Abstract
Background
Little is known about correlates of first trimester pregnancy hormones as in most studies maternal hormones have been measured later in gestation. We examined the associations of maternal characteristics and child sex with first trimester maternal concentrations of 4 hormones implicated in breast cancer: human chorionic gonadotropin (hCG), α-fetoprotein (AFP), insulin-like growth factor (IGF)-I and IGF-II.
Methods
338 serum samples donated to the Northern Sweden Maternity Cohort (NSMC), 1975–2001, during the first trimester of uncomplicated pregnancies were analyzed for the hormones of interest as a part of a case-control study. The associations between maternal characteristics and child sex with hormone concentrations were investigated by correlation, general linear regression, and multivariate regression models.
Results
In the first trimester, greater maternal age was inversely correlated with IGF-I and IGF-II. In comparison with women carrying their first child, already parous women had higher IGF-I but lower hCG. Greater maternal weight and smoking were inversely correlated with hCG. No differences in hormone levels by child sex were observed.
Conclusions
Our analyses indicated that potentially modifiable maternal characteristics (maternal weight and smoking) influence first trimester pregnancy maternal hormone concentrations.
Keywords: pregnancy, cross-sectional study, human chorionic gonadotropin (hCG), α-fetoprotein (AFP), insulin-like growth factor (IGF)-I, IGF-II
Introduction
The hormonal milieu of pregnancy is believed to underlie the effect of childbearing on cancer occurrence [1, 2]. Studies that directly relate endogenous hormones during pregnancy to cancer risk are rare because of the substantial logistic difficulties in their design and conduct [3–5]. Instead, most investigations rely on surrogate markers of the endocrine environment of pregnancy, such as pregnancy conditions (e.g. morning sickness, preeclampsia), child birth weight, maternal age, parity or smoking [6, 7]. Currently, relatively little is known about correlates of first trimester pregnancy hormones as in most studies maternal hormones have been measured in mid- or late pregnancy [8].
Early pregnancy may be a relevant period for subsequent cancer occurrence in the mother and the fetus as during that time important changes take place in both. In the mother, initial proliferation of breast epithelium sets the stage for the full breast differentiation and maturation that will culminate during the post-partum lactation period [9] and which is believed to play an important role in maternal protection from breast cancer [10]. In the fetus, this is the time when organogenesis takes place and tissue patterns and organ systems are established [11]. It has been proposed that hormonal alterations during pregnancy, specifically early in the pregnancy, may be of particular etiological importance for the development of testicular cancer in male offsprings [12].
We used data acquired from control subjects in a case-control study nested within the Northern Sweden Maternity Cohort (NSMC) [5, 13], to examine associations of maternal characteristics and child sex with first trimester maternal concentrations of human chorionic gonadotropin (hCG), α-fetoprotein (AFP) and insulin-like growth factor (IGF)-I and IGF-II. The studied hormones have diverse origin: the synthesis of hCG takes place mainly in the syncytiotrophoblast and is then secreted into the maternal circulation through the villous interface [14], AFP originates primarily from the fetal liver [14], while IGF-I and II, as in the non-pregnant state, are mostly produced by maternal liver [15]. All these hormones have been implicated in maternal breast cancer [5, 13].
Methods
Study population
The NSMC, based at the University Hospital in Umeå (Sweden), was established in November 1975 with the purpose of preserving for research purposes serum samples from pregnant women after mandatory tests for systemic infections. Cohort members are residents of one of the four northernmost counties of Sweden (total population ~800,000) who have attended a maternity health care clinic in the region during pregnancy. Blood samples are drawn mostly during the final weeks of the first trimester of pregnancy, or the early weeks of the second (weeks 6–18), and are periodically shipped frozen to a central repository at Umeå University Hospital, where they are analyzed for systemic infections and the remaining biological material is stored at −20 °C. The NSMC contains over 110,000 first-trimester serum samples from approximately 83,000 women.
Study subjects were selected among the controls included in a nested case-control study on pregnancy hormones and breast cancer risk [5, 13] from the NSMC, 1975–2001. Eligibility criteria included blood donation in gestational weeks 6 to 15 during a spontaneous, singleton pregnancy resulting in the delivery of one live or stillborn infant, no use of hormonal medication during pregnancy and no invasive cancer diagnosis before index pregnancy (except non-melanoma skin cancer). A total of 338 women were included.
Data about index pregnancy were obtained by linkages with the Swedish Birth Registry and review of the original medical records of the women, as described in detail previously [16]. The Swedish Birth Registry is based on data from standardized medical records used in all Swedish maternal care and delivery units since 1973, and more than 99% of all births in Sweden are registered [17]. In general, the quality of the information is considered high, although individual variables have variable completeness, as procedures to collect information have changed over the years. In addition, a full copy of the maternity and delivery records was requested from the hospital closest to the registered place of residence. If the medical record of a subject was not found in this hospital, requests were sent to one or two of the nearest hospitals. Data from the medical records were abstracted with duplicate entry for dates of sampling and last menstrual period. Gestational day at blood donation was calculated as the difference in days between dates at blood donation and last menstrual period. All maternal characteristics studied were measured at the time of the first visit to the maternity care unit, when a blood sample was collected.
Laboratory methods
IGF-I and IGF-II were quantified by immunoradiometric (IRMA) assays from Nichols Institute Diagnostics (San Clemente, CA) and Diagnostic System Laboratories (Webster, TX), respectively. The intra- and inter-batch coefficients of variation (CV) at 12.2 nmol/L IGF-I concentration were 6.3 % and 7.6 % and for an IGF-II concentration of 108 nmol/L were 9.0 % and 11.0%, respectively. AFP was quantified by enzyme-linked immunosorbent assay (Genentech, Inc., San Francisco, CA) with intra- and inter-assay CVs of 3.2% and 6.7%, estimated on measurements of Bio-Rad 17 μg/L AFP control samples. hCG was quantified with the Coat-a-Count IRMA assay (Diagnostic Products Corporation, Los Angeles, CA), which is highly specific for intact hCG, with low cross-reactivity to other glycoprotein hormones present in patient samples, including α and β hCG subunits. Intra- and inter-batch CVs were 2.8% and 3.8%, estimated on measurements of Bio-Rad 170 mIU/mL hCG control samples. In samples drawn in 1988 and thereafter, there was an evident reduction in measured hCG concentrations to levels incompatible with pregnancy, while no such effect on other hormone concentrations was observed. Therefore, all subjects whose baseline blood sample had been drawn after January 1, 1988, were excluded from analyses on hCG (n=56).
Statistical analyses
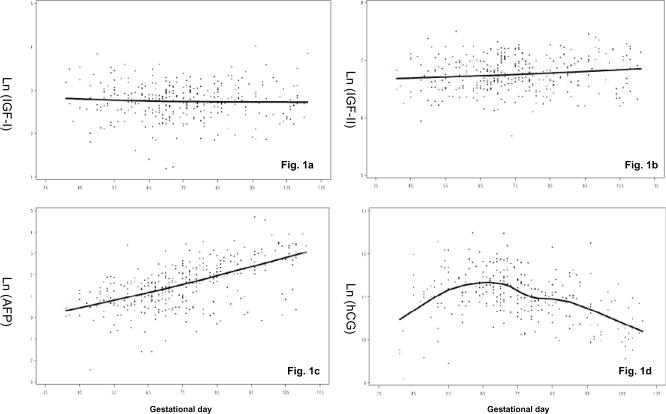
As expected, levels of hCG and AFP varied substantially according to gestational day at blood donation, while no such effect was observed for IGF-I and there was only a weak positive correlation of IGF-II with gestational day (Figure 1, the solid lines showing the progression of hormones during pregnancy, estimated by local linear regression [18]). To account for these variations, all statistical analyses for IGFs and AFP were adjusted for gestational day (linear term), while for hCG, a cubic term of gestational day was used because of the non-linear association of hCG with gestational day. Prior to analysis, original hormone levels were natural log-transformed to limit heteroscedasticity. Outliers were defined as log-hormone concentrations exceeding 3 times the interquartile range and such measurements were set to missing: 2 for IGF-I and 1 for IGF-II and AFP.
Figure 1.
Scatterplot of log-scale IGF-I (Fig.1a), IGF-II (Fig.1b), AFP (Fig.1c), and hCG (Fig.1d) by gestational day in 338 uncomplicated pregnancies from the Northern Sweden Maternity Cohort, 1975–2001. The solid line shows the progression of hormones during pregnancy, estimated by local linear regression [18].
Association between continuous variables was evaluated by calculating Spearman partial correlations (adjusted for gestational day). Variables that showed significant correlations with hormone concentrations were investigated also in tertiles (e.g. maternal age and weight). General linear regression models were used to estimate geometric means of hormones levels and compare them across exposure categories of maternal age, weight, height, parity, smoking and child sex. For continuous variables (maternal age, weight and height), tests for trend were computed by treating continuous variables as ordered categorical variables. For dichotomous variables (parity, smoking and child sex), an F-test was used to assess the significance. To explore the independent effect of maternal characteristics on hormones, multivariate regression models including maternal age, parity, weight, height and smoking were also evaluated. Estimates from models that reached either statistical significance (p < 0.05) or borderline significance (p<0.10) are presented. Addition of child sex to the adjusted model was also explored. Possible interactions between categories of some of the studied variables (e.g. maternal age and parity) were evaluated by inclusion of cross-product terms in the regression models that included the main effects for these variables, but none of them was found to be significant. SAS version 9.1 was used for all analyses.
The study was approved annually by the Regional Ethics Committees of the University of Umeå, Sweden.
Results
Maternal characteristics at enrolment in the study, child sex and hormone concentrations in the study population are presented in Table 1. Blood samples were collected during the first trimester of pregnancy (6–15 gestational weeks), 10 gestational weeks being the median time of blood draw. Maternal age ranged from 18.8 to 44.4 years (median =31.5 years) and about half of the women (52 %) donated a blood sample during their first pregnancy (primiparous). A slightly greater percentage of mothers were carrying a boy (53 vs. 47% mothers of girls). Of the women who provided information, 23% were smoking, but no detail of number of cigarettes smoked per day was available.
Table 1.
Maternal characteristics at enrollment and child sex for index birth and hormone concentrations in 338 uncomplicated pregnancies
| Characteristics* / hormone | n | Median(10th, 90th percentile) or percentage |
|---|---|---|
| Gestational week (weeks) | 338 | 10 (8–14) |
| Maternal age (years) | 338 | 31.5 (25.3–37.6) |
| Parity | ||
| Primiparous | 175 | 52% |
| Multiparous | 163 | 48% |
| Maternal weight (kg) | 338 | 60.9 (52.2–74.0) |
| Maternal height (cm) | 330 | 165 (157–172) |
| Maternal smoking | ||
| Yes | 78 | 23% |
| No | 255 | 77% |
| Child sex | ||
| Boy | 177 | 53% |
| Girl | 160 | 47% |
| Hormone concentrations ** | ||
| IGF-I (nmol/L) | 336 | 15.7 (9.4–25.4) |
| IGF-II (nmol/L) | 337 | 860 (580–1,260) |
| AFP (μg/L) | 337 | 4.6 (1.3–19.6) |
| hCG (mlU/mL)*** | 280 | 61,828 (27,734–131,098) |
Data missing: 8 women for maternal height, 5 women (1%) for smoking, and 1 woman for child sex;
IGF-I, IGF-II, AFP and hCG concentrations were geometric means;
n=280 as samples drawn in 1988 and thereafter were excluded.
Hormone concentrations were in the range expected for that period of pregnancy and their concentrations changed with increasing gestational day for AFP, hCG and slightly for IGF-II, but were stable for IGF-I, as anticipated (Figure 1, Table 2) [14, 19–21]. As expected, the strongest correlation between any two hormones was between IGF-I and IGF-II (r = 0.47). AFP was moderately directly correlated with IGF-II (r = 0.23) and IGF-I (r = 0.14). In general, continuous maternal characteristics were either weakly or not correlated with hormone concentrations (Table 2). Maternal age was inversely associated with IGF-I and IGF-II. In analyses across tertiles of maternal age, all investigated hormones showed a tendency to decrease with increasing maternal age, but significant trend was observed only for levels of IGF-II (Table 3). Maternal weight was only associated (inversely) with hCG, while maternal height was not significantly correlated with any of the hormones (Table 2).
Table 2.
Spearman partial correlation coefficients between hormone levels (log-scale) and maternal characteristics at enrollment in 338 uncomplicated pregnancies from the Northern Sweden Maternity Cohort, 1975–2001&
| Characteristics | IGF-I | IGF-II | AFP | hCG |
|---|---|---|---|---|
| Gestational day | −0.02 | 0.12 * | 0.60 ** | − 0.34 ** |
| Maternal age (years) | − 0.12 * | − 0.33 ** | −0.03 | −0.09 |
| Maternal weight (kg) | 0.06 | 0.01 | −0.04 | − 0.12 * |
| Maternal height (cm) | 0.04 | −0.01 | 0.00 | −0.01 |
| IGF-I (nmol/L) | ||||
| IGF-II (nmol/L) | 0.47 ** | |||
| AFP (μg/L) | 0.14 * | 0.23 ** | ||
| hCG (mlU/mL) | 0.07 | 0.16 * | 0.00 |
p < 0.05
p < 0.001
partial correlations for IGF-I, IGF-II, and AFP were adjusted for a linear term of gestational day; for hCG for a cubic term of gestational day.
Table 3.
Geometric mean (gestational day adjusted)* of maternal IGF-I, IGF-II, AFP and hCG levels by maternal characteristics at enrollment and child sex in 338 uncomplicated pregnancies from the Northern Sweden Maternity Cohort, 1975–2001
| Characteristic | N | IGF-I nmol/L | IGF-II nmol/L | AFP ug/L | hCG mlU/mL |
|---|---|---|---|---|---|
| Maternal age (years) | |||||
| 1st tertile (<28.6) | 112 | 16.6 | 955 | 5.3 | 62,454 |
| 2nd tertile (28.6–33.8) | 114 | 15.3 | 872 | 4.3 | 65,674 |
| 3rd tertile (≥33.8) | 112 | 15.3 | 763 | 4.4 | 57,514 |
| p-trend | 0.13 | <0.0001 | 0.13 | 0.30 | |
| Parity | |||||
| Primiparous | 175 | 15.3 | 923 | 4.6 | 66,434 |
| Multiparous | 163 | 16.2 | 797 | 4.7 | 57,936 |
| p-value | 0.23 | <0.0001 | 0.70 | 0.03 | |
| Maternal weight (kg) | |||||
| 1st tertile (<58.0) | 111 | 15.2 | 871 | 4.7 | 62,615 |
| 2nd tertile (58.0–65.3) | 114 | 16.0 | 841 | 4.6 | 66,019 |
| 3rd tertile (≥65.3) | 113 | 16.0 | 867 | 4.7 | 57,094 |
| p-trend | 0.37 | 0.93 | 0.96 | 0.25 | |
| Maternal height (cm) | |||||
| 1st tertile (<163) | 117 | 15.4 | 860 | 4.7 | 62,141 |
| 2nd tertile (163–168) | 106 | 15.2 | 846 | 4.2 | 62,042 |
| 3rd tertile (≥168) | 107 | 16.4 | 875 | 4.9 | 61,485 |
| p-trend | 0.27 | 0.68 | 0.80 | 0.90 | |
| Maternal smoking | |||||
| Yes | 78 | 16.7 | 865 | 5.1 | 54,298 |
| No | 255 | 15.4 | 861 | 4.5 | 64,584 |
| p-value | 0.13 | 0.91 | 0.27 | 0.02 | |
| Child sex | |||||
| Boy | 177 | 15.3 | 855 | 4.8 | 61,096 |
| Girl | 160 | 16.3 | 866 | 4.6 | 62,747 |
| p-value | 0.14 | 0.69 | 0.69 | 0.68 |
for IGF-I, IGF-II, and AFP, geometric mean adjusted for a linear term of gestational day; for hCG, geometric mean adjusted for a cubic term of gestational day.
In comparison with primiparous women, women who had already delivered a child had lower concentrations of IGF-II and hCG, while their levels of IGF-I and AFP were slightly higher, but the difference was significant only for IGF-II and hCG (Table 3). Mothers who smoked had significantly lower hCG concentrations than non-smoking mothers. There were no differences in hormone levels by child sex.
Multivariate regression models including baseline maternal characteristics (age, parity, weight, height and smoking) showed that age was independently associated with lower IGFs, and that multiparous women had higher IGF-I but lower hCG than primiparous women (Table 4). Maternal weight was associated inversely with hCG and smoking women had significantly lower hCG (Table 4). Addition of child sex to any of these models had only minor, negligible effect on the estimates. There was no indication of interaction between maternal age and parity for any of the 4 hormones. Analyses using local linear regression to account for variation of hormone levels with gestational age yielded almost identical results.
Table 4.
Percent change for hormone concentrations per unit increase in the independent variables (p values) from multivariate linear regression models with adjustments for maternal age, parity, weight, height, and smoking at enrollment in 338 uncomplicated pregnancies from the Northern Sweden Maternity Cohort, 1975–2001*
| Independent variable | Dependent variable (hormones) | |||
|---|---|---|---|---|
| IGF-I | IGF-II | AFP | hCG | |
| Maternal age (years) | −0.014 (0.01) | −0.017 (<0.0001) | ||
| Multiparous vs. primiparous | 0.108 (0.04) | −0.150 (0.05) | ||
| Maternal weight (kg) | −0.010 (0.01) | |||
| Maternal smoking (yes vs. no) | −0.166 (0.03) | |||
for IGF-I, IGF-II, and AFP, models were adjusted for a linear term of gestational day; for hCG, models were adjusted for a cubic term of gestational day.
Discussion
To date, few studies have investigated in detail correlates of IGF-I, IGF-II, AFP and hCG during the first trimester of pregnancy. Our analyses indicated that several maternal characteristics, including age, parity, weight, and smoking are associated with hormone concentrations.
Maternal age
Greater maternal age was inversely correlated with both IGF-I and II, and the association remained significant after adjustments for other baseline maternal characteristics. The observed relationship between age and IGF-I is consistent with the well-established effect of age on hormone concentrations staring from puberty until very old ages [22]. In contrast, no association of IGF-II with age has been reported in non-pregnant women or in men [22]. Little is known about IGF-II regulation both during and out of pregnancy and these results warrant further investigation. No effect of maternal age on hCG and AFP concentrations was observed, in line with observations for these hormones during the second trimester of pregnancy in previous studies [23].
Parity
In comparison with women carrying their first child, multiparous women had significantly lower levels of hCG, consistent with observations about the effect of parity on hCG concentrations during the second trimester of pregnancy in previous studies [23–25]. The reason for this decline, particularly for a hormone of placental origin is unknown [24]. Parous women had higher concentrations of IGF-I, which could be an effect observed only during pregnancy as IGF-I does not differ by parity in non-pregnant women [26–28]. For IGF-II, after the effect of age was taken into account, multiparous women had hormone concentrations similar to those of primiparous women. No effect of parity on AFP was observed, consistent with the observations in the second trimester in previous studies [24, 29]. To our knowledge only one study has reported first trimester AFP concentrations according to parity, which appeared to be slightly reduced only in women with more than 2 previous pregnancies, as compared to primiparous [30].
Maternal weight
Maternal weight is associated with decreased maternal concentrations of hormones of placental and fetal origin, as the amount of hormone secreted is more diluted in the larger blood volume of heavy-set women [30]. A wealth of cross-sectional studies has shown an inverse association of maternal weight with second trimester hCG and AFP [24]. Similar association have been reported for first trimester AFP and free β-hCG (the β-subunit of hCG, moderately correlated with hCG [30, 31]. In our data, we observed the expected inverse association between maternal weight at blood donation and hCG, but not with AFP. No association of maternal weight with IGF-I or IGF-II was observed, consistent with observations in non-pregnant women [32–35]. No association of pre-pregnancy BMI with second / third trimester IGF-I concentrations was reported in one other study [36].
Smoking
Mothers who reported smoking at enrollment had lower hCG concentrations than non-smoking mothers, consistent with data indicative of decreased hCG or free β-hCG both during the first [37, 38] and second trimester in smoking mothers [24, 29, 39, 40]. Interestingly, no correlation between hCG and number of cigarettes smoked per day has been observed [24, 39]. It has been proposed that morphological changes of the villus barrier and the trophoblasts in the placenta of women who smoke may account for the decrease in hCG concentrations [29, 30]. The absence of an effect of smoking on first trimester AFP is consistent with previous studies [30]. Only a negligible increase in second trimester AFP associated with smoking has been reported [24]. Similarly, the absence of an effect of smoking on first trimester IGFs is in line with observations obtained soon after childbirth [41] or outside pregnancy [36, 28, 42].
Child sex
No differences in hormone levels by child sex were observed, although women carrying a female fetus had slightly higher first trimester IGF-I and hCG (Table 3). Mothers of girls have consistently been reported to have higher hCG during the first [30, 43] and the second trimester [44] than mothers of boys. Differences in hCG by fetal sex have been observed even in very early pregnancy (3 week) [43] and might result from differential expression of genes (e.g., inactivation of X-linked genes in the placenta) that play a role in hCG metabolism [43]. The absence of an effect of child sex on first trimester AFP has been reported also by others [30, 45]. It should be noted, however, that most studies show differences during the second trimester, with mothers of girls having lower concentrations than mothers of boys [46, 47]. No differences in maternal IGF-I by fetal sex have been reported previously [48], despite that cord blood IGF-I has been found to be higher in girls than in boys [49–51]. However, IGF-I does not cross the placenta into fetal circulation [52].
The effect of pregnancy on maternal breast cancer risk is likely to be the result of the cumulative hormonal exposure throughout its duration [8, 53]. Early hormonal events would establish the basis for the more profound changes that occur later in gestation. Furthermore some degrees of correlation (usually about 0.40 or higher) between hormone concentrations during the first and second or third trimester of the same pregnancy [54] or during the first trimester of successive pregnancies have been reported by others [55, 56]. For hCG in particular, concentrations during the mid and latter parts of the first trimester maybe the most etiologically relevant in terms of breast cancer risk, as during this period of gestation they peak and the proportion of the intact, biologically active form of the hormone is highest [57]. It has been proposed that hCG plays an important role in breast differentiation during pregnancy which will ultimately result in reduced risk of maternal breast cancer [58]. Support for this hypothesis comes from animal experiments showing that short term treatment of young virgin animals with recombinant hCG induces protection similar to that conferred by pregnancy [59] and from epidemiological observations that women who took hCG as part of a weight loss program were at decreased risk of breast cancer [60] and that elevated hCG concentrations during first trimester pregnancy could be inversely related to risk of breast cancer [5]. The lower hCG concentrations in women with greater weight fit epidemiological observations of increased postmenopausal breast cancer risk associated with pregnancy weight gain [61]. The high hCG concentrations in primiparous women are in line with the important effect of the first full-term pregnancy, particularly at an early age, on maternal risk of breast cancer [62]. Most studies have not shown an association of smoking with breast cancer [63, 64], but some have indicated that smoking particularly at early age may be associated with increased risk [65, 66]. One might speculate that women who smoke at very young age (as teenagers) are more likely to continue to smoke also during pregnancy and thus experience lower hCG concentrations and less breast differentiation.
Studies within the NSMC have indicated that women with elevated IGF-I during the first trimester of pregnancy are at increased risk of breast cancer [13, 16]. IGF-I levels were higher in younger women and multiparous women, but the relevance of these findings to the pregnancy association with maternal breast cancer risk is unclear. The observation of inverse association between maternal age and IGF-II during pregnancy is novel, as very little is known about the regulation of this hormone, but awaits confirmation from further studies.
Strengths of our study are its relatively large sample size and the availability of measurements of several hormones implicated in breast cancer pathogenesis. Direct abstracting of pregnancy data from medical records ensured high quality of the information at hand. Recently we reported excellent correspondence between smoking reported in the medical record and as assessed by cotinine measurements in maternal serum (Kappa statistic of 0.84) [16]. Weaknesses include the lack of detail about smoking which precluded more in-depth dose-response analyses. Furthermore, there were no data on pregnancy conditions, such as diabetes and pre-eclampsia, which may influence maternal hormones. The long-term storage of the serum specimens at relatively high temperature (−20°C) could have caused some analyte deterioration. However, it was reassuring that hormonal variations with gestational day closely followed what has been reported in the literature [14, 19–21] and with the exception of hCG degradation in samples collected after 1988, there was no indication that duration of storage influenced hormone concentrations.
In conclusion, we observed that maternal age, parity and weight are associated with hCG or IGFs concentrations during early pregnancy. Among these, of particular importance are the effects of smoking and maternal weight as they are potentially modifiable.
Acknowledgements
This work was supported by the US National Cancer Institute [CA114329 and CA120061]. The authors are indebted to Hubert Sjodin, Soren Holmgren and Lena Selbrand for their excellent technical assistance in the conduct of the study.
Footnotes
Conflict of interest: None declared.
References
- 1.Medina D. Mammary developmental fate and breast cancer risk. Endocr Relat Cancer. 2005;12:483–495. doi: 10.1677/erc.1.00804. [DOI] [PubMed] [Google Scholar]
- 2.Russo IH, Russo J. Primary prevention of breast cancer by hormone-induced differentiation. Recent Results Cancer Res. 2007;174:111–130. doi: 10.1007/978-3-540-37696-5_11. [DOI] [PubMed] [Google Scholar]
- 3.Richardson BE, Peck JD, Wormuth JK. Mean arterial pressure, pregnancy-induced hypertension, and preeclampsia: evaluation as independent risk factors and as surrogates for high maternal serum alpha-fetoprotein in estimating breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:1349–1355. [PubMed] [Google Scholar]
- 4.Melbye M, Wohlfahrt J, Lei U, et al. alpha-fetoprotein levels in maternal serum during pregnancy and maternal breast cancer incidence. J Natl Cancer Inst. 2000;92:1001–1005. doi: 10.1093/jnci/92.12.1001. [DOI] [PubMed] [Google Scholar]
- 5.Lukanova A, Andersson R, Wulff M, et al. Human chorionic gonadotropin and alpha-fetoprotein concentrations in pregnancy and maternal risk of breast cancer: a nested case-control study. Am J Epidemiol. 2008;168:1284–1291. doi: 10.1093/aje/kwn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8:1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 7.Innes KE, Byers TE. Preeclampsia and breast cancer risk. Epidemiology. 1999;10:722–732. [PubMed] [Google Scholar]
- 8.Troisi R, Hoover RN, Thadhani R, et al. Maternal, prenatal and perinatal characteristics and first trimester maternal serum hormone concentrations. Br J Cancer. 2008;99:1161–1164. doi: 10.1038/sj.bjc.6604639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia. 2000;5:7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- 10.Russo J, Wilgus G, Russo IH. Susceptibility of the mammary gland to carcinogenesis: I Differentiation of the mammary gland as determinant of tumor incidence and type of lesion. Am J Pathol. 1979;96:721–736. [PMC free article] [PubMed] [Google Scholar]
- 11.Mullis PE, Tonella P. Regulation of fetal growth: consequences and impact of being born small. Best Pract Res Clin Endocrinol Metab. 2008;22:173–190. doi: 10.1016/j.beem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Garner M, Turner MC, Ghadirian P, et al. Testicular cancer and hormonally active agents. J Toxicol Environ Health B Crit Rev. 2008;11:260–275. doi: 10.1080/10937400701873696. [DOI] [PubMed] [Google Scholar]
- 13.Lukanova A, Toniolo P, Zeleniuch-Jacquotte A, et al. Insulin-like Growth Factor I in Pregnancy and Maternal Risk of Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2489–2493. doi: 10.1158/1055-9965.EPI-06-0625. [DOI] [PubMed] [Google Scholar]
- 14.Speroff L, Fritz M. The endocrinology of pregnancy. In: Speroff L, Fritz M, editors. Clinical Gynecologic Endocrinology and Infertility. 7 ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. pp. 259–318. [Google Scholar]
- 15.Lund PK. Insulin-Like Growth Factors: Gene Structure and Regulation. In: Kostyo JL, Goodman HM, editors. Hormonal Control of Growth. Oxford University Press; New York: 1999. pp. 537–571. [Google Scholar]
- 16.Chen T, Lukanova A, Kjell G, et al. IGF-I during primiparous pregnancy and maternal risk of breast cancer. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0519-6. Doi:10.1007/s10549-009-0519-6 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18:143–148. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland WS, Loader C. Smoothing by local regression: Priciples and Methods. In: Schimek MG, editor. Statistical Theory and Computational Aspects of Smoothing. Springer; New York: 1996. pp. 113–120. [Google Scholar]
- 19.Monaghan JM, Godber IM, Lawson N, et al. Longitudinal changes of insulin-like growth factors and their binding proteins throughout normal pregnancy. Ann Clin Biochem. 2004;41:220–226. doi: 10.1258/000456304323019596. [DOI] [PubMed] [Google Scholar]
- 20.Olajide F, Kitau MJ, Chard T. Maternal serum AFP levels in the first trimester of pregnancy. Eur J Obstet Gynecol Reprod Biol. 1989;30:123–128. doi: 10.1016/0028-2243(89)90058-0. [DOI] [PubMed] [Google Scholar]
- 21.Stenman UH, Tiitinen A, Alfthan H, Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update. 2006;12:769–784. doi: 10.1093/humupd/dml029. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Mistry J, Nicar MJ, et al. Insulin-like growth factors (IGF-I, free IGF-I and IGF-II) and insulin-like growth factor binding proteins (IGFBP-2, IGFBP-3, IGFBP-6, and ALS) in blood circulation. J Clin Lab Anal. 1999;13:166–172. doi: 10.1002/(SICI)1098-2825(1999)13:4<166::AID-JCLA5>3.0.CO;2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald NJ, Watt HC. Serum markers for Down's syndrome in relation to number of previous births and maternal age. Prenat Diagn. 1996;16:699–703. doi: 10.1002/(SICI)1097-0223(199608)16:8<699::AID-PD919>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down's syndrome. Health Technol Assess. 1998;2:i–iv. 1–112. [PubMed] [Google Scholar]
- 25.Arslan AA, Zeleniuch-Jacquotte A, Lukanova A, et al. Effects of parity on pregnancy hormonal profiles across ethnic groups with a diverse incidence of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2123–2130. doi: 10.1158/1055-9965.EPI-06-0470. [DOI] [PubMed] [Google Scholar]
- 26.Lukanova A, Toniolo P, Akhmedkhanov A, et al. A cross-sectional study of IGF-I determinants in women. Eur J Cancer Prev. 2001;10:443–452. doi: 10.1097/00008469-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Variation in plasma insulin-like growth factor-1 and insulin-like growth factor binding protein-3: personal and lifestyle factors (United States) Cancer Causes Control. 2005;16:917–927. doi: 10.1007/s10552-005-2702-3. [DOI] [PubMed] [Google Scholar]
- 28.DeLellis K, Rinaldi S, Kaaks RJ, et al. Dietary and lifestyle correlates of plasma insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3): the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1444–1451. [PubMed] [Google Scholar]
- 29.Tislaric D, Brajenovic-Milic B, Ristic S, et al. The influence of smoking and parity on serum markers for Down's syndrome screening. Fetal Diagn Ther. 2002;17:17–21. doi: 10.1159/000047999. [DOI] [PubMed] [Google Scholar]
- 30.de Graaf IM, Cuckle HS, Pajkrt E, et al. Co-variables in first trimester maternal serum screening. Prenat Diagn. 2000;20:186–189. [PubMed] [Google Scholar]
- 31.Canick JA, Lambert-Messerlian GM, Palomaki GE, et al. Comparison of serum markers in first-trimester down syndrome screening. Obstet Gynecol. 2006;108:1192–1199. doi: 10.1097/01.AOG.0000241095.19638.f2. [DOI] [PubMed] [Google Scholar]
- 32.Voskuil DW, Buenode Mesquita HB, Kaaks R, et al. Determinants of circulating insulin-like growth factor (IGF)-I and IGF binding proteins 1–3 in premenopausal women: physical activity and anthropometry (Netherlands) Cancer Causes Control. 2001;12:951–958. doi: 10.1023/a:1013708627664. [DOI] [PubMed] [Google Scholar]
- 33.Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13:113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 34.Hunt KJ, Toniolo P, Akhmedkhanov A, et al. Insulin-like growth factor II and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev. 2002;11:901–905. [PubMed] [Google Scholar]
- 35.Walker K, Fletcher O, Johnson N, et al. Premenopausal Mammographic Density in Relation to Cyclic Variations in Endogenous Sex Hormone Levels, Prolactin, and Insulin-like Growth Factors. Cancer Research. 2009;69:6490–6499. doi: 10.1158/0008-5472.CAN-09-0280. [DOI] [PubMed] [Google Scholar]
- 36.Chellakooty M, Vangsgaard K, Larsen T, et al. A longitudinal study of intrauterine growth and the placental growth hormone (GH)-insulin-like growth factor I axis in maternal circulation: association between placental GH and fetal growth. J Clin Endocrinol Metab. 2004;89:384–391. doi: 10.1210/jc.2003-030282. [DOI] [PubMed] [Google Scholar]
- 37.Lambert-Messerlian G, Palomaki GE, Canick JA. Adjustment of serum markers in first trimester screening. J Med Screen. 2009;16:102–103. doi: 10.1258/jms.2009.009028. [DOI] [PubMed] [Google Scholar]
- 38.Bestwick JP, Huttly WJ, Wald NJ. First trimester Down's syndrome screening marker values and cigarette smoking: new data and a meta-analysis on free beta human chorionic gonadotophin, pregnancy-associated plasma protein-A and nuchal translucency. J Med Screen. 2008;15:204–206. doi: 10.1258/jms.2008.008049. [DOI] [PubMed] [Google Scholar]
- 39.Crossley JA, Aitken DA, Waugh SM, Kelly T, Connor JM. Maternal smoking: age distribution, levels of alpha-fetoprotein and human chorionic gonadotrophin, and effect on detection of Down syndrome pregnancies in second-trimester screening. Prenat Diagn. 2002;22:247–255. doi: 10.1002/pd.313. [DOI] [PubMed] [Google Scholar]
- 40.Rudnicka AR, Wald NJ, Huttly W, Hackshaw AK. Influence of maternal smoking on the birth prevalence of Down syndrome and on second trimester screening performance. Prenat Diagn. 2002;22:893–897. doi: 10.1002/pd.440. [DOI] [PubMed] [Google Scholar]
- 41.Ermis B, Altinkaynak K, Yildirim A, Ozkan B. Influence of smoking on serum and milk of mothers, and their infants' serum insulin-like growth factor-I and insulin-like growth factor binding protein-3 levels. Horm Res. 2004;62:288–292. doi: 10.1159/000081974. [DOI] [PubMed] [Google Scholar]
- 42.Allen NE, Appleby PN, Kaaks R, et al. Lifestyle determinants of serum insulin-like growth-factor-I (IGF-I), C-peptide and hormone binding protein levels in British women. Cancer Causes Control. 2003;14:65–74. doi: 10.1023/a:1022518321634. [DOI] [PubMed] [Google Scholar]
- 43.Yaron Y, Lehavi O, Orr-Urtreger A, et al. Maternal serum HCG is higher in the presence of a female fetus as early as week 3 post-fertilization. Hum Reprod. 2002;17:485–489. doi: 10.1093/humrep/17.2.485. [DOI] [PubMed] [Google Scholar]
- 44.Yaron Y, Wolman I, Kupferminc MJ, et al. Effect of fetal gender on first trimester markers and on Down syndrome screening. Prenat Diagn. 2001;21:1027–1030. doi: 10.1002/pd.178. [DOI] [PubMed] [Google Scholar]
- 45.Larsen SO, Wojdemann KR, Shalmi AC, et al. Gender impact on first trimester markers in Down syndrome screening. Prenat Diagn. 2002;22:1207–1208. doi: 10.1002/pd.493. [DOI] [PubMed] [Google Scholar]
- 46.Mueller VM, Huang T, Summers AM, Winsor SH. The effect of fetal gender on the false-positive rate of Down syndrome by maternal serum screening. Prenat Diagn. 2005;25:1258–1261. doi: 10.1002/pd.1318. [DOI] [PubMed] [Google Scholar]
- 47.Spencer K. The influence of fetal sex in screening for Down syndrome in the second trimester using AFP and free beta-hCG. Prenat Diagn. 2000;20:648–651. doi: 10.1002/1097-0223(200008)20:8<648::aid-pd869>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 48.Caufriez A, Frankenne F, Hennen G, Copinschi G. Regulation of maternal IGF-I by placental GH in normal and abnormal human pregnancies. Am J Physiol. 1993;265:E572–E577. doi: 10.1152/ajpendo.1993.265.4.E572. [DOI] [PubMed] [Google Scholar]
- 49.Vatten LJ, Nilsen ST, Odegard RA, Romundstad PR, Austgulen R. Insulin-like growth factor I and leptin in umbilical cord plasma and infant birth size at term. Pediatrics. 2002;109:1131–1135. doi: 10.1542/peds.109.6.1131. [DOI] [PubMed] [Google Scholar]
- 50.Ibanez L, Sebastiani G, Lopez-Bermejo A, et al. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth. J Clin Endocrinol Metab. 2008;93:2774–2778. doi: 10.1210/jc.2008-0526. [DOI] [PubMed] [Google Scholar]
- 51.Engstrom E, Niklasson A, Wikland KA, Ewald U, Hellstrom A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005;57:605–610. doi: 10.1203/01.PDR.0000155950.67503.BC. [DOI] [PubMed] [Google Scholar]
- 52.Lagiou P, Hsieh CC, Lipworth L, et al. Insulin-like growth factor levels in cord blood, birth weight and breast cancer risk. Br J Cancer. 2009;100:1794–1798. doi: 10.1038/sj.bjc.6605074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sivaraman L, Medina D. Hormone-induced protection against breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:77–92. doi: 10.1023/a:1015774524076. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Graubard BI, Klebanoff MA, et al. Maternal hormone levels among populations at high and low risk of testicular germ cell cancer. Br J Cancer. 2005;92:1787–1793. doi: 10.1038/sj.bjc.6602545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernstein L, Lipworth L, Ross RK, Trichopoulos D. Correlation of estrogen levels between successive pregnancies. Am J Epidemiol. 1995;142:625–628. doi: 10.1093/oxfordjournals.aje.a117685. [DOI] [PubMed] [Google Scholar]
- 56.Wald NJ, Huttly WJ, Rudnicka AR. Prenatal screening for Down syndrome: the problem of recurrent false-positives. Prenat Diagn. 2004;24:389–392. doi: 10.1002/pd.890. [DOI] [PubMed] [Google Scholar]
- 57.de Medeiros SF, Norman RJ. Human choriogonadotrophin protein core and sugar branches heterogeneity: basic and clinical insights. Hum Reprod Update. 2009;15:69–95. doi: 10.1093/humupd/dmn036. [DOI] [PubMed] [Google Scholar]
- 58.Russo J, Russo IH. Hormonally induced differentiation: a novel approach to breast cancer prevention. J Cell Biochem Suppl. 1995;22:58–64. doi: 10.1002/jcb.240590809. [DOI] [PubMed] [Google Scholar]
- 59.Kappel B, Hansen K, Moller J, Faaborg-Andersen J. Human placental lactogen and dU-estrogen levels in normal twin pregnancies. Acta Genet Med Gemellol (Roma) 1985;34:59–65. doi: 10.1017/s000156600000492x. [DOI] [PubMed] [Google Scholar]
- 60.Bernstein L, Hanisch R, Sullivan-Halley J, Ross RK. Treatment with human chorionic gonadotropin and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1995;4:437–440. [PubMed] [Google Scholar]
- 61.Kinnunen TI, Luoto R, Gissler M, Hemminki E, Hilakivi-Clarke L. Pregnancy weight gain and breast cancer risk. BMC Womens Health. 2004;4:7. doi: 10.1186/1472-6874-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2:133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 63.Fink AK, Lash TL. A null association between smoking during pregnancy and breast cancer using Massachusetts registry data (United States) Cancer Causes Control. 2003;14:497–503. doi: 10.1023/a:1024922824237. [DOI] [PubMed] [Google Scholar]
- 64.Terry PD, Rohan TE. Cigarette smoking and the risk of breast cancer in women: a review of the literature. Cancer Epidemiol Biomarkers Prev. 2002;11:953–971. [PubMed] [Google Scholar]
- 65.Gram IT, Braaten T, Terry PD, et al. Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev. 2005;14:61–66. [PubMed] [Google Scholar]
- 66.Ishibe N, Hankinson SE, Colditz GA, et al. Cigarette smoking, cytochrome P450 1A1 polymorphisms, and breast cancer risk in the Nurses' Health Study. Cancer Res. 1998;58:667–671. [PubMed] [Google Scholar]