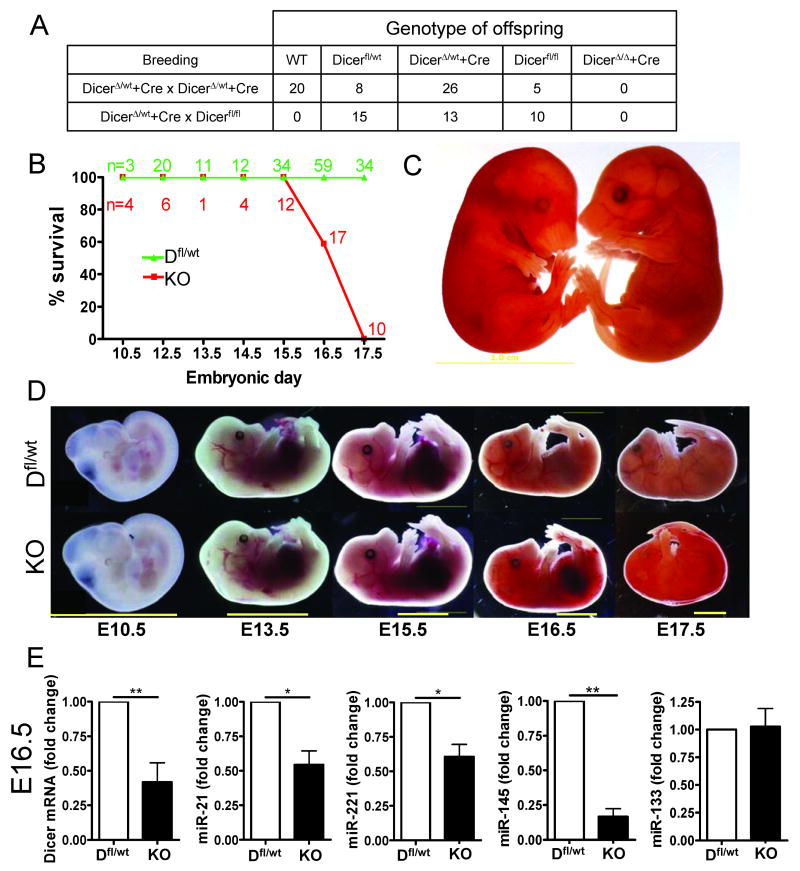
To address if SM22α-targeted deletion of Dicer influences vascular development, male SM22αCre/DicerΔ/wt mice were bred with either female Dicerfl/fl or SM22αCre/DicerΔ/wt mice. A total number of 97 pups were born with no live pups of the SM22αCre;DicerΔ/Δ genotype (SMC-Dicer KO; fig. 1A). All other combinations of genotypes were indistinguishable from WT mice. Subsequently, the timing and cause of embryonic lethality was investigated using synchronized breeding of female Dicerfl/fl mice bred to male SM22αCre/Dicer Δ/wt mice. SMC-Dicer KO embryos die between E16-E17 due to widespread hemorrhage in the skin and abdomen (fig. 1B, C and D). Surprisingly, SMC-Dicer KO embryos were indistinguishable from WT or any other genotype of embryos at E15.5. No significant difference in growth was observed in live embryos at E16.5 as measured by crown-to-rump length and wet weight (suppl. fig. 1A).
Figure 1. Loss of Dicer in smooth muscle is lethal at E16.5-17.5.
Two different breeding strategies were used to produce SMC-Dicer KO (DicerΔ/Δ + Cre) mice. The number of live pups of different genotypes is shown in A. A total number of 97 pups were genotyped. Timed mating revealed that SM22a-targeted deletion of Dicer results in embryonic lethality at E16.5 to E17.5 (B) and the embryonic lethality was associated with widespread internal hemorrhaging (C). No major difference in embryonic development was observed between Dicerfl/wt (abbreviated Dfl/wt) and SMC-Dicer KO before E16.5 (D). qPCR analysis of smooth muscle rich umbilical cords demonstrated a significant decrease in Dicer mRNA and miRNAs at E16.5 (E). Data are mean ± SEM from *p<0.05
Dicer mRNA and miRNA expression levels were examined in VSM rich umbilical cords (including the umbilical and vitelline arteries and veins). Dicer mRNA levels were decreased at E14.5 (suppl. fig. 1B) and E16.5 (fig. 1E). The residual Dicer expression is likely due to other cell types present in umbilical cords such as endothelial cells and fibroblasts. Analysis of miR-21, miR-221 and miR-145 were selected on the basis that recent studies have demonstrated that these miRNAs play a role in VSM differentiation and proliferation 6-10, 15 while miR-133 was selected based on its role in cardiac and skeletal muscle development via regulation of the transcription factor serum response factor (SRF), also important in VSM differentiation 18, 19. Expression of miR-21, miR-221 and miR-145 were significantly reduced in SMC-Dicer KO umbilical cords at E16.5 (fig. 1E). Since miR-145 and miR-143 are the only miRNAs shown to be relatively specific to SMC in the vasculature, the residual expression of miR-21 and miR-221 in the umbilical cords may represent expression in other cell types. Similarly, expression of miR-133 was unchanged suggesting that this miRNA is primarily expressed in non-SMC present in the umbilical cord.
Hemorrhage in SMC-Dicer KO embryos originates from the liver
Morphometry of E16.5 embryos revealed intraperitoneal bleeding in a majority of the SMC-Dicer KO embryos (suppl. fig. 2). In order to identify the origin of the intraperitoneal hemorrhage in these embryos, major internal organs were dissected from Dicerfl/wt and SMC-Dicer KO embryos. In SMC-Dicer KO embryos, distal parts of the liver consistently contained darker red patches suggesting that intraperitoneal hemorrhage originated from this region (suppl. fig. 3A). This correlates with a general increase in red blood cells observed in H&E stained sections of distal liver (suppl. fig. 2).
SM22αCre is highly expressed in vascular but not visceral smooth muscle
Although SM22α is considered a marker of smooth muscle it is, like most other markers for smooth muscle lineage, transiently expressed in the heart during E8-12.5 20. To investigate the specificity of SM22αCre expression in mouse embryos, SM22αCre mice were crossed to ROSA26 reporter mice. As shown in suppl fig. 3C, whole mount LacZ staining of E15.5 embryos revealed positive LacZ staining in both venous and arterial vessels in the placenta, umbilical cord and yolk sac. In paraffin sections, LacZ staining was observed specifically in VSM of the aorta (Ao), umbilical artery (UA) and cardiomyocytes with no or little staining in other tissues including visceral smooth muscle of the esophagus (Eso), trachea (Tra) and bladder (BL, suppl. fig 3C). Despite the lack of detectable SM22αCre expression or loss of Dicer in visceral and bronchial smooth muscle, a general reduction in the size of the stomach, intestine, urinary bladder, kidney and lung was observed (suppl. fig. 3A). The phenotype of SMC-Dicer KO embryos is clearly distinguishable from a model of cardiac specific Dicer KO since deletion of Dicer in cardiac progenitor cells by E8.5 results in embryonic lethality due to heart failure caused by pericardial edema and a poorly developed ventricular myocardium 21. SMC-Dicer KO did not exhibit any signs of pericardial edema however both ventricles were slightly dilated and both atria were smaller in size (suppl. fig. 3A and B). The absence of a severe cardiac phenotype in the SM22α-mediated SMC-Dicer KO embryos is not clear, but likely relates to the transient and delayed onset of Cre recombinase expression in cardiomyocytes as compared to cardiac-specific promoters such as Nkx2.5 and α-MHC.
Transmission electron microscopy revealed dramatic changes in ultrastructure of the vessel wall and medial smooth muscle cells in Dicer KO aorta
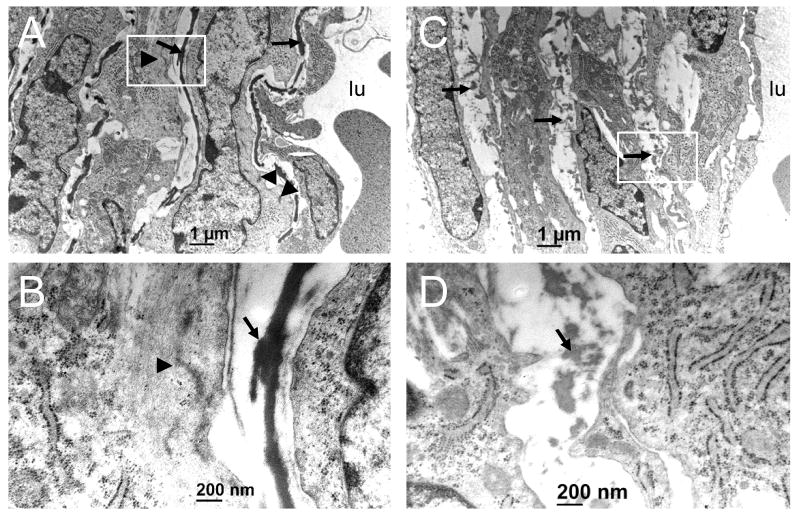
Transmission electron microscopy (TEM) was used to clarify how the loss of Dicer affects the ultrastructure of VSM and the surrounding extracellular matrix. Analysis of four independent dorsal aortae from either Dicerfl/wt or SMC-Dicer KO E15.5 embryos revealed dramatic changes in vascular ultrastructure in SMC-Dicer KO aortae. Whereas Dicerfl/wt embryos showed appropriate elastic lamellae circumscribing layers of medial SMC (fig. 2A and B), these structures were discontinuous and highly fragmented in the SMC-Dicer KO embryos (fig. 2C and D). Dense plaques and myofilament bundles, which typify the ultrastructure of differentiated SMC in Dicerfl/wt SMC, were less frequent in the SMC-Dicer KO (fig. 2 A-D). Moreover, many medial SMC in SMC-Dicer KO embryos were polygonal and poorly organized around the circumference of the dorsal aorta (suppl. fig. 4). The latter cells also appeared to be overly enriched with rough endoplasmic reticulum (fig. 2, panel D and data not shown). Together, the TEM data illustrate ultrastructural phenotypes in SMC-Dicer KO mice that are consistent with defective vascular structure and altered SMC differentiation.
Figure 2. The loss of Dicer in VSM causes structural abnormalities.
Cross-sections of Dfl/wt (A,B) and SMC-Dicer KO (C,D) dorsal aorta from E15.5 embryos. The white boxed region in panels A and C (12,000x) are depicted in panels B and D under higher magnification (60,000x). Arrows denote elastic lamellae and arrowheads point to dense plaques and myofilament arrays seen most prevalently in SMC of wildtype aorta. lu, lumen.
Altered vascular geometry in the aorta of SMC-Dicer KO embryos
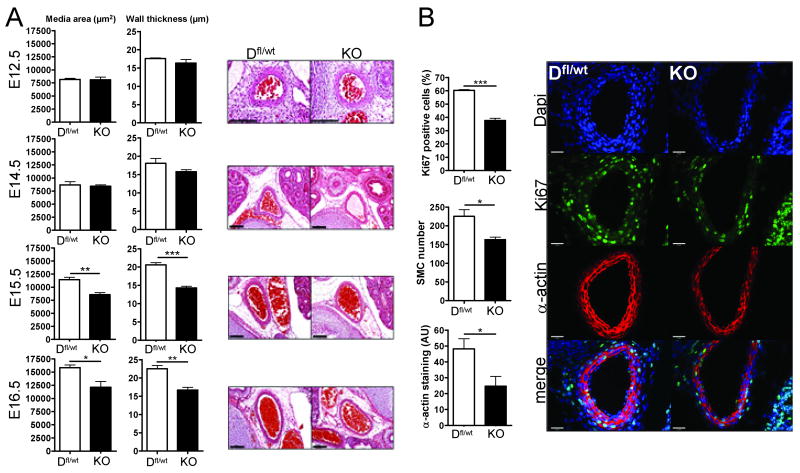
Physiologically, blood flow and capillary hydrostatic pressure is regulated by vascular contraction of arteries/arterioles that distribute oxygen and nutrients to tissues. Loss of vascular contractility in the arterial tree could potentially increase capillary pressure as systemic blood pressure increases during mid-gestation, thereby promoting hemorrhage and bleeding. It is well accepted that arterial contractile force is dependent on the differentiated state of VSM and the thickness of the vessel wall. To investigate if the loss of Dicer in VSM results in altered vascular geometry, quantitative morphometry of the aorta was performed on H&E stained sections of E12.5, E14.5, E15.5 and E16.5 embryos. As shown in figure 3A, decreased medial area (left graphs) and thickness (right graph) is evident in E15.5 and E16.5 SMC-Dicer KO mice. At E15.5 this was also associated with an increase in lumen diameter (suppl. fig. 5A).
Figure 3. SM22α-targeted deletion of Dicer causes decreased smooth muscle proliferation and aortic remodeling in vessels of E15.5 and E16.5 embryos.
Paraffin embedded sections of Dfl/wt and SMC-Dicer KO embryos were stained with hematoxylin and eosin and medial area of the vessel and wall thickness was calculated from the inner and outer media circumference in three sections from each embryo. Bars indicate 100μm. *p<0.05 (A). Paraffin embedded sections from E14.4 embryos were stained for Ki67 antigen (green), SM-α-actin (red) and DAPI (blue; B). The number of Ki67 positive nuclei within the α-actin positive area was quantified as well as the α-actin staining intensity. In a separate experiment the total number of nuclei in the aortic media was quantified in E15.5–E16.5 embryos.
Since deletion of Dicer in various cell lineages has been shown to impair cell proliferation 22-24, we tested if the reduced smooth muscle medial area in SMC-Dicer KO embryos was due to a decrease in SMC proliferation during development. As shown in figure 3B, a significant decrease in Ki67 positive aortic VSM was observed at E14.5 and E15.5 in concordance with a reduced number of aortic VSM cells found in E15.5-E16-5 embryos. In addition, the level of immunoreactive SM-α-actin was reduced in aortae of E14.5 embryos indicating a decrease in muscle specific gene expression (fig. 3B). Two important signaling pathways that regulate VSM proliferation are the mitogen activated protein kinase (MAPK) and phosphotidylinostiol 3′ kinase (PI-3K) pathways; however, the loss of Dicer in VSM did not affect activation of these pathways in umbilical cords from E16.5 embryos (suppl. fig. 5B).
Contractile function and differentiation is decreased in umbilical arteries of Dicer KO embryos
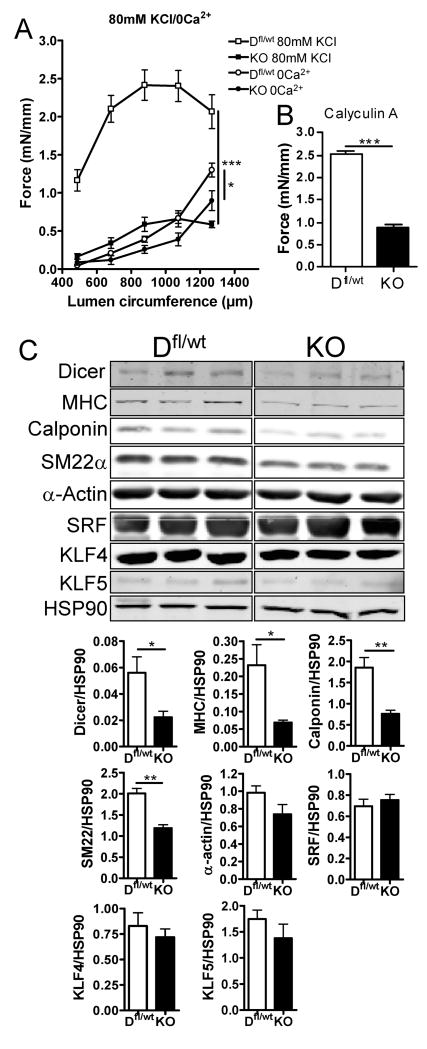
In the adult heart, inducible deletion of Dicer results in a decreased cardiac function and activation of the fetal gene program 25. To assess the contractile function of SMC-Dicer KO vessels, umbilical arterial vessels were used for functional studies. To our knowledge, analysis of contractile function in embryonic mouse umbilical arteries has not been attempted previously, likely due to the small size of these vessels. We examined both active force (due to calcium activation of actin-myosin cross bridges) in response to depolarization by 80 mM potassium (high-K) and passive force (an index of vascular compliance and/or structure) in response to nominally calcium-free buffer at increasing luminal circumferences of the embryonic vessels. As quantified in fig. 4A, the loss of Dicer in VSM markedly impairs contractile function in arteries. Reduced contractile responses to depolarization in SMC-Dicer KO vessels could be caused by altered expression or function of ion channels, such as L-type voltage gated calcium channels in the cell membrane. To test this hypothesis, we also measured contractile force in response to the protein phosphatase (PP1, PP2A) inhibitor Calyculin A, which causes calcium-independent contraction of smooth muscle by increasing myosin light chain phosphorylation 26. Similar to high-KCl, contractile force to Calyculin A (0.2μM) was reduced in SMC-Dicer KO vessels, suggesting that the cause of contractile dysfunction in the absence of miRNAs is mainly due to an effect on the contractile machinery per se and not signaling events upstream of myosin phosphorylation (fig 4B). Part of the decrease in contractile response is clearly due to a decrease in VSM mass in SMC-Dicer KO umbilical arteries (suppl. fig. 5C and D). However, while VSM mass in SMC-Dicer KO umbilical arteries was decreased by approx 33%, maximal contractile force was decreased by approximately 75%, indicating that contractile properties of the SMCs are disturbed in SMC-Dicer KO vessels. This could be a result of a decrease in actin filament content as suggested by TEM. A rightward shift in the passive length-tension relationship in SMC-Dicer KO together with a right shift in the calculated Lmax (optimal length for maximal force; lumen circumference: WT:968μm, KO;1039) suggests that loss of Dicer increases vascular compliance.
Figure 4. Umbilical vessels from SMC-Dicer KO embryos exhibit reduced contractile function and reduced expression of markers of contractile differentiation.
Umbilical arteries from Dfl/wt and SMC-Dicer KO embryos were mounted in a wire myograph. Contractile force in response to depolarization with 80mM K+ as well as passive tension in calcium free solution was analyzed at increasing lumen circumferences (A). Contractile responses to Calyculin A were also blunted in SMC-Dicer KO vessels (B). Western blot analysis of umbilical cords from Dfl/wt and SMC-Dicer KO E16.5 embryos. Original data are shown from different parts of one nitrocellulose membrane and the signal intensity was quantified and normalized to HSP90 (C). Data are mean ± SEM, *p<0.05.
To directly examine the state of differentiation of VSM, the levels of SMC-specific proteins, SM-MHC, calponin, SM22α and α-actin, were examined in Dicerfl/wt and SMC-Dicer KO cords by Western blotting. The levels of SM-MHC, calponin and SM22α were significantly reduced in SMC-Dicer KO vessels suggesting that miRNAs play an important role in SMC differentiation (fig 4C). A minor part of this effect may be due to the reduction in VSM in SMC-Dicer KO umbilical cords. However, the expression of SM α-actin was not significantly decreased in SMC-Dicer KO umbilical cords suggesting that comparable amounts of SMC from Dicerfl/wt and SMC-Dicer KO were loaded on the gel. The expression of SMC-specific genes is regulated by the transcription factor serum response factor (SRF) together with the co-activators, myocardin and myocardin related transcription factors-A/-B (MRTF-A and –B)27, 28. In addition, the transcription factors KLF4 and KLF5 repress SMC-specific gene expression by both down-regulating myocardin expression and preventing SRF/myocardin dependent gene expression 15, 29. miR145 appears to enhance myocardin expression in some studies 10 while repressing KLF 4 and KLF5 15 to reduce cell proliferation and promote VSM-specific gene expression. However, in E16.5 umbilical cords, neither the protein levels of SRF, KLF4 or KLF5 (fig. 4C), nor the mRNA levels of myocardin or KLF4 (suppl. fig. 5E) were altered in SMC-Dicer KO cords suggesting alternative mechanisms are operational in these mice.
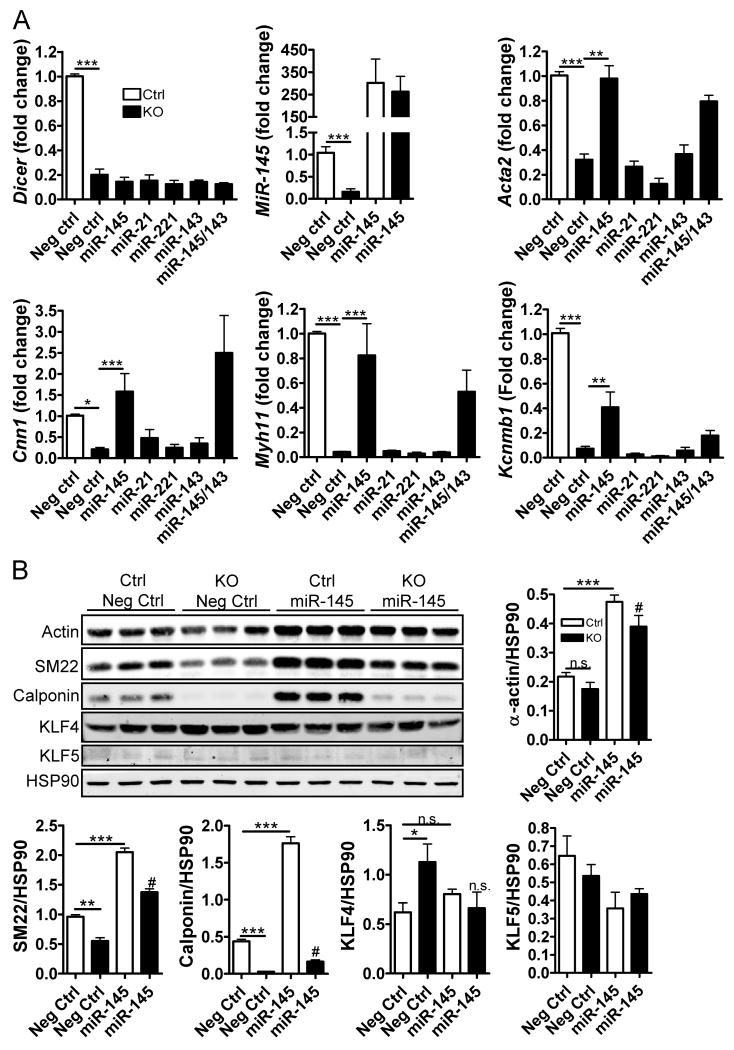
miRNA-145 rescues SMC-specific mRNA and protein expression in Dicer KO SMC
To clarify the roles of specific miRNAs for SMC differentiation, SMC-Dicer KO and control SMCs were isolated from adult, inducible SMC-Dicer KO and Cre negative (Ctrl) mice and then transfected with miRNA mimics (see Methods). Dicer mRNA levels were reduced by approximately 75% in Dicer KO SMC while miR-145 levels were reduced by approximately 85% compared to Ctrl. Interestingly, transfection of miR-145 completely rescued the expression of SMC-specific genes in Dicer KO SMCs (Acta2:SM-α-actin, Myh11:SM-myosin heavy chain and Cnn1: calponin; fig 5A). miR-143 did not significantly alter the effect of miR-145 when added in combination and the fold increase in SMC gene expression was similar in Dicer KO and Ctrl SMC transfected with miR-145 (data not shown), suggesting that miR-145 functions independently of other miRNAs. No significant difference in SMC-specific gene transcription was observed with transfection of miR-21, miR-221 or miR-143 alone in Ctrl or Dicer KO SMCs (fig 5A and data not shown). As shown in suppl. fig 6A, mRNA levels of myocardin, KLF4 and KLF5 were not affected by transfection of miR-145, miR-21 or miR-221 mimics.
Figure 5. miR-145 rescues SMC-specific gene and protein expression in Dicer KO SMC.
(A) Ctrl and Dicer KO SMCs were transfected for 96h with 60nM negative control (Neg Ctrl), miR-145, miR-21, miR-221 or miR-143 mimics. The expression of SMC specific genes (Acta-2: SM-α-actin, Cnn1: calponin, and Myh11: myosin heavy chain), Kcnmb1: B1-subunit of MaxiK channel, Dicer, and miR-145 was analyzed by qPCR and normalized to control VSM cells. 18S was used as internal control for SMC specific genes and Dicer, 5S was used as internal control for miR-145. (B) SMC protein markers and transcription factors were analyzed by Western blotting in Ctrl and Dicer KO SMC transfected with Neg Ctrl or miR-145 mimics. Original data are shown from different parts of one nitrocellulose membrane and the signal intensity was quantified and normalized to HSP90. Data are mean ± SEM, * p<0.05, # p<0.05 vs. KO (Neg Ctrl).
Recently it was demonstrated that the gene encoding for the β1-subunit of the large conductance calcium-activated potassium (MaxiK) channels channel, Kcnmb1, is transcriptionally regulated by myocardin-SRF 30. We found that expression of Kcnmb1 was nearly abolished in Dicer KO SMC and that this effect was partly rescued by transfection of miR-145, confirming that Kcnmb1 is regulated by similar mechanisms as SMC-specific contractile genes.
To test if miRNAs are required for myocardin activity, human myocardin was over expressed in Dicer KO SMCs using adenoviral-mediated gene transfer. As shown in suppl. fig. 6B, over expression of myocardin significantly increased the expression of VSM-specific genes in Dicer KO SMCs, demonstrating that myocardin is functional in a setting of reduced miRNA levels.
Since miRNAs often target translation of mRNA to protein, the effects of some miRNA are only observed at the protein level. We thus quantified the effect of miR-145 on SMC protein markers and transcription factors by Western blotting. As shown in fig 5B, SM22 and calponin protein levels were significantly reduced in Dicer KO SMC vs. ctrl and all SM markers were upregulated in response to miR-145. In addition, we found an increased expression of KLF4 in Dicer KO SMC, which was reduced by addition of miR-145. Surprisingly, SMC proliferation rate, in vitro, was increased in Dicer KO vs. ctrl and this effect was not sensitive to miR-145 transfection (suppl fig 6C). The cause of the discrepancies in Dicer KO SMC proliferation rates as well as KLF4 expression in vivo and in vitro are not clear but may depend on differences in environmental cues.
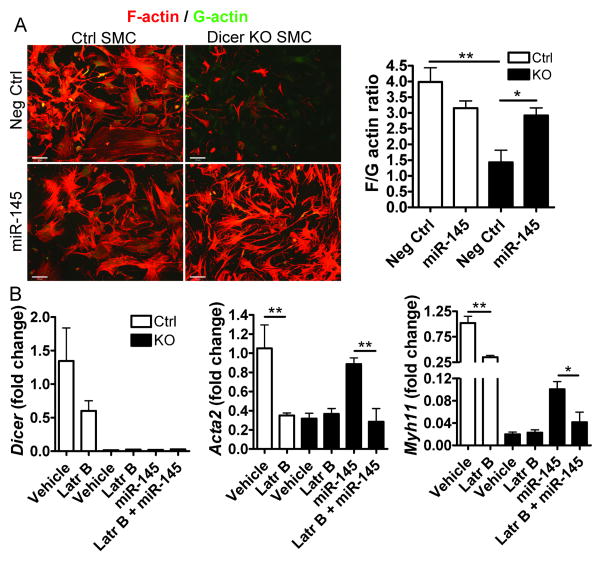
miR-145 induces SMC-specific gene transcription by promoting actin polymerization
A possible mechanism for the reduced vascular contractility and differentiation of SMC-Dicer KO may be perturbed actin dynamics. Naturally, an increased number of actin filaments will promote smooth muscle contractile function but it is also well known that increased actin polymerization promotes SMC specific gene transcription and protein synthesis in vitro and ex vivo 31, 32. Globular (monomeric) G-actin binds to the SRF co-factor MRTF-A or MAL, thereby preventing its translocation to the nucleus 33. An increase in actin filament polymerization results in a reduction of the G-actin pool, nuclear translocation of MAL/MRTF-A and activation of SMC specific gene transcription. It was recently suggested that miR-143/145 regulates the expression of several factors involved in smooth muscle actin dynamics 13. To analyze actin filament structure, Dicer KO and control SMC were isolated and stained with fluorescent Phalloidin (F-actin) and DNase1 (G-actin). Initial experiments were performed using non-transfected SMC and showed that loss of Dicer results in decreased stress fiber formation compared to control cells (suppl fig 7). In following experiments, ctrl and Dicer KO SMC were transfected with either negative ctrl or miR-145 mimic (fig 6A). Surprisingly, addition of miR-145 to Dicer KO SMC rescued the loss of actin filaments indicating that this miRNA plays an important role in actin dynamics. Moreover, we found that depolymerization of actin filaments using Latrunculin B (Latr B) prevents the increase in SMC-specific gene expression induced by miR-145 (fig 6B). This suggests that miR-145 promotes SMC differentiation via an increased actin polymerization.
Figure 6. Decreased stress fiber formation in Dicer KO SMC results in loss of SMC-specific gene expression.
(A) Ctrl and Dicer KO (KO) SMC and were transfected with negative control (Neg ctrl) or miR-145 mimic for 96h. Actin filaments (F-actin) and monomeric actin (G-actin) were visualized using rhodamine phalloidine (red) and Alexa 488 DNase1 (green), respectively. The staining intensity of F- and G-acting in 12-16 fields from 4 separate slides was quantified and shown as the F/G-actin ratio. (B) Ctrl and Dicer KO SMC were pretreated with vehicle or Latrunculin B (Latr B, 250nM) in order to depolymerize actin filaments. The cells were then transfected with negative control or miR-145 mimic for 24h. The expression of SMC specific genes (Acta-2: SM-a-actin and Myh11: myosin heavy chain) and Dicer was analyzed by qPCR and normalized to Ctrl SMC. 18S was used as internal control. Data are mean ± SEM, * p<0.05
Discussion
The present study demonstrates that miRNAs are essential for VSM development and function by regulating SMC differentiation and proliferation. The loss of miRNAs in VSM during early development results in hemorrhage and embryonic lethality. The phenotype of the SMC-Dicer KO mouse is clearly distinguishable from Dicer KO in cardiomyocytes 21, 34. We also found a decreased medial thickness (E15.5-16.5) in the aorta of SMC-Dicer KO embryos caused by a decrease proliferation of SMC at E14.5. In addition, unique measurements of vascular function in E16.5 embryonic arteries revealed a nearly abolished contractile force in SMC-Dicer KO likely caused by a combination of a decreased SMC mass, decreased expression of contractile proteins and decreased formation of actin filaments. We suggest that the loss of SMC proliferation and arterial contractile function results in hemorrhage and embryonic lethality due to dysregulation of blood flow into the microcirculation. Although over expression of miR-145 and myocardin restored SMC specific gene expression in Dicer KO SMC, our results indicate that additional miRNA dependent mechanisms must be operational during VSM development since the loss of Dicer in SMC is lethal whereas the global loss of miR-145 is not.
During vascular development, VSM proliferate and mature into the differentiated, contractile state to maintain pressure and flow throughout the growing embryo. Postnatally, VSM is in its differentiated state expressing markers such as myocardin, SM-MHC, calponin and SM22α and remains quiescent until injury or disease processes such as atherosclerosis promotes a less differentiated and proliferative VSM phenotype. Recent data suggests that miRNAs may differentially modulate the proliferative versus the differentiated state of VSM. Growth factor upregulation of miR-221 promotes VSM proliferation via targeting the negative regulators of the cell cycle, p27 and p57 7, 8. On the other hand, both miR-21 and miR-145 promote VSM differentiation. miR-21 negatively regulates programmed cell death 4 (PDCD4) promoting VSM differentiation while miR-145 enhances myocardin expression/activity, possibly via inhibition of the myocardin negative regulators, KLF 4 and 5 6, 10, 15. In support of previous findings, we show that addition of miR-145 in Dicer KO SMC is sufficient to partly or completely restore SMC-specific gene transcription (fig 5A and B). Thus miR-145 seems to play an important role in SMC differentiation. However, recent studies of miR-143/145 KO mice show that these miRNAs are not necessary for VSM development in vivo 11-13. In fact, in contrast to other reports on miR-143/145 KO mice 11, 12, Olson and coworkers did not detect a difference in the expression of SMC markers between WT and miR143/145 KO aorta 13. In light of these studies, our data showing that the loss of miRNAs in VSM results in embryonic lethality, we surmise that multiple miRNAs and their targets are involved in fine tuning VSM proliferation and differentiation during development.
VSM growth and differentiation, though distinct processes, are not always mutually exclusive events 32, 35. In our model, the net effect of the loss of Dicer in VSM results in impaired growth, differentiation and contractility consistent with miRNAs as regulators of both processes. In E16.5 umbilical cords enriched in VSM, we did observe marked reductions in Dicer, miR-21, miR-221 and miR-145 levels, decreases in SM-MHC, SM22 and calponin proteins, but did not observe differences in the levels of SRF or KLF4/5 proteins (fig. 4C) suggesting that other mechanisms may be involved in miRNA-dependent SMC specific gene transcription in vivo. Recently, it was shown that miR-143/145 regulate the expression of several factors that control actin polymerization 13, which has a profound effect on SRF-dependent gene transcription via control of nuclear translocation of the SRF co-factor, MAL/MRTF-A 33. Herein, we show that stress fiber formation in isolated Dicer KO SMCs is severely impaired in accordance with a loss of actin filaments in vivo in aortas of E15.5 SMC-Dicer KO embryos. Interestingly, the loss of actin filaments was rescued by transfection of miR-145. In addition, miR-145 was not able to promote SMC gene expression in the presence of Latrunculin B, an inhibitor of actin polymerization. These results suggest that miR-145 is involved in the control of actin dynamics and these data are congruent with those of Olson and colleagues who showed impaired actin cyto-architecture and reduced cytoskeletal genes accompanying an apparent defect in VSM cell migration in the miR-143/145 KO 13. Future experiments elucidating the repertoire of miRNAs that negatively regulate VSM-specific contractile/regulatory gene function (including the VSM master regulatory factor, myocardin) will enhance our understanding of miRNA regulation of vascular function in health and disease. For these studies, the SMC-Dicer KO model may be of great value where individual miRNAs can be reintroduced without the confounding effect of endogenous miRNAs.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported in part by grants from the National Institute of Health (R01 HL 064793, RO1 HL 061371, R01 HL 081190, R01 HL 096670, PO1 HL 70295, Contract No. N01-HV-28186 (NHLBI-Yale Proteomics Contract) to W.C.S. and HL 62572 and HL 091168 to J.M.M.); a Swedish Research Council and the Swedish Heart Lung Foundation Award to S.A, and a Scientist Development Award from the American Heart Association to Y.S.
Footnotes
Disclosure: There are no conflicts of interest.
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 4.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 5.Suarez Y, Fernandez-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci U S A. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 10.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O'Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 18.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 19.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78:188–195. doi: 10.1161/01.res.78.2.188. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez Y, Fernandez-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 25.da Costa Martins PA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, Molkentin JD, De Windt LJ. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118:1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 26.Ishihara H, Ozaki H, Sato K, Hori M, Karaki H, Watabe S, Kato Y, Fusetani N, Hashimoto K, Uemura D, Hartshorne DJ. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989;250:388–396. [PubMed] [Google Scholar]
- 27.Miano JM. Serum response factor: toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 28.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 29.Bagi Z, Frangos JA, Yeh JC, White CR, Kaley G, Koller A. PECAM-1 mediates NO-dependent dilation of arterioles to high temporal gradients of shear stress. Arterioscler Thromb Vasc Biol. 2005;25:1590–1595. doi: 10.1161/01.ATV.0000170136.71970.5f. [DOI] [PubMed] [Google Scholar]
- 30.Long X, Tharp DL, Georger MA, Slivano OJ, Lee MY, Wamhoff BR, Bowles DK, Miano JM. The smooth muscle cell-restricted KCNMB1 ion channel subunit is a direct transcriptional target of serum response factor and myocardin. J Biol Chem. 2009;284:33671–33682. doi: 10.1074/jbc.M109.050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 32.Albinsson S, Nordstrom I, Hellstrand P. Stretch of the vascular wall induces smooth muscle differentiation by promoting actin polymerization. J Biol Chem. 2004;279:34849–34855. doi: 10.1074/jbc.M403370200. [DOI] [PubMed] [Google Scholar]
- 33.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SH, Hungerford JE, Little CD, Iruela-Arispe ML. Proliferation and differentiation of smooth muscle cell precursors occurs simultaneously during the development of the vessel wall. Dev Dyn. 1997;209:342–352. doi: 10.1002/(SICI)1097-0177(199708)209:4<342::AID-AJA2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.