Abstract
Objective
This study was intended to examine variations in electroencephalographic (EEG) complexity in response to photic stimulation (PS) during aging to test the hypothesis that the aging process reduces physiologic complexity and functional responsiveness.
Methods
Multiscale entropy (MSE), an estimate of time-series signal complexity associated with long-range temporal correlation, is used as a recently proposed method for quantifying EEG complexity with multiple coarse-grained sequences. We recorded EEG in 13 healthy elderly subjects and 12 healthy young subjects during pre-PS and post-PS conditions and estimated their respective MSE values.
Results
For the pre-PS condition, no significant complexity difference was found between the groups. However, a significant MSE change (complexity increase) was found post-PS only in young subjects, thereby revealing a power-law scaling property, which means long-range temporal correlation.
Conclusions
Enhancement of long-range temporal correlation in young subjects after PS might reflect a cortical response to stimuli, which was absent in elderly subjects. These results are consistent with the general “loss of complexity/diminished functional response to stimuli” theory of aging.
Significance
Our findings demonstrate that application of MSE analysis to EEG is a powerful approach for studying age-related changes in brain function.
Keywords: Multiscale entropy, Aging, Electroencephalography, Complexity, Long-range temporal correlation, Photic stimulation
1. Introduction
Physiological systems are regulated by multiple couplings and feedback loops. Their output signals exhibit complex fluctuations, which are not simply attributable to noise but which rather include complexities associated with long-range temporal correlation (Goldberger et al., 2002). A general concept that has emerged from investigations of aging is that it may diminish physiological complexity and reduce an organism’s adaptive capacity (Goldberger et al., 2002; Kyriazis, 2003; Lipsitz and Goldberger, 1992; Pincus, 2001). A diminished functional response to stimuli with aging has therefore been proposed as a generic feature of age-related pathology (Kyriazis, 2003; Lipsitz and Goldberger, 1992). One of main features of physiological aging, then, could be described as “loss of complexity/diminished functional response to stimuli”.
Brain activity is characterized by dynamic processes with various interactions among several brain regions (reviewed in Sporns et al., 2000), consisting of both stochastic and deterministic processes (reviewed in Tononi et al., 1998). In this context, quantification of nonlinear dynamical electroencephalographic (EEG) activity might be a powerful approach to understanding changes in patterns of brain activity that occur with aging (reviewed in Stam, 2005; reviewed in Tononi et al., 1998).
One of the traditional entropy-based analyses of physiological time series is dynamic entropy methods. Kolmogorov and Sinai extended the statistic Shannon entropy to a dynamical version, i.e., entropy production rate over time, termed KS entropy (Kolmogorov, 1958). As KS entropy assumes stationary, long and fine-resolution time-series, KS entropy has limited application to real data. Based upon the investigations of chaotic time-series nonlinearity (Eckmann et al., 1986; Grassberger and Procaccia, 1983a,b; Pawelzik and Schuster, 1987), Pincus (1991) proposed approximate entropy (ApEn; Pincus, 1991, 1995) as a technically tractable KS entropy. Approximate entropy is a measure of the irregularity/predictability of finite length time-series. Subsequently, Richman and Moorman (2000) developed sample entropy (SampEn), which is KS entropy as defined by correlation entropy instead of Shannon entropy. Their work demonstrated that SampEn is more tractable than ApEn (Richman and Moorman, 2000). These two entropy indices are useful for analyzing short and noisy real physiological signals (Pincus and Goldberger, 1994) and have been usefully applied to EEG data (Abásolo et al., 2005; Bouillon et al., 2004; Burioka et al., 2003, 2005; Ferenets et al., 2006; Papadelis et al., 2007).
Complexity that is inherent in physiological systems has a more restrictive concept: complex systems are neither completely regular nor absolutely random (Zhang, 1991; Costa et al., 2005; Goldberger et al., 2002; reviewed in Tononi et al., 1998). Thus, although measures of entropy index “irregularity/predictability”, they are not direct estimates of physiological complexity (Costa et al., 2005; Goldberger et al., 2002). Both ApEn and SampEn give high values with random data, even though the data does not comprise intrinsic physiological complexity (Costa et al., 2002, 2005). Thus, ApEn and SampEn as a measure of irregularity/predictability do not imply an intrinsic physiological complexity (Costa et al., 2002, 2005). Costa et al. (2002) introduced multiscale entropy analysis (MSE), which is an assessment of entropy across multiple scales, based on SampEn measures of entropy. This procedure permits the detection of signal fluctuations that may be due to the presence of memory effects in physiological systems that exist across multiple time scales, through the use of multiple coarse-grained sequences. The particular pattern of entropy values across the varying scales permits a differentiation between noise and meaningful complexity. Further, because of the coarse-graining procedure, MSE can also detect long-range temporal correlations, thereby assessing whether there are “memory” or “history” effects in underlying signal dynamics (Costa et al., 2005). Such approaches have been fruitfully applied to physiologic data, for instance, in evaluating heart rate variability to distinguish normal from pathologic rhythms (Chialvo, 2002; Costa et al., 2005; Ferrario et al., 2006). MSE may provide similar insights regarding the complex dynamics of other physiological signals such as EEG neural activity and how these are changed by aging and clinical disorders (Escudero et al., 2006).
Photic stimulation (PS) is a commonly used cerebral activation method in routine EEG examination and can be used to probe for underlying brain dysfunction (Takahashi, 1987). Most previous studies employing PS have examined the so-called photic driving response during PS. It is a harmonic response to the PS frequency (Kikuchi et al., 2002a,b, 2003; Wada et al., 1998). Though there are no previous reports examining neural responses to PS in the post-PS interval, we chose to examine the post-PS responses since they may be more amenable to the nonlinear measures of brain signal complexity employed in this study. Harmonic responses evoked during PS are linear in nature and may obscure naturally occurring nonlinearities in brain signals. These harmonic responses dominate during the administration of PS, while any nonlinear brain activations may only manifest more clearly in the interval just following PS. Therefore, we focused on a nonlinear analysis of post-PS effects in the brain activities of young vs. elderly subjects, thus providing a novel approach for understanding of brain activity and identifying neurophysiologic signatures associated with aging or disease states.
The present pilot study used MSE to investigate EEG complexity variations in response to PS in young vs. elderly subjects. We employed MSE as a novel approach for characterizing age-related changes in cortical network integrity and functional responsiveness.
2. Methods and materials
2.1. Subjects
The study was performed at the Department of Neuropsychiatry, University of Fukui, Japan. In this study, 13 healthy young subjects (7 male, 6 female, average age 29.2 years, SD = 3.8 years, range = 21–34 years) participated along with 15 healthy elderly subjects (7 male, 8 female, average age of 64.5 years, SD = 4.2 years, range = 56–71 years). All subjects were nonsmokers and medication-free. Subjects with major medical or neurological conditions including epilepsy or head trauma in the past, or a lifetime history of alcohol or drug dependence were excluded. Conventional MRI was performed to exclude subjects with major brain abnormalities. In addition, subjects with scores on the Mini-Mental State Examination of less than 27 were excluded. After a complete explanation of the study, written informed consent was obtained from each subject. The Ethics Committee of the University of Fukui approved the study protocol.
2.2. EEG recording and photic stimulation (PS)
The subjects were studied while seated in an electrically shielded, soundproof, light-controlled recording room. Standard scalp electrodes were placed in accordance with the International 10–20 System. Subjects’ EEGs were recorded using an 18-channel electroencephalograph (EEG-4518; Nihon Kohden Corp., Tokyo, Japan) at 14 electrode sites: F3, F4, F7, F8, C3, C4, P3, P4, T3, T4, T5, T6, O1, and O2 referenced to linked ear lobe electrodes. Eye movements were monitored using bipolar electro-oculographic (EOG) derivations. The EEG signals (200 Hz sampling frequency) were recorded with a time constant of 0.3 s, a high cut-off frequency of 120 Hz, and a sensitivity of 50 μV/7 mm. Impedance was less than 5 kΩ for each electrode. No other software was used for filtering or reconstruction. The data were stored on a magnetic optical disk for off-line analyses.
PS consisted of flashes of white flicker at 3, 6, 10, 15, 20, and 30 Hz with a flash intensity of 14,000 cd/m2. Each frequency was applied for 10 s, with a 10-s pause between stimuli. The PS stimuli were delivered by a photostimulator and stroboscopic lamp (EEG-4518; Nihon Kohden Corp.) placed 25 cm from the subject’s eyelids. All subjects were instructed to relax and keep their eyes closed throughout the recording period. Selection of segments recorded during the eyes-closed but awake state, was based on visual inspection of EEG and EOG recordings. The subject was considered to be fully awake when alpha activity appeared predominantly over the posterior regions in conjunction with fast eye movements in the EOG channel (Wada et al., 1996). Continuous 20-s, artifact-free epochs were selected manually, immediately before PS and immediately after all frequencies (i.e., 3, 6, 10, 15, 20, and 30 Hz) of PS were performed. To obtain artifact-free epochs, PS was started after sufficient duration of an absolute artifact-free resting condition epoch (more than 20-s) was attained. We used these two epochs (pre- and post-PS) for MSE analyses. One subject from the young group and two subjects from the elderly group were eliminated from subsequent MSE analyses because their EEG recordings included eye or body movement artifacts during the post-PS epoch. Thus, results for 12 healthy young subjects (6 male, 6 female, average age 29.2 years, SD = 4.0 years, range = 21–34 years) and 13 healthy elderly subjects (5 male, 8 female, average age 64.3 years, SD = 4.5 years, range = 56–71 years) are presented.
2.3. Multiscale entropy (MSE)
Multiscale entropy analysis is described as following. Considering an EEG time-series {x1,x2, …} as observations of the stochastic variable x, dynamic sample entropy is defined as (Richman and Moorman, 2000):
where Cm(r) = {number of pairs (i, j) with , i ≠ j}/{number of all probable pairs, i.e., (N − m + 1)(N − m)}. Therein, xm is a vector of mmembers time series of (N − m) length, and denotes the distance between points and in the space of dimension m, and r means the effective filter for measuring consistency of time series.
We first embedded the time series into a m dimensional space in the form of a vector and counted the points which stay around within the distance r. Then, we summed up all counts to produce the numerator of Cm(r). Cm(r) means a correlation degree by its definition, which is popular as a correlation integral for fractal analysis. −loge[Cm(r)] is information content, and the difference of m and m + 1 information contents: hsamp(r,m,N) = (−loge[Cm+1(r)]) − (−loge[Cm(r)]) defines the rate of loss of information content, or alternatively, is a measure of entropy production rate. In the case we adopt general entropy, Renyi entropy of order q, generalized KS entropies are also defined (Pawelzik and Schuster, 1987). Sample entropy corresponds to second order (q = 2) KS entropy, and approximate entropy, first (q = 1) order KS entropy.
The original EEG time series {x1,x2, …,xN} is coarse-grained by the scale factor τ, with non-overlapping windows (sometimes referred to as creating an aggregated time series):
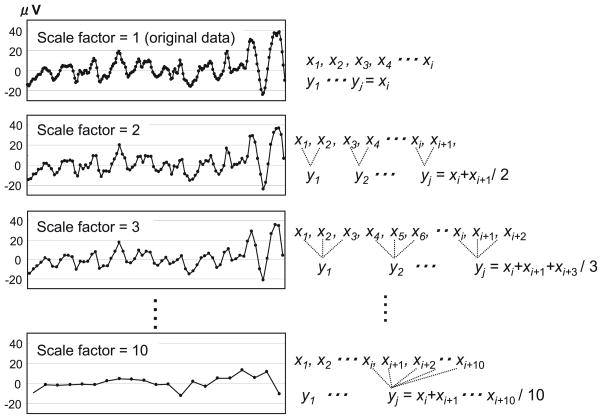
Fig. 1 shows a schematic illustration of the coarse-grained procedure (adapted from Eckmann et al., 1986).
Fig. 1.
Schematic illustration of the coarse-graining procedure for scale factors 1, 2, 3, and 10. Adapted from Costa et al. (2005).
Various theoretical and clinical applications have shown that m = 1 or 2, and r = 0.1–0.25 of the standard deviation of the data points provides good statistical validity for SampEn. For the present analyses, we used a time series of length N = 4000 (i.e., 20-s × 200 Hz) with m = 2 and r = 0.2, which are values that have been applied in preceding EEG studies (Burioka et al., 2005; Chialvo, 2002). Because the length of each coarse-grained sequence is τ times shorter than the length of the original signal, for τ = 20, the coarse-grained sequence has only 200 points in this study. On the other hand, SampEn requires signal lengths of 10m to 20m points to provide reliable estimation (Richman and Moorman, 2000). For that reason, we also calculated MSE values with m = 1 to facilitate comparison with MSE with m = 2. Consequently, MSEs of both m = 1 and m = 2 were highly correlated. We used m = 2 to calculate MSE values.
2.4. Power analysis
In addition to MSE analysis, we performed power analysis of EEG measurements using a computer program specifically designed for EEG, ‘BIMUTAS II’ (Kissei-Comtec). A Hanning window was applied to each artifact-free 2.56-s epoch (sampling rate 200 Hz), and the spectral density was calculated using a fast Fourier transform (FFT). From the consecutive 20-s which are used for MSE analyses, a total of 7 artifact-free epochs were continuously selected to calculate absolute EEG power (μV2). Then the frequency spectrum was collapsed into frequency bands of delta (2–6 Hz), theta (6–8 Hz), alpha (8–13 Hz), beta (13–30 Hz) and gamma (30–40 Hz). For each frequency band, we then calculated a measure of relative power hange (power in each frequency divided by total power across all frequency bands) for statistical analyses.
2.5. Statistical analysis
Statistical analyses were carried out using software (SPSS Windows ver. 16; SPSS Japan Inc., Tokyo, Japan). Some SampEn values for each scale factor were found to have a skewed distribution and were therefore log-transformed to achieve a normal distribution. A two-tailed α level of 0.05 was considered statistically significant for all analyses. The Greenhouse–Geisser adjustment was applied to the degrees of freedom for all analyses.
For MSE nalyses, repeated measures analysis of variance (ANOVA) was performed with groups (young vs. elderly) as between-subject factors. Then the hemisphere (left vs. right), condition (pre-PS vs. post-PS), and scale factor (τ: 20 scales), as within-subject factors, were used to test differences in MSE among paired electrode sites. In addition to the each electrode site, we calculated averaged MSE values (averageMSE) for each hemisphere and performed ANOVA with groups (young vs. elderly) as between-subject factors, and the hemisphere (left vs. right), condition (pre-PS vs. post-PS), and scale factor (τ: 20 scales), as within-subject factors.
Post-hoc ANOVA was performed with groups (young vs. elderly) as between-subject factors. Then within-subject factors analyses for hemisphere (left vs. right) and scale factor (τ: 20 scales) were performed to test for group differences in MSE for each condition (pre-PS, post-PS). Additionally, post-hoc ANOVA, with condition (pre-PS vs. post-PS), hemisphere (left vs. right), and scale factor (τ: 20 scales) as within-subject factors, were used to test for condition-dependent differences in MSE for each group (young, elderly). For significant group-by-scale factor or condition-by-scale factor interactions, independent or paired t-tests were used to compare group and condition differences separately for each scale factor.
The MSE curve tends to be a straight line over scale factors of between τ = 10 and 20. Therefore, we calculated the regression coefficient (i.e., scale factor as the independent variable and SampEn value as the dependent variable) to characterize the profile of the MSE slope over the scale factor of 10–20. The regression coefficients were distributed normally. Then we performed ANOVA with groups (young vs. elderly) as between-subject factors, and condition (pre-PS vs. post-PS) as within-subject factors to explore the change of MSE slope after PS.
For relative power analysis, repeated measures ANOVA were performed with groups (young vs. elderly) as between-subject factors. Then the hemisphere (left vs. right), condition (pre-PS vs. post-PS) as within-subject factors, were used to test differences for each frequency band (delta, theta, alpha, beta and gamma), among paired electrode sites. Additionally, independent and paired t-tests were used to compare group and condition differences separately for each frequency band and electrode sites.
3. Results
3.1. Multiscale entropy value
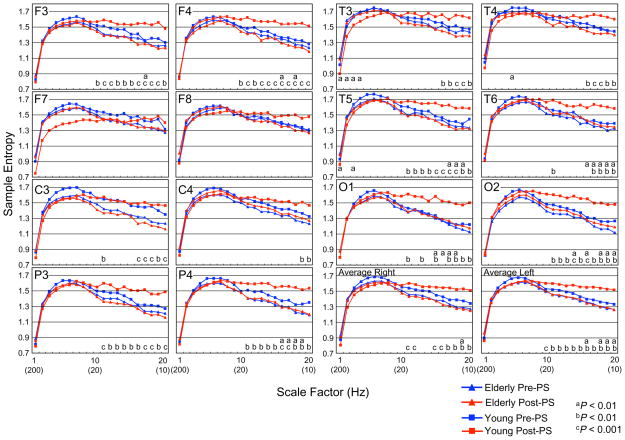
Results of ANOVA revealed no group-by-hemisphere-by-condition-by-scale factor interaction in any paired electrode sites. However, a significant group-by-condition-by-scale factor interaction was identified for each paired electrode site [F3/4, F(19,437) = 7.4, P = 0.0002; F7/8, F(19,437) = 3.9, P = 0.014; C3/4, F(19,437) = 5.4, P = 0.003; P3/4, F(19,437) = 7.0, P = 0.0006; T3/4, F(19,437) = 8.0, P = 0.00008; T5/6, F(19,437) = 9.0, P = 0.00003; O1/2, F(19,437) = 5.0, P = 0.008] and AverageMSE [F(19,437) = 8.0, P = 0.00008].
Post-hoc ANOVAs for testing group differences in the pre-PS condition among paired electrode sites revealed no significant interaction or main effect, although the post-PS condition identified significant group-by-scale factor interactions at all paired electrode sites [F3/4, F(19,437) = 7.2, P = 0.002; F7/8, F(19,437) = 4.3, P = 0.025; C3/4, F(19,437) = 7.7, P = 0.0008; P3/4, F(19,437) = 10.3, P = 0.0001; T3/4, F (19,437) = 8.8, P = 0.001; T5/6, F(19,437) = 8.3, P = 0.0007; O1/2, F(19,437) = 6.4, P = 0.002] and AverageMSE [F(19,437) = 12.6, P = 0.0001] (Fig. 2). No significant main effect was found for the hemisphere at any paired electrode sites. Independent follow-up t-tests were conducted for each electrode site in the post-PS condition to assess the group-by-scale factor interactions in the post-PS condition. Significant group differences were found for the higher scale factors except for the F7 and F8 electrode sites (Fig. 2).
Fig. 2.
Multiscale entropy (MSE) analysis of 13 elderly subjects (triangle) and 12 young subjects (square) in both pre (blue)-PS and post (red)-PS condition for each site and average of each site. Each panel presents the average of the left and right MSE values. Post-hoc comparisons between the pre-PS and post-PS conditions in young subjects: aP < 0.01. Post-hoc comparisons between young and elderly subjects in the post-PS condition: bP < 0.01, cP < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
Significant condition-by-scale factor interactions were found using ANOVAs for testing condition-based differences in the young group at all paired electrode sites [F3/4, F(19,209) = 11.7, P = 0.0004; F7/8, F(19,209) = 3.7, P = 0.042; C3/4, F(19,209)=7.4, P = 0.003; P3/4, F(19,209) = 13.4, P = 0.0001; T3/4, F(19,209) = 11.6, P = 0.00007; T5/6, F (19,209) = 15.7, P = 0.000007; O1/2, F (19,209) = 13.8, P = 0.0001] and AverageMSE [F(19,437) = 11.6, P = 0.00007]. In the elderly group, no significant condition-by-scale factor interaction was identified (Fig. 2). No significant main effect existed for hemispheres at any paired electrode site. Follow-up paired t-tests were conducted for each electrode site in the young group to assess the condition-by-scale factor interaction in the young group (Fig. 2). Significant condition differences were found for lower and higher factor scales, except for the F7 and F8 electrode sites (Fig. 2).
3.2. Slope of multiscale entropy
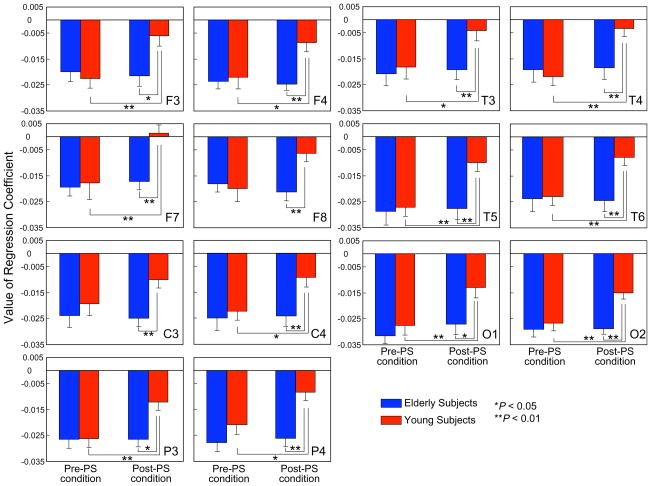
We also analyzed regression coefficients for MSE slopes over scale factors of 10–20 to clarify the slope of MSE (i.e., over a scale factor 10–20) further.
Results of ANOVA revealed no group-by-hemisphere-by-condition, group-by-hemisphere, or hemisphere-by-condition interaction in any paired electrode sites. No significant main effect was found for hemisphere at any paired electrode site. However, significant group-by-condition interactions were identified for all paired electrode sites except for C3/4 [F3/4, F(1,23) = 11.9, P = 0.002; F7/8, F(1,23) = 11.3, P = 0.003; P3/4, F(1,23) = 9.5, P = 0.005; T3/4, F(1,23) = 11.0, P = 0.003; T5/6, F(1,23) = 21.0, P = 0.0001; O1/2, F(1,23) = 9.1, P = 0.006, but not in C3/4, F(1,23) = 3.8, P = 0.06] (Fig. 3). Results of paired t-tests comparing condition effects for the after-PS condition showed a significant increase of the regression coefficient in the young subjects in F3, F4, F7, C4, P3, P4, T3, T4, T5, T6, O1, and O2, but not in elderly subjects (Fig. 3). Results of unpaired t-tests comparing group effects revealed that the young subjects had significantly higher regression coefficients than the elderly subjects in Post-PS conditions in all sites, but not for Pre-PS (Fig. 3).
Fig. 3.
Regression coefficients for MSE slopes over scale factor 10–20 for each site. Each panel presents the average of the regression coefficients of 13 elderly subjects (blue) and 12 young subjects (red) in both the pre-PS and post-PS conditions (error bars indicate the standard error). Post-hoc comparisons between the pre-PS and post-PS condition in young subjects: *P < 0.05, **P < 0.01. Post-hoc comparisons of young and elderly subjects in the post-PS condition; *P < 0.05, **P < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
3.3. Power analysis
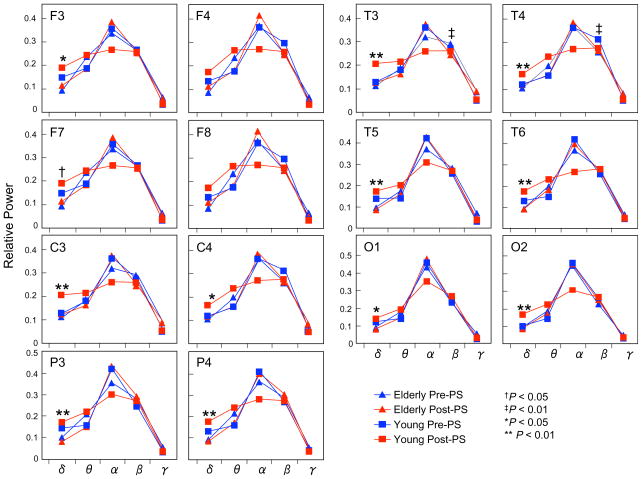
Results of ANOVA revealed no group-by-hemisphere-by-condition interaction or main effect in any paired electrode sites for each frequency band. Post-hoc ANOVAs for testing group and condition-dependent differences revealed significant group-by-condition interaction for theta band in F3/4, C3/4 and P3/4 [F(1,23) = 9.6, P = 0.005; F(1,23) = 8.0, P = 0.01; F(1,23) = 6.1, P = 0.020], and alpha band in P3/4 and T5/6 [F(1,23) = 4.8, P = 0.039; F(1,23) = 4.8, P = 0.038]. Main effect for group were identified for delta band in F3/4, C3/4, P3/4, T3/4, T5/6 and O1/2 [F(1,23) = 6.9, P = 0.015; F(1,23) = 13.5, P = 0.001; F(1,23) = 12.9, P = 0.002; F(1,23) = 12.9, P = 0.002; F(1,23) = 17.9, P = 0.0003; F(1,23) = 6.6, P = 0.017]. No main effect for condition was identified. Results of independent or paired t-tests were demonstrated in (Fig. 4).
Fig. 4.
Relative power analysis of 13 elderly subjects (triangle) and 12 young subjects (square) in both pre (blue)-PS and post (red)-PS condition for each site. Each panel presents the average of the left and right relative power values. Post-hoc comparisons between the pre-PS and post-PS conditions in young subjects: †P < 0.05, ‡P < 0.01. Post-hoc comparisons between young and elderly subjects in the post-PS condition: *P < 0.05, **P < 0.01. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
4. Discussion
In this study, we employed multiscale entropy to evaluate EEG signal complexity in young and elderly subjects in response to PS. The recently proposed SampEn-MSE method is excellent for analyzing short and noisy experimental datasets (reviewed in Pincus, 2006). Our findings from MSE analysis suggest that EEG complexity apparently increased after PS in young but not in elderly subjects. This EEG change after PS in young subjects reflects a good cortical response to stimuli: elderly subjects displayed an attenuated response that was consistent with the hypothesis that the ability to respond dynamically to an external stimulus, decreases with age (Goldberger et al., 2002; Kyriazis, 2003; Pincus, 2001). These results agree well with a general “loss of complexity/diminished functional response to stimuli” theory of aging and demonstrate the potential utility of MSE for investigating the pathophysiology of aging based on EEG measurements.
Several prior studies have applied ApEn or SampEn analysis to EEG data. Burioka et al. (2003, 2005) demonstrated the utility of ApEn in estimating the sleep stage and brain activity. Papadelis et al. (2007) measured EEG irregularity using ApEn in various hypoxic conditions. They revealed that irregularity decreases with hypoxia. Ferenets et al. (2006) and Bouillon et al. (2004) demonstrated the usefulness of ApEn in evaluating the depth of sedation induced by anesthetic agents. Abásolo et al. (2005) demonstrated decreased irregularity of EEG background activity of Alzheimer’s disease using ApEn. However, these studies are based on a single scale factor (i.e., calculated on only the original data), rendering it difficult to infer the existence of long-range temporal correlations associated with intrinsic physiological system complexity. A few studies have demonstrated the usefulness of MSE in exploring brain activity. Escudero et al. (2006) applied MSE to examine background EEG activity in Alzheimer’s disease patients. They found significantly different MSE slopes between Alzheimer’s disease patients and age-matched controls indicating that Alzheimer’s patients showed markedly decreased EEG complexity. Bhattacharya et al. (2005) investigated the nature of spontaneous fluctuations of single neurons from the human hippocampal–amygdala complex using MSE, finding evidence for long-range temporal correlations in the neural firing pattern.
The slopes of the MSE curve therefore provide a valuable approach for interpreting EEG data (Bhattacharya et al., 2005; Escudero et al., 2006). In particular, a gradual monotonic decrease in SampEn values with increasing scale factor represents an independent random time sequence. In this study, MSE values tended to increase with smaller scale factors and to decrease or remain constant in higher scale factors (Fig. 2). Multiscale entropy slopes over a scale factor of 10–20 tended to be straight lines. Closer inspection revealed that the regression coefficient approaches zero in young subjects after PS (Fig. 3). In other words, SampEn values in higher scale factors tended to be constant values: parallel to the x-axis (Fig. 2). Constant SampEn values suggest the existence of a power-law scaling property. The power-law scaling property is characteristic of nonlinearity (Bak et al., 1987; Stanley et al., 1994) associated with intrinsic complexity in physiological systems (Goldberger et al., 2002). Further, long-range temporal correlations of MSE represent a “memory” or “history” effect in global neuroanatomical network activity in the brain, which means that EEG activities are not fully temporally independent: they share mutual relations (Bhattacharya et al., 2005; Lowen et al., 1997). In summary, investigation of MSE analysis provides an indication of nonlinear dynamics in EEG, which cannot be found using single scale factor dynamic entropies.
Power analyses demonstrated that young subjects showed modulation of their spectral profiles that was lacking in the elderly subjects, consistent with previous reports (Kikuchi et al., 2002a). Considering the fact that MSE analysis provide information of nonlinear dynamics in EEG, we can assume that enhancement of long-range temporal correlations could be the neuropathological background of EEG power alterlations in young subject with visual stimuli.
A common finding in age-related change in electrophysiological studies using visual stimuli, is a frontal distribution of alterations (Amenedo and Díaz, 1998; Fabiani et al., 1998; Friedman et al., 1997; reviewed in Friedman, 2003). In this study, changes in MSE values post-PS in young subjects were observed throughout widespread brain regions (Figs. 2 and 3), possibly reflecting global alterations across neural networks. However, considering the fact that electrical activity in each scalp electrode might not have its origin in the brain area directly underneath the electrode, it is difficult to infer the precise of spatial distribution of regional changes. Complementary techniques with a better spatial resolution such as magnetoencephalogram are necessary to obtain more precise inferences of scalp distribution.
The existence of PS-related harmonic activity during the post-PS interval or arousal effects after PS must be considered. However, no specific SampEn value change was observed in the specific frequency associated with flicker frequency across all the scale factors. In attempts to control for arousal effects, we eliminated any EEG epochs that indicated drowsiness under visual inspection (Wada et al., 1996). Additionally, power analysis of pre-PS condition also demonstrated no difference between groups. These results might explain the irrelevance of harmonic response to post-PS conditions and arousal effects. Another important question is why no significant difference of SampEn was found between the two groups in the pre-PS condition. Linear EEG studies, such as those involving spectral power analyses, indicate a global slowing of the background EEG with age (Klimesch, 1999). On the other hand, Shigeta et al. (1995) reported that aged but healthy individuals showed no slowing of the background EEG in their longitudinal study. Accordingly, we found no significant power spectrum difference between the two groups in the pre-PS condition.
This pilot study is a first step in the inspection of the age-related EEG variation using MSE with small sample size. Additional longitudinal studies, with several kinds of stimuli and increased sample number, including individuals from various age groups, are necessary to extend the current findings. Nevertheless, our findings support the potential usefulness of MSE analysis in characterizing variability in EEG signal complexity across different populations. The findings suggest that MSE can help elucidate age-related decline in brain function through the detection of intrinsic complexity associated with long-range temporal correlation in EEG signals.
Acknowledgments
We thank Dr. A. Umezawa for providing statistical advice for this study.
This study was supported by Grants-in-Aid for Young Scientists (B) No. 20790833 from the Japan Society for the Promotion of Science (T.T.) and Scientific Research Grant from Fukui prefecture (T.T.).
Footnotes
Conflicts of interest
We state that there are no actual or potential conflicts of interest that could inappropriately influence this work. The study protocol was approved by the Ethics Committee of the University of Fukui.
References
- Abásolo D, Hornero R, Espino P, Poza J, Sánchez CI, de la Rosa R. Analysis of regularity in the EEG background activity of Alzheimer’s disease patients with Approximate Entropy. Clin Neurophysiol. 2005;116:1826–34. doi: 10.1016/j.clinph.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Amenedo E, Díaz F. Aging-related changes in processing of nontarget and target stimuli during an auditory oddball task. Biol Psychol. 1998;48:235–67. doi: 10.1016/s0301-0511(98)00040-4. [DOI] [PubMed] [Google Scholar]
- Bak P, Tang C, Wiesenfeld K. Self-organized criticality: an explanation of the 1/f noise. Phys Rev Lett. 1987;9(5):381–4. doi: 10.1103/PhysRevLett.59.381. [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Edwards J, Mamelak AN, Schuman EM. Long-range temporal correlations in the spontaneous spiking of neurons in the hippocampal–amygdala complex of humans. Neuroscience. 2005;131:547–55. doi: 10.1016/j.neuroscience.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Bouillon TW, Bruhn J, Radulescu L, Andresen C, Shafer TJ, Cohane C, et al. Pharmacodynamic interaction between propofol and remifentanil regarding hypnosis, tolerance of laryngoscopy, bispectral index, and electroencephalographic approximate entropy. Anesthesiology. 2004;100:1353–72. doi: 10.1097/00000542-200406000-00006. [DOI] [PubMed] [Google Scholar]
- Burioka N, Cornelissen G, Halberg F, Kaplan DT, Suyama H, Sako T, et al. Approximate entropy of human respiratory movement during eye-closed waking and different sleep stages. Chest. 2003;23:80–6. doi: 10.1378/chest.123.1.80. [DOI] [PubMed] [Google Scholar]
- Burioka N, Miyata M, Cornelissen G, Halberg F, Takeshima T, Kaplan DT, et al. Approximate entropy in the electroencephalogram during wake and sleep. Clin EEG Neurosci. 2005;36:21–4. doi: 10.1177/155005940503600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chialvo DR. Physiology: unhealthy surprises. Nature. 2002;419:263. doi: 10.1038/419263a. [DOI] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E. 2005;71:021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- Eckmann J, Kamphorst SO, Ruelle D, Ciliberto S. Liapunov exponents from time series. Phys Rev A. 1986;34:4971–9. doi: 10.1103/physreva.34.4971. [DOI] [PubMed] [Google Scholar]
- Escudero J, Abásolo D, Hornero R, Espino P, López M. Analysis of electroencephalograms in Alzheimer’s disease patients with multiscale entropy. Physiol Meas. 2006;27:1091–106. doi: 10.1088/0967-3334/27/11/004. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35:698–708. [PubMed] [Google Scholar]
- Ferenets R, Lipping T, Anier A, Jäntti V, Melto S, Hovilehto S. Comparison of entropy and complexity measures for the assessment of depth of sedation. IEEE Trans Biomed Eng. 2006;53:1067–77. doi: 10.1109/TBME.2006.873543. [DOI] [PubMed] [Google Scholar]
- Ferrario M, Signorini MG, Magenes G, Cerutti S. Comparison of entropy-based regularity estimators: application to the fetal heart rate signal for the identification of fetal distress. IEEE Trans Biomed Eng. 2006;53:119–25. doi: 10.1109/TBME.2005.859809. [DOI] [PubMed] [Google Scholar]
- Friedman D. Cognition and aging: a highly selective overview of event-related potential (ERP) data. J Clin Exp Neuropsychol. 2003;25:702–20. doi: 10.1076/jcen.25.5.702.14578. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M. An overview of age-related changes in the scalp distribution of P3b. Electroencephalogr Clin Neurophysiol. 1997;104:498–513. doi: 10.1016/s0168-5597(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Goldberger AL, Peng CK, Lipsitz LA. What is physiologic complexity and how does it change with aging and disease? Neurobiol Aging. 2002;23:23–6. doi: 10.1016/s0197-4580(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Grassberger P, Procaccia I. Estimation of the Kolmogorov entropy from a chaotic signal. Phys Rev A. 1983a;28:2591–3. [Google Scholar]
- Grassberger P, Procaccia I. Measuring the strangeness of strange attractors. Physica D. 1983b;9:189–208. [Google Scholar]
- Kikuchi M, Wada Y, Koshino Y. Differences in EEG harmonic driving responses to photic stimulation between normal aging and Alzheimer’s disease. Clin Electroencephalogr. 2002a;33:86–92. doi: 10.1177/155005940203300208. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Wada Y, Takeda T, Oe H, Hashimoto T, Koshino Y. EEG harmonic responses to photic stimulation in normal aging and Alzheimer’s disease: differences in interhemispheric coherence. Clin Neurophysiol. 2002b;113:1045–51. doi: 10.1016/s1388-2457(02)00129-3. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Wada Y, Koshino Y. Sequential EEG analysis during intermittent photic stimulation in never-medicated patients with schizophrenia. Clin Electroencephalogr. 2003;34:201–6. doi: 10.1177/155005940303400407. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29:169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kolmogorov AN. New metric invariant of transitive dynamical systems and endomorphisms of Lebesgue spaces. Dokl Russ Acad Sci. 1958;119:861–4. [Google Scholar]
- Kyriazis M. Practical applications of chaos theory to the modulation of human ageing: nature prefers chaos to regularity. Biogerontology. 2003;4:75–90. doi: 10.1023/a:1023306419861. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Goldberger AL. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267:1806–9. [PubMed] [Google Scholar]
- Lowen SB, Cash SS, Poo M, Teich MC. Quantal neurotransmitter secretion rate exhibits fractal behavior. J Neurosci. 1997;17:5666–77. doi: 10.1523/JNEUROSCI.17-15-05666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadelis C, Kourtidou-Papadeli C, Bamidis PD, Maglaveras N, Pappas K. The effect of hypobaric hypoxia on multichannel EEG signal complexity. Clin Neurophysiol. 2007;118:31–52. doi: 10.1016/j.clinph.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Pawelzik K, Schuster HG. Generalized dimensions and entropies from a measured time series. Phys Rev A. 1987;35:481–4. doi: 10.1103/physreva.35.481. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA. 1991;88:2297–301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy (ApEn) as a complexity measure. Chaos. 1995;5:110–7. doi: 10.1063/1.166092. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Assessing serial irregularity and its implications for health. Ann N Y Acad Sci. 2001;954:245–67. doi: 10.1111/j.1749-6632.2001.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Pincus SM. Approximate entropy as a measure of irregularity for psychiatric serial metrics. Bipolar Disord. 2006;8:430–40. doi: 10.1111/j.1399-5618.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266:1643–56. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:2039–49. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Shigeta M, Julin P, Almkvist O, Basun H, Rudberg U, Wahlund LO. EEG in successful aging: a 5 year follow-up study from the eighth to ninth decade of life. Electroencephalogr Clin Neurophysiol. 1995;95:77–83. doi: 10.1016/0013-4694(95)00034-v. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Network. 2000;13:909–22. doi: 10.1016/s0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol. 2005;116:2266–301. doi: 10.1016/j.clinph.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Stanley HE, Buldyrev SV, Goldberger AL, Goldberger ZD, Havlin S, Mantegna RN, et al. Statistical mechanics in biology: how ubiquitous are long-range correlations? Physica A. 1994;205:214–53. doi: 10.1016/0378-4371(94)90502-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T. Activation methods. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: basic principles, clinical applications and related fields. Baltimore: Urban & Schwarzenberg; 1987. pp. 209–27. [Google Scholar]
- Tononi G, Edelman GM, Sporns O. Complexity and coherency: integrating information in the brain. Trends Cogn Sci. 1998;2:474–84. doi: 10.1016/s1364-6613(98)01259-5. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nanbu Y, Koshino Y, Shimada Y, Hashimoto T. Inter- and intrahemispheric EEG coherence during light drowsiness. Clin Electroencephalogr. 1996;27:84–8. doi: 10.1177/155005949602700207. [DOI] [PubMed] [Google Scholar]
- Wada Y, Nanbu Y, Kikuchi M, Koshino Y, Hashimoto T, Yamaguchi N. Interhemispheric EEG coherence in never-medicated patients with paranoid schizophrenia: analysis at rest and during photic stimulation. Clin Electroencephalogr. 1998;29:170–6. doi: 10.1177/155005949802900408. [DOI] [PubMed] [Google Scholar]
- Zhang YC. Complexity and 1/f noise. A phase space approach. J Phys I (France) 1991;1:971–7. [Google Scholar]