Abstract
Dynamin family members are large GTPases that assemble into multimeric spirals. These spirals promote membrane fission or fusion, or they inhibit processes such as viral replication. Two new studies identify interactions between subunits of dynamin spirals, advancing mechanistic understanding of dynamin function (Chappie et al., 2010; Gao et al., 2010).
The GTPase dynamin is involved in membrane scission during endocytic vesicle formation. 15 years ago, two papers showed that dynamin self-assembles into multimeric spirals with 20 subunits per rung (Hinshaw and Schmid, 1995; Takei et al., 1995). The size of the dynamin spirals immediately suggested how they might function in endocytosis, because similarly-sized electron-dense collars were known to wrap the necks of budding vesicles in a Drosophila dynamin mutant (Kosaka and Ikeda, 1983). Since that time, many mechanisms have been proposed for dynamin-mediated scission, including ratcheting, twisting, popping, signaling, crimping, dissociation and partial insertion into the membrane. Not all of these proposals are mutually exclusive, but there is still no clear front-runner. Two new papers in Nature describe partial structures of dynamin and of the dynamin related protein MxA (Chappie et al., 2010; Gao et al., 2010). These structures identify interfaces between different subunits of dynamin complexes, and offer new views consistent with the ratcheting and crimping models for scission.
Dynamin itself and related dynamin family members each contain a GTPase domain followed by two elongated regions, a Middle domain (MD) and a GTPase Effecter domain (GED), that fold back on each other. The MD and the GED are connected by a variable sequence that primarily functions in targeting (Figure 1A). In dynamin, this targeting sequence is a Pleckstrin Homology (PH) domain that binds to the plasma membrane. Dynamin also has a carboxy-terminal Proline-Rich domain (PRD) that can bind to other proteins involved in endocytosis. Dynamin cycles on and off membranes during each round of vesicle formation. The cytosolic pool of dynamin consists of tetramers with a low basal rate of GTP hydrolysis. Assembly into spirals leads to a 100-fold increase in the GTP hydrolysis rate. The question of how dynamin actually functions in membrane scission can broken into several parts: How does the tetramer form a spiral? How does assembly stimulate GTP hydrolysis? How does GTP hydrolysis drive scission? How does the cycle end? Figuring out answers to all of these questions will require a better understanding of interactions between different subunits of dynamin spirals.
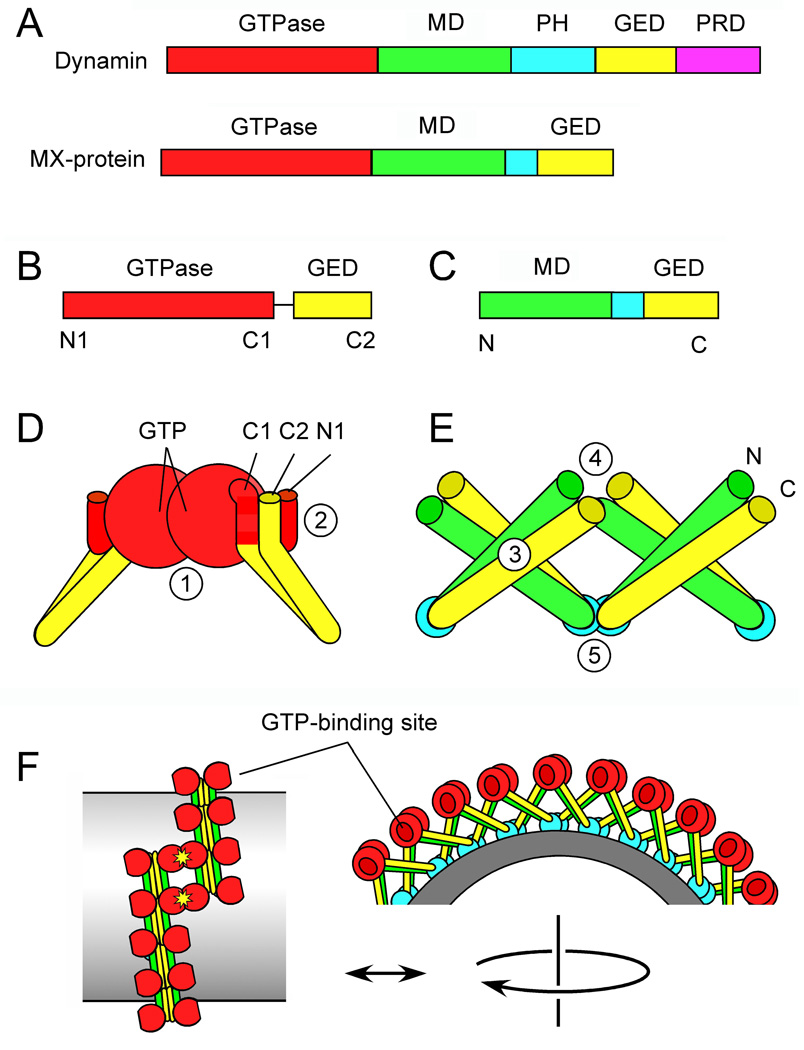
Figure 1. Dynamin protein domains and the newly solved structures.
(A) Protein domains in Dynamin and MxA. (B) Dynamin GTPase-GED fusion protein crystallized by Chappie et al. (2010). Amino and carboxy terminal α-helices are indicated by N1, C1 and C2. (C) MxA fragment crystallized by Gao et al. (2010). (D) Key features of the GTPase-GED structure, showing dimerization of two GTPase domains. The interface with GTP-binding sites (1) is buried in this drawing. The BSE is formed by interactions between N1, C1 and C2 helices (2). (E) Key features of the MD-GED structure, showing interactions with adjacent subunits at three different points (3–5) (F) Hypothetical arrangement of dynamin subunits in a spiral suggested by the newly identified interactions and by cryoEM.
So far, crystallographic studies of dynamin have been limited to separate protein domains or monomers. The arrangement of multimers in a spiral on lipid tubules has only been viewed at low resolution by cryo-electron microscopy (cryoEM). These studies showed outer and inner rings of electron density, with the crystal structures of the GTPase domain fitting in the outer ring and the PH domain fitting in the inner ring (Mears et al., 2007). The MD and the GED form stalks connecting these two rings. Adding GTP to these preparations constricts the spirals through crimping, which may be caused by kinks in the spiral backbone (Mears et al., 2007). Whether crimping is enough for scission remains unclear, especially in light of findings showing rapid twisting motion during the scission process (Roux et al., 2006).
The two new studies use truncated versions of dynamin and MxA, a dynamin family member, for structural analysis (Figure 1B–C). Chappie et al. (2010) fused the dynamin GTPase domain to the GED and crystallized this fusion in the presence of the transition state mimic GDP.AlF4-. The resulting structure revealed fusion proteins forming dimers with extensive contacts along the GTP binding cleft of the GTPase domains (Figure 1D). Similar dimerization was previously observed with several other GTPases, including some distant dynamin family members (Gasper et al., 2009). In some of these other GTPase dimers the transition state of GTP is stabilized by direct contact of the nucleotides with each other, and in others by a specific arginine residue called an arginine finger, which is present in traditional GTPase activating proteins. In dynamin, however, the active site is a composite, with a loop from one GTPase stabilizing the transition state of GTP in the other. The arginine finger in other structures is replaced here by a monovalent cation. Stabilization of the transition state through dimerization suggests that dynamin GTPase domains mediate reciprocal activation of GTP hydrolysis. This implies that assembly-stimulated GTP hydrolysis is due to contacts between different GTPases in a dynamin spiral.
The second interaction observed by Chappie et al. (2010) was intra-molecular. Two short α-helices in the GTPase domain (one at the amino-terminus and one at the carboxy-terminus) align to form a hydrophobic groove away from the GTP binding cleft. This hydrophobic groove binds to an α-helix at the carboxy terminus of the GED (Figure 1D). This helix bundle was previously termed the Bundle Signal Element (BSE), because it transmits a signal from the GED to the GTPase domain to induce assembly-stimulated GTP hydrolysis (Chappie et al., 2009). It seems likely that the BSE also transmits signals in the other direction, inducing conformational changes in the GED or the MD, consistent with the crimping model of membrane scission and dissociation of dynamin subunits after GTP hydrolysis.
Gao et al. (2010) crystallized a fragment of MxA containing the MD and GED (Figure 1C). These two domains were previously shown to fold back on each other, forming the stalk between membrane and the GTPase domain in cryoEM reconstructions. The crystallized fragment shows the expected folding pattern but, surprisingly, the stalks also concatemerize in a crisscross arrangement with contacts at three different sites. Monomers contact other monomers at their crossing point to form dimers, while dimers contact other dimers at both ends to form a chain (Figure 1E). This type of chain may constitute the backbone of the dynamin spiral. It is unclear whether the angles or other conformational aspects of stalk polymers change during the GTP hydrolysis cycle but, if so, the distance between dynamin subunits in the spiral could change, again consistent with a crimping mechanism.
There is, however, a big wrinkle in this story. The first structure shows dimerization at the GTP binding cleft (1 in Figure 1D). The second structure shows stalks interacting near the BSE (4 in Figure 1E, corresponding to 2 in Figure 1D) and modeling based on this structure suggests that the GTP-binding clefts of the GTPase domains face away from each other along the spine of stalk polymers. It is possible that GTPases within a rung activate each other or, alternatively, GTPases from different rungs of the spiral might dimerize as depicted in Figure 1F (Chappie et al., 2010; Gao et al., 2010). GTP-dependent interactions between different rungs of the spiral would be compatible with a ratchet model for constriction (Smirnova et al., 1999). Such interactions might also serve as a sensor for completion of the spiral. GTP hydrolysis could then be used to induce crimping through conformational changes (Mears et al., 2007) or cause dissociation of terminal subunits in the spiral as part of a ratcheting process. Time will tell whether either of these models, or a combination of them, is correct. The idea that GTP hydrolysis is triggered by interactions between rungs of the dynamin spiral can, however, explain how assembly induces GTP hydrolysis. These studies therefore provide important new insights into a critical step of the constriction process. Future studies with larger pieces of dynamin or MxA will show how the GTPase domain controls conformational changes in the stalk region, which may ultimately lead to a complete understanding of this beautiful molecular machine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chappie JS, Acharya S, Leonard M, Schmid SL, Dyda F. Nature. 2010 doi: 10.1038/nature09032. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappie JS, Acharya S, Liu YW, Leonard M, Pucadyil TJ, Schmid SL. Mol Biol Cell. 2009;20:3561–3571. doi: 10.1091/mbc.E09-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, von der Malsburg A, Paeschke S, Behlke J, Haller O, Kochs G, Daumke O. Nature. 2010 doi: 10.1038/nature08972. In Press. [DOI] [PubMed] [Google Scholar]
- Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. Nat Rev Mol Cell Biol. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- Hinshaw JE, Schmid SL. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. J Neurobiol. 1983;14:207–225. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- Mears JA, Ray P, Hinshaw JE. Structure. 2007;15:1190–1202. doi: 10.1016/j.str.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Uyhazi K, Frost A, De Camilli P. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Newman-Smith ED, Pishvaee B, van der Bliek AM. J Biol Chem. 1999;274:14942–14947. doi: 10.1074/jbc.274.21.14942. [DOI] [PubMed] [Google Scholar]
- Takei K, McPherson PS, Schmid SL, De Camilli P. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]